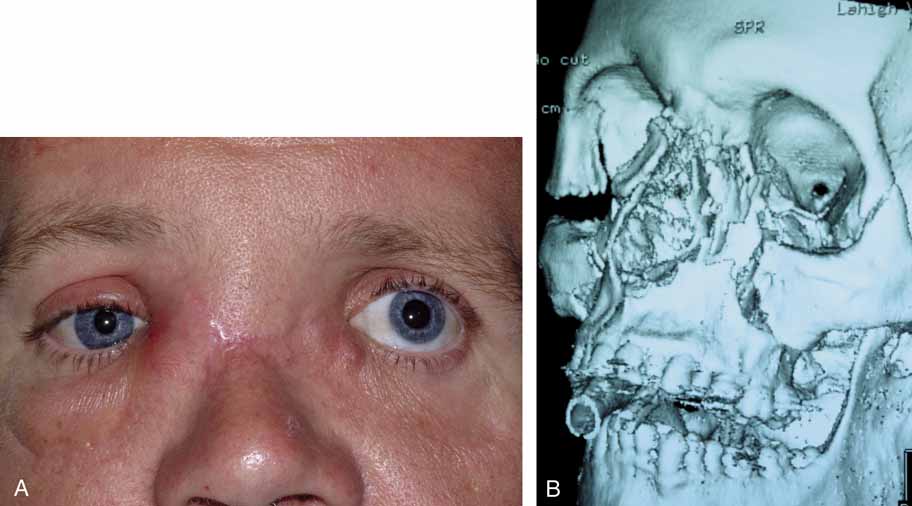
| The objective of lacrimal drainage surgery is to establish flow of tears
from the cul-de-sac to the nasal cavity. The techniques
used in children and adults are virtually the same. There are, however, some
specific differences that will be reviewed. SURGICAL MANAGEMENT OF LOWER SYSTEM BLOCKS Pediatric Irrigation and Probing Under General Anesthesia When pediatric general anesthesia is safely available and is selected by
a surgeon, a face mask usually will suffice. However, a laryngeal mask
is preferred because it is an excellent device that allows free access
to the nose for retrieval of dye and irrigated fluids. Furthermore, it
maintains the airway, even if it is necessary to perform Silastic
intubation of the nasolacrimal system.54 An intravenous drip should be established before the surgeon performs
the actual probing and irrigation. Endotracheal intubation should be done
if there is any question about the child's airway. When the child
is asleep, careful examination of the canaliculi should be done with
gentle dilation of the punctum, as previously described, and careful
passage of a lacrimal cannula (blunt 23-gauge cannula) attached
to a 3-mL Luer-Lok syringe containing several
milliliters of 2% fluorescein solution. The surgeon should
feel for veils and strictures in each canaliculus and at the internal
common punctum, before attempting to probe the nasolacrimal duct. The
surgeon can attempt to use hydraulic pressure irrigation while occluding
the opposite punctum as the initial effort to open the lower (distal) portion
of the nasolacrimal system. If this maneuver fails, the
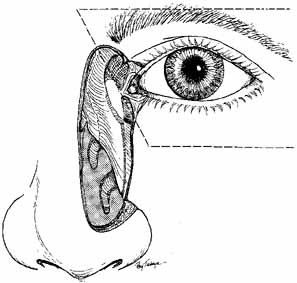
lacrimal cannula can be used as a probe because it is between a
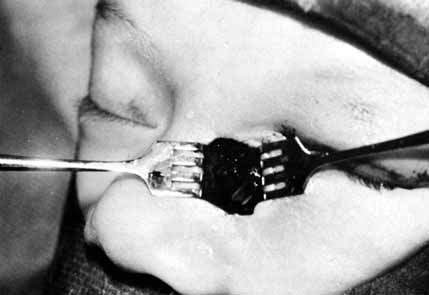
number 0 and a number 00 Bowman probe in size (Fig. 19). After the cannula is shaped into a slight curve, this instrument
can be passed gently into the nasolacrimal duct by the upper canaliculus. The
lower canaliculus should be manipulated as little as possible. If
any resistance is encountered because the cannula is too large, the
surgeon should withdraw this instrument immediately and use a smaller
Bowman probe. The probing instrument is slipped down into the nasolacrimal
duct until it meets resistance at the lower end. The surgeon
then should turn the curve of the cannula medially, and with a gentle
but firm push, enter the inferior meatus. When the cannula is passed through
the duct, any scraping sensation, rather than a smooth, sliding
sensation, usually indicates a false passage or the presence of extensive
scar tissue. In such instances, the instrument should be withdrawn, and
the surgeon should make a gentle attempt to find the normal channel. If
passage into the nose is not achieved because of a presumed false
passage, it is preferable to stop the procedure and reschedule a
probing and irrigation under a general anesthetic several weeks later
to allow the operative inflammation to subside. The resolution of inflammation
is aided by irrigation with 0.5 mL of an antibiotic steroid solution. 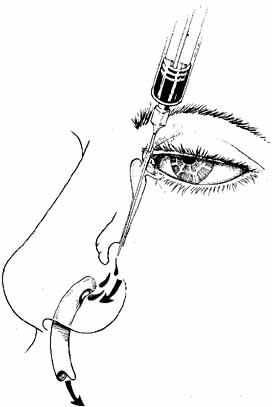 Fig. 19 Probing and irrigation of the nasolacrimal system. Hydraulic pressure is
used in an attempt to force fluid through the obstruction at the valve
of Hasner. If this attempt is unsuccessful, the cannula is slipped
down to the point of obstruction and pushed through gently but firmly. Dye
is injected from the syringe, and the patency of the system is confirmed
by suctioning the dye from the inferior meatus with a soft pediatric-size
plastic suction catheter. Fig. 19 Probing and irrigation of the nasolacrimal system. Hydraulic pressure is
