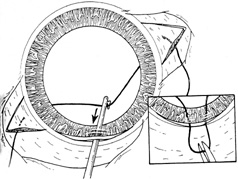
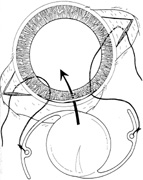
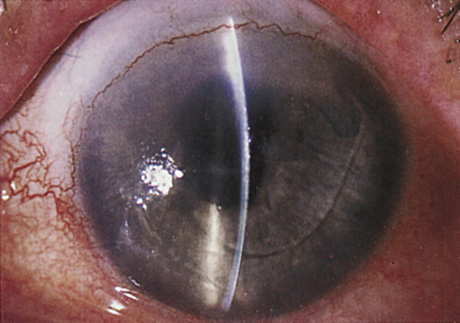
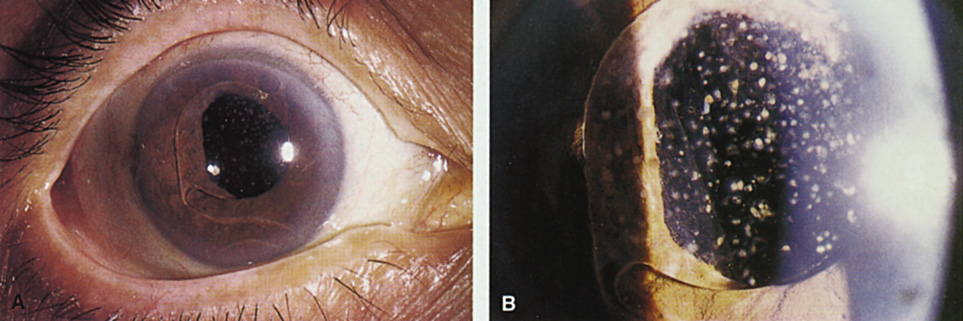
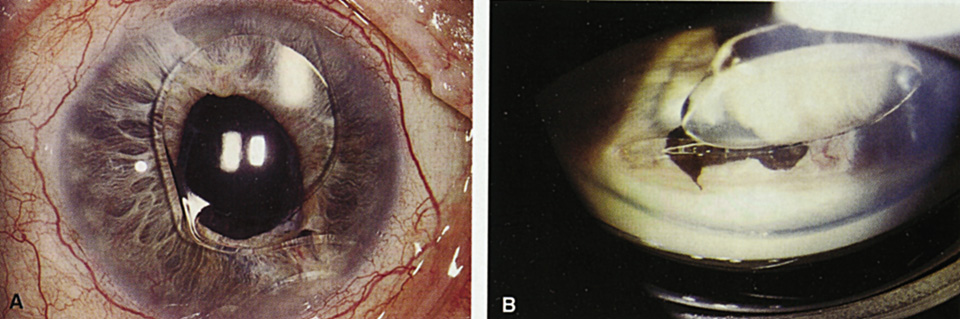
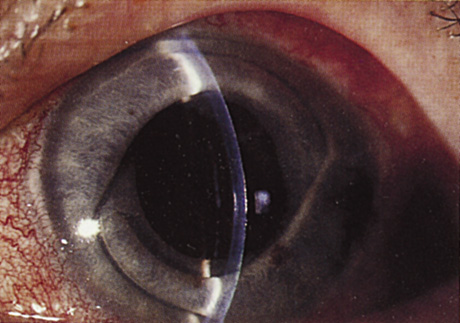
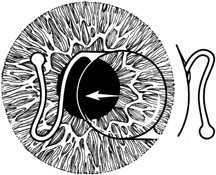
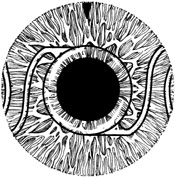
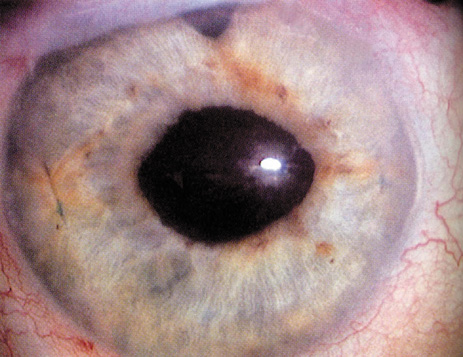
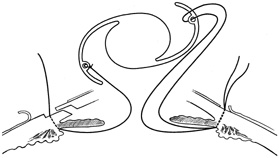
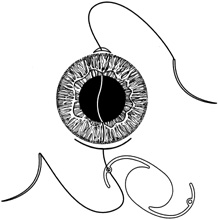
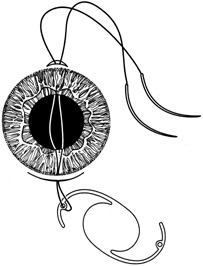
1. Kaufman HE: The correction of aphakia. XXXVI Edward Jackson Memorial Lecture. Am J Ophthalmol 89:1, 1980 2. Barraquer JI: Modification of refraction by means of intracorneal inclusions. Int Ophthalmol Clin 6:53, 1966 3. McDonnell PJ, Dietze T, Durrie DS, Schanzlin DJ: Surgical correction of aphakia: Intraocular lens implantation and epikeratophakia. J Refract Surg 3:209, 1987 4. Lane SL, Lindstrom RL, Cameron JD, et al: Polysulfone corneal lenses. J Cataract Refract Surg 12:50, 1986 5. Horgan SE, Fraser SG, Choyce DP, Alexander WL: Twelve year follow-up of unfenestrated polysulfone intracorneal lenses
in human sighted eyes. J Cataract Refract Surg 22:1045, 1996 6. Steinert RF, Storie B, Smith P, et al: Hydrogel intracorneal lenses in aphakic eyes. Arch Ophthalmol 114:135, 1996 7. Wong SK, Koch DD, Emery JM: Secondary intraocular lens implantation. J Cataract Refract Surg 13:17, 1987 8. Ellington FT: Complications with Choyce Mark VIII and Mark IV anterior chamber implants: Uveitis, glaucoma, hyphema. Am Intraocular Implant Soc J 3:199, 1977 9. Apple DJ, Mamalis N, Lotfield K, et al: Complications of intraocular lenses: A historical and histopathological
review. Surv Ophthalmol 29:1, 1984 10. Kraff MC, Sanders DR, Lieberman HL: Monitoring for continuing endothelial cell loss with cataract extraction
and intraocular lens implantation. Ophthalmology 98:30, 1982 11. Sugar A, Meyer RF, Heidemann D, et al: Specular microscopic followup of corneal grafts for pseudophakic bullous
keratopathy. Ophthalmology 92:325, 1985 12. Busin M, Arffa RO, McDonald MB, et al: Intraocular lens removal during penetrating keratoplasty for pseudophakic
bullous keratopathy. Ophthalmology 94:505, 1987 13. Smith PW, Wong SK, Stark WJ, et al: Complications of semiflexible closed-loop anterior chamber intraocular
lenses. Arch Ophthalmol 105:52, 1987 14. Speaker MG, Lugo M, Laibson PR, et al: Penetrating keratoplasty for pseudophakic bullous keratopathy: Management
of intraocular lens. Ophthalmology 95:1260, 1988 15. Cohen EJ, Brady SE, Leavitt K, et al: Pseudophakic bullous keratopathy. Am J Ophthalmol 106:264, 1988 16. Waring GO: The 50-year epidemic of pseudophakic corneal edema. Arch Ophthalmol 107:657, 1989 17. Insler S, Kook MS, Kaufmann HE: Penetrating keratoplasty for pseudophakic bullous keratopathy associated
with semi-flexible, close-loop anterior chamber intraocular lenses. Am J Ophthalmol 107:252, 1989 18. Koenig SB, McDermott ML, Hyndluk RA: Penetrating keratoplasty and intraocular lens exchange for pseudophakic
bullous keratopathy associated with a closed-loop anterior chamber intraocular
lens. Am J Ophthalmol 108:43, 1989 19. Kornmehl EW, Steinert RF, Odrich MG, et al: Penetrating keratoplasty for pseudophakic bullous keratopathy associated
