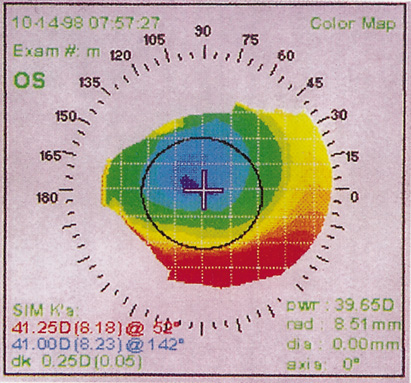
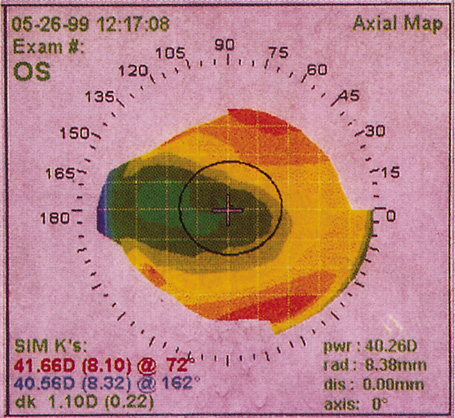
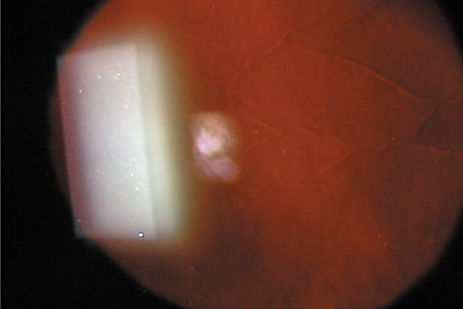
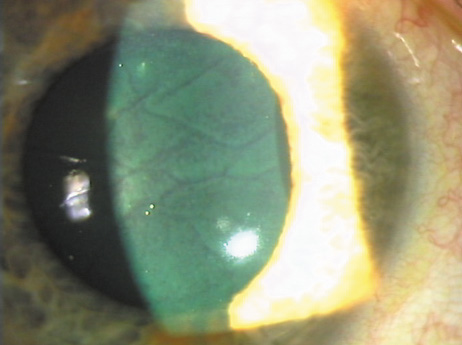
1. Burlamacchi P: Laser sources. In Lasers in Biology and Medicine. New York: Plenum, 1980:1 2. Rhodes CK: Excimer Lasers, 2nd ed. New York: Springer-Verlag, 1984 3. Special issue on excimer laser. IEEE J Quantum Electronics OE 15:337, 1979 4. Searles SK, Hart GA: Stimulated emission at 281.8 nm from XeBr. Appl Phys Lett 27:243, 1975 5. McKee T, Nilson JA: Excimer applications. Laser Focus 18:51, 1982 6. Srinivasan R, Leigh WJ: Ablative photodecomposition on poly(ethylene terephthalate) films. J Am Chem Soc 104:6784, 1982 7. Deutsch TF, Geis MW: Self-developing UV photoresist using excimer laser exposure. J Appl Phys 54:7201, 1983 8. Koren G, Yet JT: Emission spectra, surface quality, and mechanism of excimer laser etching
of polyamide films. Appl Phys Lett 44:1112, 1984 9. Srinivasan R, Mayne-Banton V: Self-developing photoetching of poly(ethylene terephthalate) films
by far-ultraviolet excimer laser radiation. Appl Phys Lett 41:576, 1983 10. Garrison BJ, Srinivasan R: Microscopic model for the ablative photodecomposition of polymers by far-ultraviolet
radiation (193 nm). Appl Phys Lett 44:489, 1984 11. Jellinek HH, Srinivasan R: Theory of etching of polymers by far-violet, high-intensity pulsed laser
and long-term irradiation. J Phys Chem 88:3048, 1984 12. Melcher RL: Thermal and acoustic techniques for monitoring pulsed laser processing. Springer Series Chem Phys 39:418, 1984 13. Srinivasan R: Kinetics of ablative photodecomposition of organic polymers in the far-ultraviolet (193 nm). J Vac Sci Tech 11:923, 1983 14. Srinivasan R, Braren B: Ablative photodecomposition of polymer films by far-violet (193 nm) laser
radiation: Dependence of etch depth on experimental conditions. J Polym Sci Polym Chem Ed 22:2601, 1984 15. Gorodetsky G, Kazyaka TG, Melcher RL, et al: Calorimetric and acoustic study of ultraviolet laser ablation of polymers. Appl Phys Lett 46:828, 1985 16. Slavin W: Stray light in ultraviolet, visible, and near-infrared spectral photometry. Anal Chem 35:561, 1963 17. Beaven GH, Holiday ER: Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem 7:319, 1952 18. Loofbourow JR, Gould BS, Sizer IW: Studies on the ultraviolet absorption spectra of collagen. Arch Biochem 22:406, 1949 19. Smith KC: Ultraviolet radiation effects on molecules and cells. In The Science of Photobiology. New York: Plenum, 1985:113 20. Stone AL: Optical rotary dispersion of mucopolysaccharides: III. Ultraviolet circular
dichroism and conformational specificity in amide groups. Biopolymers 10:739, 1971 21. O'Brien WJ: Measurement of corneal DNA content. Invest Ophthalmol Vis Sci 18:538, 1979 22. Dougherty AM, Causley GC, Johnson WC: Flow dichroism evidence for tilting of the bases when DNA is in solution. Proc Natl Acad Sci USA 80:2194, 1983 23. Ringvold A: Cornea and ultraviolet radiation. Acta Ophthalmol 58:63, 1980 24. McCann J, Ames BN: Detection of carcinogens as mutagens in the salmonella/microsome test: Assay