used in an attempt to force fluid through the obstruction at the valve
of Hasner. If this attempt is unsuccessful, the cannula is slipped
down to the point of obstruction and pushed through gently but firmly. Dye
is injected from the syringe, and the patency of the system is confirmed
by suctioning the dye from the inferior meatus with a soft pediatric-size
plastic suction catheter.
|
Location of the probe in the inferior meatus can be confirmed by metal-to-metal
contact with a larger second probe placed through
the nares. Direct visualization of the probe is particularly useful
in complicated cases. This can be accomplished by using a head lamp, endoscope
and nasal vasoconstrictor such as 4% cocaine solution
or oxymetazoline (Afrin, Schering, Liberty Corners, NJ). In
uncomplicated cases, a simple method of confirmation is to inject dye
through the syringe of the cannula, using fluorescein on one side and
methylene blue on the other side. The dye is collected from the inferior
meatus of the nose with a soft, plastic suction catheter (number 10F
Argyle oxygen catheter; Brunswick, St. Louis, MO) of pediatric
size. The use of different dyes is convenient in confirming the
patency of the system after irrigation and probing. Fluorescein dye should
be used on any eye that has a high risk of needing Silastic intubation
because methylene blue may stain the tubing. After these steps are
completed, a careful intranasal examination should be performed. If
the inferior turbinate seems to be impacted on the nasolacrimal duct
opening at the initial examination, it can be pushed medially by infracturing
the turbinate toward the septum with a Freer elevator or a Crawford
hook (Fig. 20).43 After probing and irrigation, 1 mL of antibiotic steroid solution is irrigated
through the upper canaliculus of each nasolacrimal system. The
parents are instructed to use an antibiotic–steroid drop four
times daily for the next week. The patient is seen in the office 2 to 3 weeks
postoperatively. If one attempt at simple irrigation and probing
for soft tissue obstruction of the duct is not successful, a repeat
irrigation and probing is done. The secondary procedure may involve a
Silastic intubation or balloon dacryoplasty. Also, the probing and irrigation
procedure is combined with an inferior turbinate infracture if
a distal obstruction is present at the valve of Hasner. 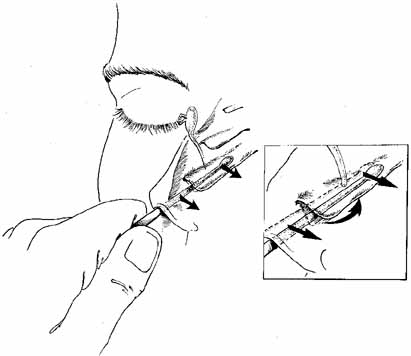 Fig. 20 Infracture of the inferior turbinate. A periosteal elevator is slipped
into the inferior meatus and advanced along the length of the inferior
turbinate. The patient's head is stabilized and the turbinate is
pushed medially by distributing force along the entire length of the
turbinate rather than just at one point. This step creates a larger space
for the exit of tears from the nasolacrimal duct and minimizes trauma. The
instillation of oxymetazoline (Afrin, Schering, Liberty
Corners, NJ) into the inferior meatus before this maneuver will reduce
intraoperative and postoperative bleeding. Fig. 20 Infracture of the inferior turbinate. A periosteal elevator is slipped
into the inferior meatus and advanced along the length of the inferior
turbinate. The patient's head is stabilized and the turbinate is
pushed medially by distributing force along the entire length of the
turbinate rather than just at one point. This step creates a larger space
for the exit of tears from the nasolacrimal duct and minimizes trauma. The
instillation of oxymetazoline (Afrin, Schering, Liberty
Corners, NJ) into the inferior meatus before this maneuver will reduce
intraoperative and postoperative bleeding.