with closed-loop anterior chamber intraocular lenses. Ophthalmology 97:407, 1990 20. Binkhorst CD: Corneal and retinal complications after cataract extraction: The mechanical
aspect of endophthalmodonesis. Ophthalmology 87:609, 1980 21. Drews RC: Intermittent touch syndrome. Arch Ophthalmol 110:1440, 1982 22. Stark WJ, Worthen DM, Holladay JT, et al: The FDA report on intraocular lenses. Ophthalmology 90:311, 1983 23. McDonnell PJ, Green WR, Maumenee AE, et al: Pathology of intraocular lenses in 33 eyes examined postmortem. Ophthalmology 90:386, 1983 24. Champion R, McDonnell PJ, Green WR: Intraocular lenses: Histopathologic characteristics of a large series of
autopsy eyes. Surv Ophthalmol 30:1, 1985 25. Apple DJ, Olson RJ: Closed-loop anterior chamber lenses. Arch Ophthalmol 105:52, 1987 26. Apple DJ, Brems RN, Park RB, et al: Anterior chamber lenses. Part I: Complications and pathology and a review
of design. J Cataract Refract Surg 13:157, 1987 27. Lorusso V, Moramarco A, Pacella E, Balacco-Gabrieli C: Intraocular lens complications. Ann Ophthalmol 22:377, 1990 28. Champion R, Green WR: Intraocular lenses: A histopathologic study of eyes, ocular tissue and
intraocular lenses obtained surgically. Ophthalmology 92:1628, 1985 29. Maynor RCJ: Five cases of severe anterior chamber lens implant complications. Am Intraocular Implant Soc J 10:223, 1984 30. Lyle WA, Jin JC: Secondary intraocular lens implantation: Anterior chamber vs. posterior
chamber lenses. Ophthal Surg 24:375, 1993 31. Price FWJ, Whitson WE, Collins K, Johns S: Changing trends in explanted intraocular lenses: A single center study. J Cataract Refract Surg 18:470, 1992 32. Lindstrom RL, Harris WS: Secondary anterior chamber lens implantation. CLAO J 10:133, 1984 33. Weene LE: Flexible open-loop anterior chamber intraocular lens implants. Ophthalmology 100:1636, 1993 34. Waring GO, Stulting RD, Street D, et al: Penetrating keratoplasty for pseudophakic corneal edema with exchange of
intraocular lenses. Arch Ophthalmol 105:58, 1987 35. Lim ES, Apple DJ, Tsai JC, et al: An analysis of flexible anterior chamber lenses with special reference
to the normalized rate of lens explantation. Ophthalmology 98:243, 1991 36. Solomon KD, Apple DJ, Mamalis N, et al: Complications of intraocular lenses with special reference to an analysis
of 2500 explanted intraocular lenses (IOLs). Eur J Implant Refract Surg 3:195, 1991 37. Auffarth GU, Wesendahl , TA, Brown SJ, Apple DJ: Are there acceptable anterior chamber intraocular lenses for clinical use
in the 1990s? An analysis of 4104 explanted anterior chamber
intraocular lenses. Ophthalmology 101:1913, 1994 38. Perez-Santonja JJ, Iradier MT, Sanz-Iglesias L, et al: Endothelial changes in phakic eyes with anterior chamber intraocular lenses
to correct high myopia. J Cataract Refract Surg 22:1017, 1996 39. Soong HK, Musch DC, Kowal V, et al: Implantation of posterior chamber intraocular lenses in the absence of
lens capsule during penetrating keratoplasty. Arch Ophthalmol 107:660, 1989 40. Drews RC: Posterior chamber lens implantation during keratoplasty without posterior