of 300 chemicals. Discussion. Proc Natl Acad Sci USA 73:950, 1976 25. Nuss RC, Puliafito CA, Dehm EJ: Unscheduled DNA synthesis following excimer laser ablation of the cornea
in vivo. Invest Ophthalmol Vis Sci 28:287, 1987 26. Kochevar IE: Cytotoxicity and mutagenicity of excimer laser radiation. Lasers Surg Med 9:440, 1989 27. Muller-Stolzenburg NW, Muller GJ, Buchwald HJ, et al: UV exposure of the lens during 193-nm excimer laser corneal surgery. Arch Ophthalmol 108:915, 1990 28. Trokel SL, Srinivasan R, Braren B: Excimer laser surgery of the cornea. Am J Ophthalmol 96:710, 1983 29. Puliafito CA, Steinert RF, Deutsch TF, et al: Excimer laser ablation of the cornea and lens: Experimental studies. Ophthalmology 92:741, 1985 30. Kerr-Muir MG, Trokel SL, Marshall J, et al: Ultrastructural comparison of conventional surgical and argon fluoride
excimer laser keratectomy. Am J Ophthalmol 103:448, 1987 31. Peyman GA, Kuszak JR, Weckstrom K, et al: Effects of XeCL excimer laser on the eyelid and anterior segment structures. Arch Ophthalmol 104:118, 1986 32. Krueger RR, Trokel SL: Quantitation of corneal ablation by ultraviolet laser light. Arch Ophthalmol 103:1741, 1985 33. Krueger RR, Trokel SL, Schubert HD: Interaction of ultraviolet laser light with the cornea. Invest Ophthalmol Vis Sci 26:1455, 1985 34. Puliafito CA, Wong K, Steinert RF: Quantitative and ultrastructural studies of excimer laser ablation of the
cornea at 193 and 248 nanometers. Lasers Surg Med 7:155, 1987 35. Fantes EE, Waring GO: Effects of excimer laser radiant exposure on uniformity of ablated corneal
surface. Lasers Surg Med 9:533, 1989 36. Berns MW, Liaw L-H, Oliva A, et al: An acute light and electron microscopic study of ultraviolet 193-nm excimer
laser corneal incisions. Ophthalmology 95:1422, 1988 37. Dehm EJ, Puliafito CA, Adler CM, et al: Corneal endothelial injury following excimer laser ablation and 193 and 248 nm. Arch Ophthalmol 104:1364, 1986 38. Hanna KD, Pouliquen Y, Waring GO, et al: Corneal stromal wound healing in rabbits after 193-nm excimer laser surface
ablation. Arch Ophthalmol 107:895, 1989 39. Zabel R, Tuft S, Marshall J: Excimer laser photorefractive keratectomy: Endothelial morphology following
area ablation of the cornea. Invest Ophthalmol Vis Sci 29(suppl):390, 1988 40. Puliafito CA, Stern D, Krueger RR, et al: High-speed photography of the excimer laser ablation of the cornea. Arch Ophthalmol 105:1255, 1987 41. Kahle G, Stadter H, Seiler T, et al: Gas chromatograph/mass spectrometer analysis of excimer and erbium-YAG
laser ablated human corneas. Invest Ophthalmol Vis Sci 32(suppl):996, 1991 42. Fagerholm P: Phototherapeutic keratectomy: 12 years of experience. Acta Ophthalmol Scand 81:19, 2003 43. Autrata R, Rehurek J, Vodickova K: Phototherapeutic keratectomy in children: 5-year results. J Cataract Refract Surg 30:1909, 2004 44. Morad Y, Haviv D, Zadok D, et al: Excimer laser phototherapeutic keratectomy for recurrent corneal erosion. J Cataract Refract Surg 24:451, 1998 45. Jain S, Austin DJ: Phototherapeutic keratectomy for treatment of recurrent corneal erosion. J Cataract Refract Surg 25:1610, 1999 46. Lohmann CP, Sachs H, Marshall J, Gabel VP: Excimer laser phototherapeutic keratectomy for recurrent erosions: A clinical