|
Timing of Irrigation and Probing for Congenital Dacryostenosis The correct timing for initial probing of a child with nasolacrimal duct
obstruction has been controversial.55–57 Based on a large study by Katowitz and Welsh,57 we recommend that initial probing be done when the patient is approximately 1 year
of age. In this study of 572 eyes, the success rate with
initial probing was 97% in patients younger than 13 months. In
patients older than 13 months, however, the mean success rate was 54.7%. When
subdivided into smaller age categories, a stepwise progression
was observed from 76.4% for patients between 13 and 18 months
to 33.4% for patients older than 24 months. In addition, the
number and complexity of subsequent procedures appeared to correlate
with the age of the initial probing. It is important to consider the
actual gestational age of the child; a premature infant should be given
a period of postnatal time to respond to conservative therapy that
is equivalent to that for a full-term infant. A major point of controversy regarding congenital dacryostenosis is whether
to probe infants in the office or under a general anesthetic in a
hospital setting.22,26,58,59 This controversy primarily applies to the initial probing procedure. All
children who have had failed probings should have this procedure repeated
under general anesthesia. When deciding whether a general anesthetic should be used for the initial
probing, the most obvious concern is the risk associated with general
anesthesia. This risk is especially concerning in children less than 6 months
of age. Fortunately, it is unnecessary to perform probings
in children younger than 6 months for routine nasolacrimal duct obstruction. Parents
should be full participants in any decision regarding the
choice of office surgery or the use of a general anesthetic. With the
increasing availability of good pediatric anesthesia staff, we typically
recommend general anesthesia for initial probings. It is difficult to attempt irrigation of the infant nasolacrimal system
in the office. Probing of the duct alone, however, can be accomplished
more easily.19,22,24,26 Some surgeons prefer the lower canaliculus for office probing, but as
a general rule, it is best to avoid the risks of traumatizing this channel. A
distinction should be made between therapeutic and diagnostic
probing. An office probing in an infant is primarily therapeutic because
there is little control over the child and, therefore, less time to
feel for veils or strictures carefully at various points along the lacrimal
drainage system. A diagnostic probing in an infant requires general
anesthesia. The technique of probing an infant's nasolacrimal system must be gentle
because the infant's punctum is delicate. Wide dilators can
be traumatic as the punctum can easily tear. The Jones punctal dilator (OP 7002, V. Mueller & Co., Chicago, IL) or a safety pin
that will not dilate past a number 1 Bowman probe size should be used. Bowman
probe sizes vary depending on the manufacturer, and therefore, the
physician should be familiar with the actual width of the probe
in use.22 In office probings, the child does not need to be mummified. The child's
head can be held firmly against an assistant's knees, and
the surgeon can sit at the child's feet, performing the probing
from this position. Jones and Wobig19,43,60 prefer to use a number 0 or smaller probe in an infant and to leave the
probe in place for 5 minutes after confirming its presence in the inferior
meatus of the nose. They believe that this practice allows the
mucosa to retract around the probe, and thus, facilitates a successful
result. We consider office probing in patients older than 3 months unnecessarily
traumatic to the child, the parents, and the surgeon. With the availability
of an excellent pediatric anesthesia department, probing under
general anesthesia is preferred. General anesthesia allows the surgeon
to perform a complete diagnostic and therapeutic probing as well as
an examination of the nasal passage. Examination under anesthesia also