lens capsule support. Cornea 6:38, 1987 41. Koch DD: New optic hole configuration for iris fixation of posterior chamber lenses. Arch Ophthalmol 106:163, 1988 42. Menezo JL, Cisneros AL, Cervera M, et al: Iris claw phakic lens: Intermediate and long-term corneal endothelial changes. Eur J Implant Ref Surg 6:195, 1994 43. Menezo JL, Martinez MC, Cisneros AL: Iris-fixated Worst claw versus sulcus-fixated posterior chamber lenses
in the absence of capsular support. J Cataract Refract Surg 22:1476, 1996 44. Wong SK, Stark WJ, Gottsch JD, et al: Use of posterior chamber lenses in pseudophakic bullous keratopathy. Arch Ophthalmol 105:856, 1987 45. Carlson AN, Stewart WC, Tso PC: Intraocular lens complications requiring removal or exchange. Surv Ophthalmol 42:417, 1998 46. Davis RM, Best D, Gilbert GE: Comparison of intraocular lens fixation techniques performed during keratoplasty. Am J Ophthalmol 111:743, 1991 47. Lindquist TD, Agapitos PJ, Lindstrom RL, et al: Transscleral fixation of posterior chamber intraocular lenses in the absence
of capsular support. Ophthalmic Surg 20:769, 1989 48. Evereklioglu C, Er H, Bekir NA, et al: Comparison of secondary implantation of flexible open-loop anterior chamber
and scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg 29:30, 2003 49. Wagoner MD, Cox TA, Ariyasu RG, et al: Intraocular lens implantation in the absence of capsular support, a report
by the American Academy of Ophthalmology. Ophthalmology 110:840, 2003 50. Assia EI, Nemet A, Sach D: Bilateral spontaneous subluxation of scleral-fixated intraocular lenses. J Cataract Refract Surg 28:2214, 2002 51. Shammas HJ, Milkie CF: Cystoid macular edema following secondary lens implantation. Am Intraocular Implant Soc J 7:40, 1981 52. Hara T, Hara T, Yamada Y: Equator ring for maintenance of the completely circular contour of
the capsular bag equator after cataract removal. Ophthalmic Surg 22:358, 1991 53. Cionni RJ, Osher RH: Management of zonular dialysis with the endocapsular ring. J Cataract Refract Surg 21:245, 1995 54. Cionni RJ, Osher RH: Management of profound zonular dialysis or weakness with a new endocapsular
ring designed for scleral fixation. J Cataract Refract Surg 24:1299, 1998 55. Lam DS, Young AL, Leung AT, et al: Scleral fixation of a capsular tension ring for severe ectopia lentis. J Cataract Refract Surg 26:609, 2000 56. Cionni RJ, Osher RH, Marques DM, et al: Modified capsular tension ring for patients with congenital loss of zonular
support. J Cataract Refract Surg 29:1668, 2003 57. McCannel MA: A retrievable suture idea for anterior uveal problems. Ophthalmic Surg 7:98, 1976 58. Siepser SB: The closed chamber slipping suture technique for iris repair. Ann Ophthalmol 26:71, 1994 59. Zeh WG, Price FW: Iris fixation of posterior chamber intraocular lenses. J Cataract Refract Surg 26:1028, 2000 60. Stutzman RD, Stark WJ: Surgical technique for suture fixation of an acrylic intraocular lens in
the absence of capsule support. J Cataract Refract Surg 29:1658, 2003 61. Condon GP: Simplified small-incision peripheral iris fixation of an AcrySof intraocular
lens in the absence of capsule support. J Cataract Refract Surg 29:1663, 2003 62. Chu MW, Font RL, Koch DD: Visual results and complications following posterior iris-fixated posterior