study. Ophthal Surg Lasers 27:768, 1996 47. O'Brart DP, Muir MG, Marshall J: Phototherapeutic keratectomy for recurrent corneal erosions. Eye 8:378, 1994 48. Orndahl MJ, Fagerholm PP: Phototherapeutic keratectomy for map-dot-fingerprint corneal dystrophy. Cornea 17:595, 1998 49. Cavanaugh TB, Lind DM, Cutarelli PE, et al: Phototherapeutic keratectomy for recurrent erosion syndrome in anterior
basement membrane dystrophy. Ophthalmology 106:971, 1999 50. Dausch D, Landesz M, Klein R, Schroder E: Phototherapeutic keratectomy in recurrent corneal epithelial erosion. Refract Corneal Surg 9:419, 1993 51. Ohman L, Fagerholm P, Tengroth B: Treatment of recurrent corneal erosions with the excimer laser. Acta Ophthalmol 72:461, 1994 52. Bernauer W, De Cock R, Dart JK: Phototherapeutic keratectomy in recurrent corneal erosions refractory to
other forms of treatment. Eye 10:561, 1996 53. Forster W, Atzler U, Ratkay I, Busse H: Therapeutic use of the 193-nm excimer laser in corneal pathologies. Graefes Arch Clin Exp Ophthalmol 235:296, 1997 54. Fagerholm P, Fitzsimmons TD, Orndahl M, et al: Phototherapeutic keratectomy: Long-term results in 166 eyes. Refract Corneal Surg 9:S76, 1993 55. Moniz N, Fernandez ST: Efficacy of phototherapeutic keratectomy in various superficial corneal
pathologies. J Refract Surg 19:S243, 2003 56. Cavanaugh TB, Lind DM, Cutarelli PE, et al: Phototherapeutic keratectomy for recurrent erosion syndrome in anterior
basement membrane dystrophy. Ophthalmology 106:971, 1999 57. Dogru M, Katakami C, Miyashita M, et al: Ocular surface changes after excimer laser phototherapeutic keratectomy. Ophthalmology 107:1144, 2000 58. Sridhar MS, Rapuano CJ, Cosar CB, Cohen EJ, Laibson PR: Phototherapeutic keratectomy versus diamond burr polishing of Bowman's
membrane in the treatment of recurrent corneal erosions associated
with anterior basement membrane dystrophy. Ophthalmology 109:674, 2002 59. Rosa N, Cennamo G: Phototherapeutic keratectomy for relief of pain in patients with pseudophakic
bullous keratopathy. J Refract Surg 18:276, 2002 60. Maini R, Sullivan L, Snibson GR, Taylor HR, Loughnan MS: A comparison of different depth ablations in the treatment of painful bullous
keratopathy with phototherapeutic keratectomy. Br J Ophthalmol 85:912, 2001 61. Koksal M, Kargi S, Gurelik G, Akata F: Phototherapeutic keratectomy in Schnyder crystalline corneal dystrophy. Cornea 23:311, 2004 62. Germundsson J, Fagerholm P: Phototherapeutic keratectomy in Salzmann's nodular dystrophy. Acta Ophthalmol Scand 82:148, 2004 63. Stewart OG, Pararajasegaram P, Cazabon J, Morrell AJ: Visual and symptomatic outcome of phototherapeutic keratectomy (PTK) for
corneal dystrophies. Eye 16:126, 2002 64. Kornmehl EW, Steinert RF, Puliafito CA: A comparison study of masking fluids for excimer laser phototherapeutic