provides the opportunity for retinoscopy and a dilated fundus examination. However, a
number of respected surgeons prefer office probing in
patients as old as 8 months.19,22,26,61 Adult Probing and Irrigation of the Nasolacrimal Duct The adult patient in some ways is easier to treat than the pediatric patient. This
is because of the physician's ability to perform a more
thorough examination in the office and create an appropriate treatment
plan. Proximal probing and irrigation of the canaliculus is a diagnostic
procedure only and is done in the office with topical anesthetics
as previously discussed. The canalicular probing will qualitatively
and quantitatively define the point of pathology in the proximal or distal
portion of the nasolacrimal system. Office probing of the full nasolacrimal
system, even with topical anesthetic, is uncomfortable for
the patient and therefore should not be performed. It is uncommon to
perform a probing and irrigation of the nasolacrimal duct as an isolated
therapeutic procedure. Rather, it is typically performed with balloon
dacryoplasty or Silastic intubation in the adult population to treat
partial nasolacrimal obstruction. Unfortunately, these two procedures
are usally only temporizing because the stenosis recurs and these patient
may eventually require DCR surgery. The exception to this rule is
the patient with punctual stenosis. In these patients, probing and irrigation
with Silastic intubation has a high success rate. Silastic Intubation of the Nasolacrimal Duct The major difficulty in dealing with persistent dacryocystostenosis is
deciding how to proceed if all of these measures fail. Before progressing
to the more aggressive DCR,22,62–67 a secondary procedure should be considered like passing Silastic tubing
through the entire nasolacrimal system into the nose (Fig. 21). Although Silastic intubation of the nasolacrimal system appears
to be a more conservative and reasonable approach, it can be a traumatic
procedure, especially in a young child. Therefore, caution is essential
to minimize complications. Furthermore, the procedure is not indicated
for bony obstruction or severe scarring from previous procedures. In
addition to Silastic intubation, balloon dacryoplasty of the nasolacrimal
duct is a viable secondary option. 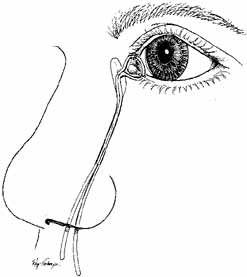 Fig. 21 Silastic tubing is placed through the entire nasolacrimal system. Fig. 21 Silastic tubing is placed through the entire nasolacrimal system.
|
INTUBATION MATERIALS. For many years, bicanalicular Silastic intubation has been the gold standard
for secondary treatment of failed probings.68 Silastic tubing comes packaged in several ways. The important features
to consider in choosing a packaged set for nasolacrimal intubation are
that the metal probe be malleable enough to minimize the trauma to the
nasolacrimal system and that the tubing be bonded sufficiently to the
probes to avoid slipping during passage through the system. Free tubing
can be loaded onto a Quickert probe and passed. Tubing (Silastic, Dow
Corning, New York, NY; tubing 5941, Storz, St. Louis, MO) of 0.025 mm
external diameter is preferred for pediatric and adult
use. The tubing can be glued in place with silicone bonding glue. It is
stretched over a number 0 tapered probe (Quickert 4220, Storz) before
autoclaving. Packaged intubation sets like Crawford Silastic
set (28–0185; hook, 28–0186, Jedmed Instrument Co., St. Louis, MO), O'Donoghue lacrimal tubes (Visitec, Sarsota, FL), and
Guibor lacrimal tubing also are available. They
are more expensive, but avoid the need to prepare the probes with tubing. In 1988, monocanalicular intubation sets became available (Monaka
tubes). Fayet and co-workers69 developed and studied the success rate of monocanalicular stenting. His
group showed a similar success rate between bicanalicular stenting and
monocanalicular stenting. Kaufman and co-workers70 had a 79% overall success with monocanalicular stenting. At Children's
Hospital of Philadelphia, we had a 91% success rate
in our first 35 cases71 and then proceeded to coleect a prospective series of 101 patients which