chamber lenses at penetrating keratoplasty. Ophthalmic Surg 23:608, 1992 63. Stark WJ, Goodman G, Goodman D, Gottsch J: Posterior chamber intraocular lens implantation in the absence of posterior
capsular support. Ophthalmic Surg 19:240, 1988 64. Gess LA: Scleral fixation for intraocular lenses. Am Intraocular Implant Soc J 9:453, 1983 65. Malbran ES, Malbran EJ, Negir I: Lens guide suture for transport and fixation in secondary IOL implantation
after intracapsular extraction. In Ophthalmol 9:161, 1986 66. Cowden JW, Hu BV: A new surgical technique for posterior chamber lens fixation during penetrating
keratoplasty in the absence of capsular or zonular support. Cornea 7:231, 1988 67. McGill J, Liakos G: Complications of anterior chamber intraocular lenses and their effect on
the endothelium. Trans Ophthalmol Soc UK 104:273, 1985 68. Moses L: Complications of rigid anterior chamber implants. Ophthalmology 91:819, 1984 69. Passo MS, Van Buskirk EM: Pupillary block with flexible anterior chamber intraocular lenses. Am J Ophthalmol 99:603, 1985 70. Apple DJ, Reidy JJ, Googe JM, et al: A comparison of ciliary sulcus and capsular bag fixation of posterior chamber
intraocular lenses. Am Intraocular Implant Soc J 11:44, 1985 71. Sharpe MR, Biglan AW, Gerontis CC: Scleral fixation of posterior chamber intraocular lenses in children. Ophthalmic Surg Lasers 27:337, 1996 72. Buckley EG: Scleral fixated (sutured) posterior chamber intraocular lens
implantation in children. J AAPOS 3:289, 1999 73. Mittelviefhaus H, Mittelviefhaus K, Gerling J: Transscleral suture fixation of posterior chamber intraocular lenses in
children under 3 years. Graefes Arch Clin Exp Ophthalmol 238:143, 2000 74. Pavlin CJ, Rootman D, Arshinoff S, et al: Determination of haptic position of transsclerally fixated posterior chamber
intraocular lenses by ultrasound biomicroscopy. J Cataract Refract Surg 19:573, 1993 75. Bellucci R, Marchini G, Morselli S, et al: Scleral fixation re-examined by ultrasound biomicroscopy. Eur J Implant Refract Surg 7:326, 1995 76. Manabe S, Oh H, Amino K, et al: Ultrasound biomicroscopic analysis of posterior chamber intraocular lenses
with transscleral sulcus suture. Ophthalmology 107:2172, 2000 77. Campbell D, Davis R, Ferguson J: Ciliary sulcus anatomical dimensions. Invest Ophthalmol Vis Sci 29(suppl):34, 29 (abstract) 78. Duffey RJ, Holland EJ, Agapitos PJ, Lindstrom RL: Anatomic study of transsclerally sutured intraocular lens implantation. Am J Ophthalmol 108:300, 1989 79. Woodhams JT, Lester JC: Pigmentary dispersion glaucoma secondary to posterior chamber intraocular
lenses. Ann Ophthalmol 16:852, 1984 80. Smith JP: Pigmentary open-angle glaucoma secondary to posterior chamber intraocular
lens implantation and erosion of the iris pigment epithelium. Am Intraocular Implant Soc J 11:174, 1985 81. Huber C: The gray iris syndrome: An iatrogenic form of pigmentary glaucoma. Arch Ophthalmol 102:397, 1984 82. Samples JR, Van Buskirk EM: Pigmentary glaucoma associated with posterior chamber intraocular lenses. Am J Ophthalmol 100:385, 1985 83. Masket S: Pseudophakic posterior iris chafing syndrome. J Cataract Refract Surg 12:252, 1986 84. Lieppman ME: Intermittent visual white out: A new intraocular lens complication. Ophthalmology 89:109, 1982 85. Lane SS, Lubniewski AJ, Holland EJ: Transsclerally sutured posterior chamber lenses: Improved lens designs