keratectomy. Arch Ophthalmol 109:860, 1991 65. Alio JL, Belda JI, Shalaby AM: Correction of irregular astigmatism with excimer laser assisted sodium
hyaluronate. Ophthalmology 108:1246, 2001 66. Katsanevaki VJ, Ginis HS, Naoumidi II, Pallikaris IG: The PALM technique: histologic findings of masked phototherapeutic keratectomy
on rabbit corneas. BMC Ophthalmol 3:4, 2003 67. Kremer F, Aronsky M, Bowyer B, Stevens SX: Treatment of corneal surface irregularities using biomask as an adjunct
to excimer laser phototherapeutic keratectomy. Cornea 21:28, 2002 68. Maloney RK, Thompson V, Ghiselli G, et al: A prospective multicenter trial of excimer laser phototherapeutic keratectomy
for corneal vision loss. The Summit Phototherapeutic Keratectomy
Study Group. Am J Ophthalmol 122:149, 1996 69. Sher NA, Bowers RA, Zabel RW, et al: Clinical use of the 193-nm excimer laser in the treatment of corneal scars. Arch Ophthalmol 109:491, 1991 70. Stark WJ, Chamon W, Kamp MT, et al: Clinical follow-up of 193-nm ArF excimer laser photokeratectomy. Ophthalmology 99:805, 1992 71. Campos M, Nielsen S, Szerenyi K, et al: Clinical follow-up of phototherapeutic keratectomy for treatment of corneal
opacities. Am J Ophthalmol 115:433, 1993 72. Rapuano CJ: Excimer laser phototherapeutic keratectomy: Long-term results and practical
considerations. Cornea 16:151, 1997 73. Stark WJ, Gilbert ML, Gottsch GD, Munnerlyn C: Optical pachymetry in the measurement of anterior corneal disease: An evaluative
tool for phototherapeutic keratectomy. Arch Ophthalmol 108:12, 1990 74. Stewart OG, Morrell AJ: Management of band keratopathy with excimer laser phototherapeutic keratectomy: Visual, refractive, and symptomatic outcome. Eye 17:233, 2003 75. Amm M, Duncker GI: Refractive changes after phototherapeutic keratectomy. J Cataract Refract Surg 23:839, 1997 76. McGhee CNJ, Bryce IG: Natural history of central topographic islands following excimer laser
photorefractive keratectomy. J Cataract Refract Surg 22:1151, 1996 77. Rapuano CJ: Excimer laser phototherapeutic keratectomy in eyes with anterior corneal
dystrophies: preoperative and postoperative ultrasound biomicroscopic
examination and short-term clinical outcomes with and without antihyperopia
treatment. Trans Am Ophthalmol Soc 101:371, 2003 78. Katsube N, Wang R, Okuma E, Roberts C: Biomechanical response of the cornea to phototherapeutic keratectomy when
treated as a fluid-filled porous material. J Refract Surg 18:S593, 2002 79. Porges Y, Ben-Haim O, Hirsh A, Levinger S: Phototherapeutic keratectomy with mitomycin C for corneal haze following
photorefractive keratectomy for myopia. J Refract Surg 19:40, 2003 80. Dinh R, Rapuano CJ, Cohen EJ, Laibson PR: Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology 106:1490, 1999 81. Miller A, Solomon R, Bloom A, et al: Prevention of recurrent Reis-Bucklers dystrophy following excimer laser
phototherapeutic keratectomy with topical mitomycin C. Cornea 23:732, 2004 82. Pfister RR: Permanent corneal edema resulting from the treatment of PTK corneal haze