indicated a 93% success rate.72 The use of monocanalicular stenting has become our preferred method of
placing Silastic tubing during a probing and irrigation. It effectively
stents the system like bicanalicular stents but has two major advantages. First, monocanalicular stents self anchor in the puncta, thus only
one canaliculus is subjected to probing and potential injury. Second, these
stents can easily be removed in the office without the need
for a general anesthetic. TECHNIQUES OF INSERTION. Passage and retrieval of the probes may be difficult. It is important to
recognize that intubation of the nasolacrimal system requires the physician
to negotiate several right-angle turns, particularly when
entering through the lower canaliculus. Furthermore, as the probe emerges
near the floor of the nose, it can be difficult to withdraw, especially
in the young child. This problem occurs because the inferior
meatus in a child is flat, and there is little space between the floor
of the nose, the lateral nasal wall, and the inferior turbinate (Fig. 22).73 Silastic intubation of the nasolacrimal duct can be particularly traumatic
if the probes are not malleable (Fig. 23). By the age of 3 years, more space has developed, but it is still
less than half of that present in the adult. For this reason, it can
be difficult to reach in and withdraw the probe with Silastic tubing
from the inferior of the nose without causing trauma to the nasolacrimal
duct in the interior of the nose. Enlarging this space by using a nasal
vasoconstrictor (4% cocaine or oxymetazoline) and
infracturing the turbinate toward the nasal septum, as previously described
facilitates visualization and removal of the probe. 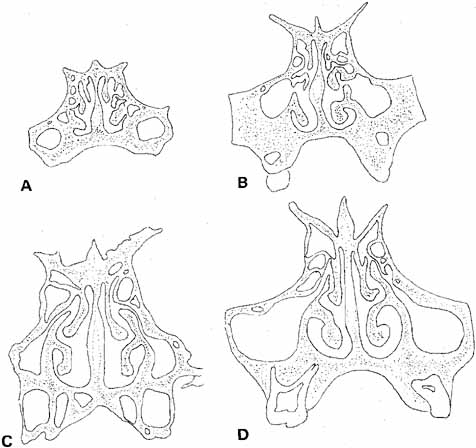 Fig. 22 Coronal sections through the nasal fossa and sinuses showing their sizes
and relationships through various ages. A. Age 5 weeks. B. Age 3½ years. C. Age 7 years. D. Age 9 years. (Bernstein L: Pediatric sinus problems. Otolaryngol
Clin North Am 4:128, 1971) Fig. 22 Coronal sections through the nasal fossa and sinuses showing their sizes
and relationships through various ages. A. Age 5 weeks. B. Age 3½ years. C. Age 7 years. D. Age 9 years. (Bernstein L: Pediatric sinus problems. Otolaryngol
Clin North Am 4:128, 1971)
|
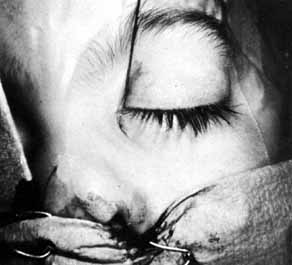 Fig. 23 Trauma to the inferior meatus and turbinate caused by difficulties in removing
a stiff stainless-steel type probe from the inferior meatus
with a hemostat. Fig. 23 Trauma to the inferior meatus and turbinate caused by difficulties in removing
a stiff stainless-steel type probe from the inferior meatus
with a hemostat.
|
Many methods have been devised in an attempt to improve the original grooved
director retrieval method of Quickert and Dryden.63 A straight hemostat often can be used successfully in a larger child, particularly
when the turbinate is infractured. However, the malleable
Crawford-type probes offer an advantage in that they can be retrieved, even
in a small infant, by using the corresponding hook Fig. 24).64,74 It is important to recall the intranasal anatomy while retrieving the
malleable probe. The nasal floor, which is also the palate, is the inferior
limit of the inferior meatus. The inferior turbinate arises from
the lateral nasal wall. Thus, by staying parallel to the palate and directing
the hook laterally towards the ear, the inferior meatus is easily
located. After entering the inferior meatus, tactile movement is
required to locate and retrieve the Crawford probe. Detachment of the
Silastic from the probe may occur when the probe is retrieved from the
inferior meatus of the nose and the junction of the tubing and the probe
is pulled through the punctum into the nasolacrimal system. Usually, dilation
of the punctum before insertion of the probe and application
of an ointment for lubrication at the junction of the metal and Silastic
will prevent this detachment. 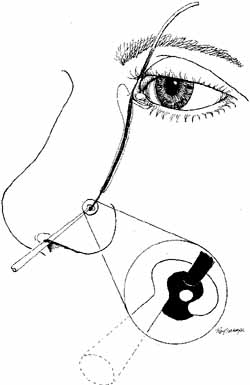 Fig. 24 Crawford probe insertion and removal with a crochet-type hook. Fig. 24 Crawford probe insertion and removal with a crochet-type hook.