and techniques to maximize lens stability and minimize suture erosion. Semin Ophthalmol 7:245, 1992 86. Heidemann DG, Dunn SP: Visual results and complications of transsclerally sutured intraocular
lenses in penetrating keratoplasty. Ophthalmic Surg 21:609, 1990 87. Teichmann KD, Teichmann IA: The torque and tilt gamble. J Cataract Refract Surg 23:413, 1997 88. Sivak JG, Kreuzer RO, Hildebrand T: Intraocular lenses, tilt and astigmatism. Ophthalmic Res 17:54, 1985 89. Durak A, Oner HF, Kocak N, Kaynak S: Tilt and decentration after primary and secondary transsclerally sutured
posterior chamber intraocular lens implantation. J Cataract Refract Surg 27:227, 2001 90. Leo RJ, Palmer DJ: Episcleritis and secondary glaucoma after transscleral fixation of a posterior
chamber intraocular lens. Arch Ophthalmol 109:617, 1991 91. Small EA, Solomon JM, Prince AM: Hypotonus cyclodialysis cleft following suture fixation of a posterior
chamber intraocular lens. Ophthalmic Surg 25:107, 1994 92. Lane SS, Lindquist TD, Spigelman AV: Scleral fixation complications. Invest Ophthalmol Vis Sci 29(suppl):34, 1988 93. Stark WJ, Gottsch JD, Goodman DF, et al: Posterior chamber intraocular lens implantation in the absence of capsular
support. Arch Ophthalmol 107:1078, 1989 94. Johnson SM: Results of exchanging anterior chamber lenses with sulcus-fixated posterior
chamber IOLs without capsular support in penetrating keratoplasty. Ophthalmic Surg 20:465, 1989 95. Price FW, Whitson WE: Suprachoroidal hemorrhage after placement of a scleral-fixated lens. J Cataract Refract Surg 16:514, 1990 96. Kershner RM: Simple method of transscleral fixation of a posterior chamber intraocular
lens in the absence of the lens capsule. J Refract Corneal Surg 10:647, 1994 97. Kay MD, Epstein RJ, Torczynski E: Histopathology of acute intraoperative suprachoroidal hemorrhage associated
with transscleral intraocular lens fixation. J Cataract Refract Surg 19:83, 1993 98. Solomon K, Gussler JR, Gussler C, Van Meter WS: Incidence of management of complications of transsclerally sutured posterior
chamber lenses. J Cataract Refract Surg 19:488, 1993 99. Heilskov T, Joondeph BC, Olsen KR, Blankenship GW: Late endophthalmitis after transscleral fixation of a posterior chamber
intraocular lens. Arch Ophthalmol 107:1427, 1989 100. Schechter RJ: Suture-wick endophthalmitis with sutured posterior chamber intraocular
lenses. J Cataract Refract Surg 16:755, 1990 101. Epstein E: Suture problems. J Cataract Refract Surg 16:116, 1989 102. Helal M, El Sayyad F, Elsherif Z, et al: Transscleral fixation of posterior chamber intraocular lenses in the absence
of capsular support. J Cataract Refract Surg 22:347, 1996 103. Girard IJ: PC IOL implantation in the absence of posterior capsular support. Ophthalmic Surg 19:680, 1988 104. Spigelman AV, Lindstrom RL, Nichols BD, et al: Implantation of a posterior chamber lens without capsular support during