with mitomycin: A case report. Cornea 23:744, 2004 83. Szentmary N, Langenbucher A, Hafner A, Seitz B: Impact of phototherapeutic keratectomy on the outcome of subsequent penetrating
keratoplasty in patients with stromal corneal dystrophies. Am J Ophthalmol 137:301, 2004 84. Abbas UL, Hersh PS: Natural history of corneal topography after excimer laser photorefractive
keratectomy. Ophthalmology 108(5):953, 2001 85. Marques EF, Leite EB, Cunha-Vaz JG: Corticosteroids for reversal of myopic regression after photorefractive
keratectomy. J Refract Surg 11:S302, 1995 86. Pop M, Aras M: Photorefractive keratectomy retreatments for regression: One-year follow-up. Ophthalmology 103:96, 1979 87. Gartry DS, Larkin DF, Hill AR, et al: Retreatment for significant regression after excimer laser photorefractive
keratectomy: A prospective, randomized, masked trial. Ophthalmology 105:131, 1998 88. Lee HK, Kim JK, Kim EK, Kim GO, Lee IS: Phototherapeutic keratectomy with amniotic membrane for severe subepithelial
fibrosis following excimer laser refractive surgery. J Cataract Refract Surg 29:1430, 2003 89. Seiler T, Schmidt-Petersen H, Wollensak J: Complications after myopic photorefractive keratectomy primarily with the
Summit excimer laser. In Corneal Laser Surgery. St. Louis: Mosby, 1995:131 90. Alkara N, Genth U, Seiler T: Diametral ablation: A technique to manage decentered photorefractive keratectomy
for myopia. J Refract Surg 15:436, 1999 91. Lim-Bon-Siong R, Williams JM, Steinert RS, Pepose JS: Retreatment of decentered excimer photorefractive keratectomy ablations. Am J Ophthalmol 123:122, 1997 92. Talamo JH, Wagoner MD, Lee SY: Management of ablation decentration following excimer laser photorefractive
keratectomy. Arch Ophthalmol 113:706, 1995 93. Rachid MD, Yoo SH, Azar DT: Phototherapeutic keratectomy for decentration and central islands after
photorefractive keratectomy. Ophthalmology 108:545, 2001 94. Pallikaris I, Siganos D: LASIK complications management. In The Excimer Manual. Boston: Little, Brown, 1997:227 95. Kapadia MS, Wilson SE: Transepithelial photorefractive keratectomy for treatment of thin flaps
or caps after complicated laser in situ keratomileusis. Am J Ophthalmol 126:827, 1998 96. Leu G, Hersh P: Phototherapeutic keratectomy for the treatment of diffuse lamellar keratitis. J Cataract Refract Surg 28:1471, 2002 97. Rojas MC, Manche EE: Phototherapeutic keratectomy for anterior basement membrane dystrophy after
laser in situ keratomileusis. Arch Ophthalmol 120:722, 2002 98. Steinert RF, Ashrafzadeh A, Hersh PS: Results of phototherapeutic keratectomy in the management of flap striae
after LASIK. Ophthalmology 111:740, 2004 99. Wiesinger-Jendritza B, Knorz MC, Hugger P, Liermann A: Laser in situ keratomileusis assisted by corneal topography. J Cataract Refract Surg 24:166, 1998 100. Vinciguerra P, Camesasca FI: Custom phototherapeutic keratectomy with intraoperative topography. J Refract Surg 20:S555, 2004 101. Vinciguerra P, Camesasca FI: One year follow-up of custom phototherapeutic keratectomy. J Refract Surg 20:S705, 2004 |