|
As for the monocanalicular stents, our preference is to use the Ritleng
probe for passage. This hollow probe has a directed groove at the distal
tip (Fig. 25A and 25B). The prolene portion on the Silastic tubing is fed down the probe
and recovered from the nose (Fig. 26). Sometimes the directional groove will lead the prolene portion
directly out the nostril. It is helpful to feed a majority of the prolene
down the probe to facilitate distal recovery. The Ritleng hook, Crawford
hook, or myringotomy forceps can be utilized to retrieve the prolene
portion from the nose. If any trouble is encountered retrieving
the prolene feeder, the surgeon should feel for the Ritleng probe and
hook it. Once the hook is around the probe, the probe should be rotated 180 degrees
because this will position the distal opening of the probe
posteriorly. In addition, the probe should be slightly withdrawn. The
hook will engage the prolene feeder when the hook is pulled anteriorly. It
is important to note that the prolene thread has two portions, a
light blue section and a dark blue section. The darker portion has
a thicker diameter. When all the dark prolene has been inserted down the
probe, the light blue, thinner portion should be passed outside the
slit on the side of the probe. Simultaneously, the probe should be removed
proximally from the nasolacrimal system. |
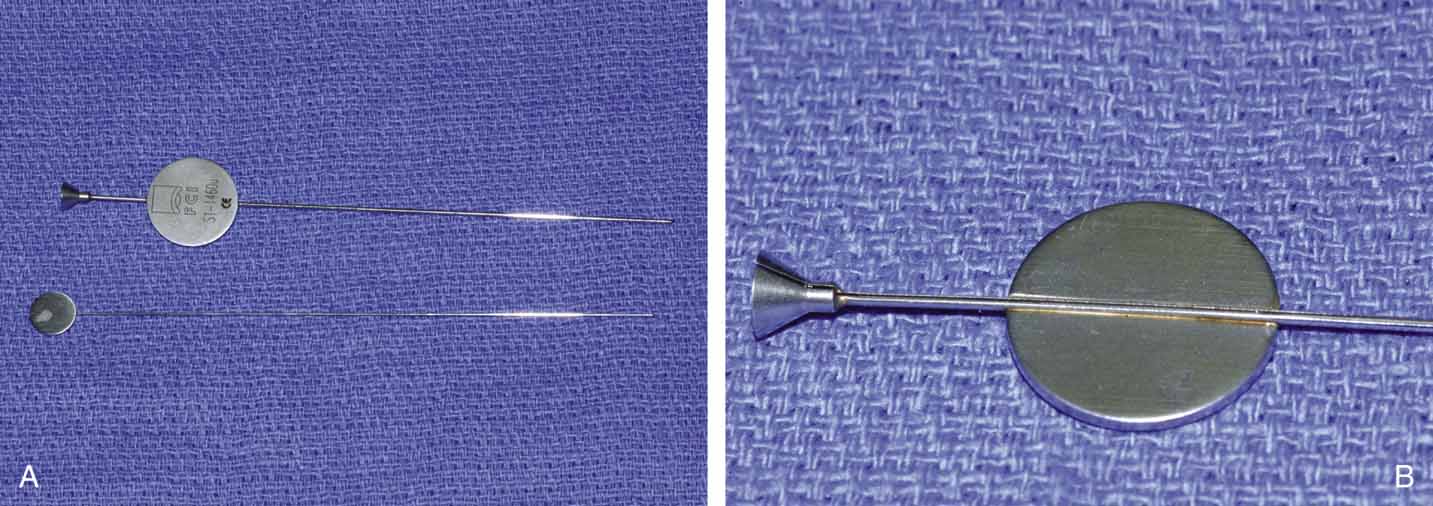 Fig. 25 A and B. Ritleng probe with hollow lumen and stylette used while passing the probe. A
slit exits along the side to remove prolene lead attached to Silastic
tubing distally.