penetrating keratoplasty or as a secondary lens implant. Ophthalmic Surg 19:396, 1988 105. Shin DH, Hu BV, Hong Y, et al: Posterior chamber lens implantation in the absence of posterior capsular
support. Ophthalmic Surg 19:606, 1988 106. Shin DH: Implantation of a posterior chamber lens without capsular support during
penetrating keratoplasty or as a secondary lens. Ophthalmic Surg 19:755, 1988 107. Pannu JS: A new suturing technique for ciliary sulcus fixation in the absence of
posterior capsule. Ophthalmic Surg 19:751, 1989 108. Malbran ES, Malbran E, Aranguren AN: Scleral fixated intraocular lenses. Arch Ophthalmol 106:1347, 1988 109. Smiddy WE: Dislocated posterior chamber intraocular lens. Arch Ophthalmol 107:1678, 1989 110. Smiddy WE, Sawusch MR, O'Brien TP, et al: Articles: Implantation of scleral-fixated posterior chamber intraocular
lenses. J Cataract Refract Surg 16:691, 1990 111. Lubniewski AJ, Holland EJ, Van Meter WS, et al: Histologic study of eyes with transsclerally sutured posterior chamber
intraocular lenses. Am J Ophthalmol 110:237, 1990 112. Koplin RS, Colquhoun J: Surgical technique for managing a subluxed posterior chamber lens implant. J Cataract Refract Surg 14:685, 1988 113. McDonnell PJ, Champion R, Green WR: Location and composition of haptics of posterior chamber intraocular lenses: Histopathologic
study of postmortem eyes. Ophthalmology 94:136, 1987 114. Mannarino AP, Hannush SB: A new technique for transscleral fixation of a posterior chamber intraocular
lens in the absence of capsular support during penetrating keratoplasty. J Refract Corneal Surg 6:353, 1990 115. Hu BV, Shin DH, Gibbs KA, Hong YJ: Implantation of posterior chamber lens in the absence of capsular and zonular
support. Arch Ophthalmol 106:416, 1988 116. Schein OD, Kenyon KR, Steinert RF, et al: A randomized trial of intraocular lens fixation techniques with penetrating
keratoplasty. Ophthalmology 100:1437, 1993 117. Mittelviefhaus H, Wiek J: A refined technique of transscleral suture fixation of posterior chamber
lenses developed for cases of complicated cataract surgery with vitreous
loss. Ophthalmic Surg 24:698, 1993 118. Lewis JS: Ab externo sulcus fixation. Ophthalmic Surg 22:692, 1991 119. Bergren RL: Four-point fixation technique for sutured posterior chamber intraocular
lenses. Arch Ophthalmol 112:1485, 1994 120. Lewis JS: Sulcus fixation without flaps. Ophthalmology 100:1346, 1993 121. Leon CS, Leon JA: Microendoscopic ocular surgery: A new intraoperative, diagnostic and therapeutic
strategy. Part I: Endoscopic equipment/methodology applied to
cataract surgery with intraocular lens implantation. J Cataract Refract Surg 17:568, 1991 122. Tsai JC, Rowsey JJ, Fouraker BD, et al: Use of a mirror needle holder with transsclerally sutured posterior chamber
intraocular lenses. Ophthalmic Surg Lasers 27:720, 1996 123. Kora Y, Fukado Y, Yaguchi S: Sulcus fixation of posterior chamber lenses by transscleral sutures. J Cataract Refract Surg 17:636, 1991 124. Anand R, Bowman RW: Simplified technique for suturing dislocated posterior chamber intraocular
lens to the ciliary sulcus. Arch Ophthalmol 108:1205, 1990 125. Friedberg MA, Berler DK: Scleral fixation of posterior chamber intraocular lenses combined with
vitrectomy. Ophthalmic Surg 23:18, 1992 126. Bucci FAJ, Holland EJ, Lindstrom RL: Corneal autografts for external knots in transsclerally sutured posterior
chamber lenses. Am J Ophthalmol 112:353, 1991 127. Choyce DP: The latest facts and figures on anterior chamber lens implants. Contact Intraoc Lens Med J 2:60, 1976 128. Drolsum L: Long-term follow-up of secondary flexible, open-loop, anterior chamber
intraocular lenses. J Cataract Refract Surg 29:498, 2003 129. Werner L, Izak AM, Pandey SK, et al: Correlation between different measurements within the eye relative to phakic
intraocular lens implantation. J Cataract Refract Surg 30:1982, 2004. 130. Allemann N, Chamon W, Tanaka HM, et al: Myopic angle-supported intraocular lenses: two-year follow-up. Ophthalmology 107:1549, 2000 131. de Souza RF, Forseto A, Nose R, et al: Anterior chamber intraocular lens for high myopia: five year results. J Cataract Refract Surg 27:1248, 2001 132. Perez-Santonja JJ, Iradier MT, Sanz-Iglesias L, et al: Endothelial changes in phakic eyes with anterior chamber intraocular lenses
to correct high myopia. J Cataract Refract Surg 22:1017, 1996 133. Perez-Santonja JJ, Iradier MT, Benitez del Castillo JM, et al: Chronic subclinical inflammation in phakic eyes with intraocular lenses
to correct myopia. J Cataract Refract Surg 22:183, 1996 134. Friedman NJ, Koch DD: Scleral tunnel incisions: Principles and methods. In Ophthalmic Surgery: Principles and Practice, 3rd ed. Philadelphia: Saunders, 2002:51 135. Dangel M, Keates R: The adjustable slide knot: An alternative technique. Ophthalmic Surg 11:843, 1980 136. Kraff MC, Sanders DR, Lieberman HL, Kraff J: Secondary intraocular lens implantation. Ophthalmology 90:324, 1983 137. Shammas HJ, Milkie CF: Secondary implantation of anterior chamber lenses. Am Intraocular Implant Soc J 9:313, 1983 138. Cozean CHJ: A longer view of secondary intraocular lens implantation with special emphasis
on the role of the vitreous. Am Intraocular Implant Soc J 6:361, 1980 139. Kraff MC, Lieberman HL, Sanders DR: Secondary intraocular lens implantation: Rigid/semi-rigid versus flexible
lenses. J Cataract Refract Surg 13:21, 1987 140. Heller MD, Straatsma BR, Foos RY: Detachment of the posterior vitreous in phakic and aphakic eyes. Mod Probl Ophthalmol 10:23, 1972 141. McCluskey P, Harrisberg B: Long-term results using scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg 20:34, 1994 142. Maus M, Sivalingam E: Alternative method for sulcus fixation of posterior chamber lenses in the
absence of capsular support. Ophthalmic Surg 20:476, 1989 143. Price FWJ, Wellemeyer M: Transscleral fixation of posterior chamber intraocular lenses. J Cataract Refract Surg 21:567, 1995 144. Bleckmann H, Kaczmarek U: Functional results of posterior chamber lens implantation with scleral
fixation. J Cataract Refract Surg 20:321, 1994 145. Brunette I, Stulting RD, Rinne JR, et al: Penetrating keratoplasty with anterior or posterior chamber intraocular
lens implantation. Arch Ophthalmol 112:1311, 1994 146. Steinert RF: Cataract Surgery: Techniques, Complications and Management, 2nd ed. Philadelphia: Saunders, 2004:433. |