Fig. 25 A and B. Ritleng probe with hollow lumen and stylette used while passing the probe. A
slit exits along the side to remove prolene lead attached to Silastic
tubing distally.
|
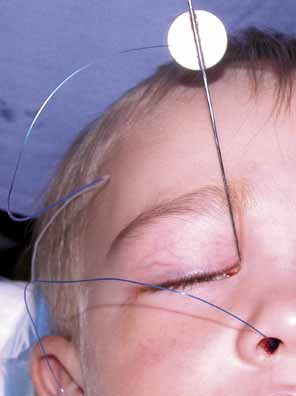 Fig. 26 Monocanalicualr stent with Silastic tubing attached to prolene being placed
with a Ritleng probe. Fig. 26 Monocanalicualr stent with Silastic tubing attached to prolene being placed
with a Ritleng probe.
|
FIXATION OF TUBING. Monocanalicular stents are easy to fix in place. Once placed, the tubing
has a collarette with a punctal anchor on the proximal end that nicely
seats itself in the puncta similar to a punctal plug. The collarette
has two components: a flat surface that lies on the lid margin, and
a small bump under the plate, that anchors into the puncta (Fig. 27A and 27B). The colarette comes in two sizes, 3 mm and 4 mm. We prefer the
smaller 3-mm–sized stents for use in infants and toddlers.72 For adolescents and adults, we recommend using the 4-mm–sized
tubes. The stent is placed over a Ritleng probe as previously described. Of
note, the difficulty with these tubes is not fixation but rather
the passage through the nasolacrimal system. Once the stent is in
place, a small punctal plug seating device is inserted into a small
hole in the anchoring plate. The surgeon can facilitate seating the stent
by simultaneously pulling the distal prolene potion and pushing the
anchor with the seating device. A small pop is felt when then anchor
locks into the punctum. If the punctal plug seating device is not available, a
number 0000 Bowman probe or a fine tip punctal dilator can be
used instead. |
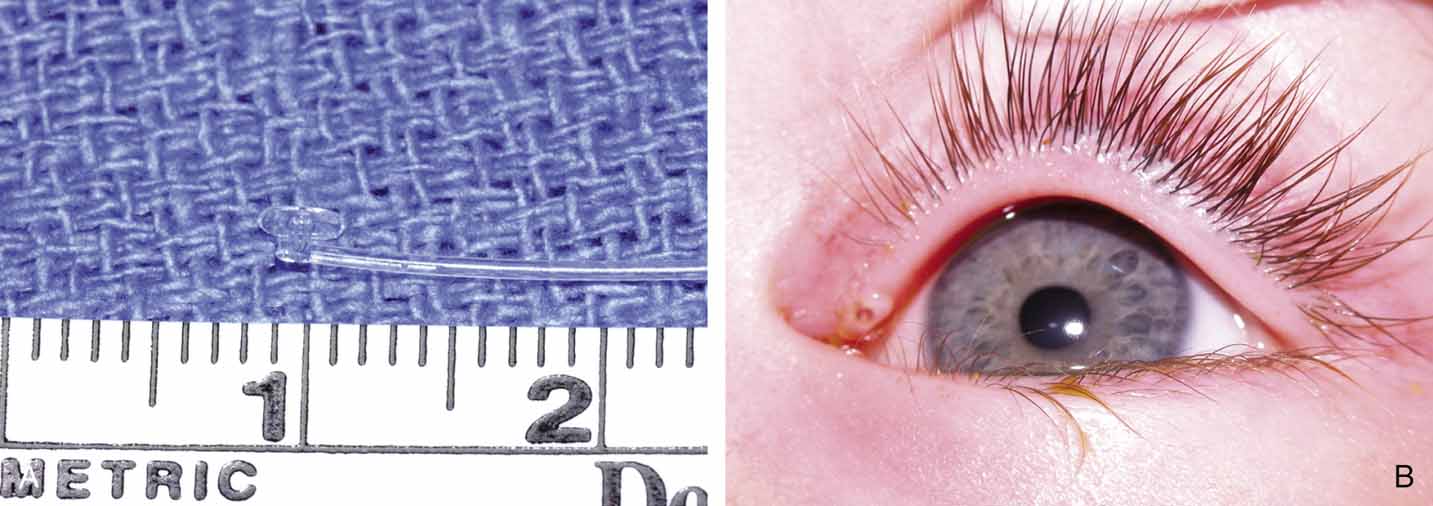 Fig. 27 A and B. Monocanalicular stent with collarette. Note placement when appropriately
seated in puncta.
Fig. 27 A and B. Monocanalicular stent with collarette. Note placement when appropriately
seated in puncta.
|
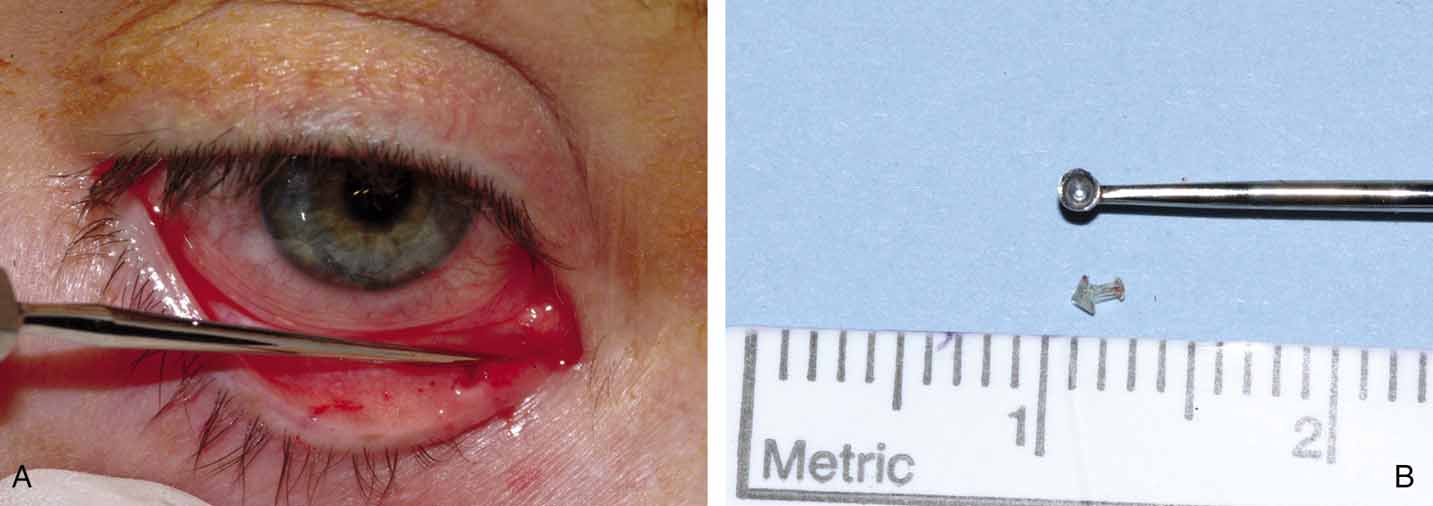
One caution is not to overdilate the punctum prior to placement. An enlarged
punctum may not hold the anchoring device in place. Also, it is
advisable not to use the monocanalicular stent with a punctoplasty because
the anchoring device will not anchor and may retract into the canaliculus. The
monocanalicular stent may be placed through either the upper
or lower puncta. Our preference is to utilize the upper puncta in
routine cases of nasolacrimal duct obstruction. Finally, when trimming
the tubing, it is important to leave the tubing long enough such that
the Silastic does not retract proximal to the valve of Hasner because
this would defeat the purpose of stenting the nasolacrimal duct. Fixation of the bicanalicular Silastic tubing has been controversial.75 The use of multiple square knots alone for fixation in the pediatric age
group allows a potential problem to develop if the child grasps the
loop in the medial canthus and pulls the tubing and knots into the nasolacrimal
duct and sac (Fig. 28). This problem will be seen as a long loop of the tubing visible
at the medial canthus and extending toward the cornea or actually lying
on the cornea. This complication can be remedied by cutting the tubing
and pulling the knots through the common internal punctum and upper
canaliculus, but only if a simple square knot or two have been tied so
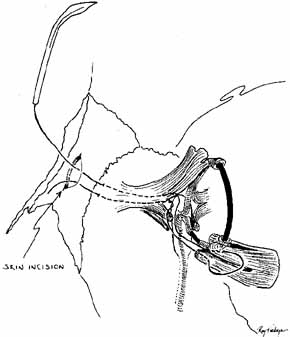
that the knot is not large enough to damage the canaliculus. If general
anesthesia is utilized, then our preferred method is to cut the loop
at the medial canthal angle and wedge a small probe into the lumen
of the Silastic tubing (Fig. 29) that emerges from the upper punctum. This probe, attached to the
tubing (in effect, a reversal of the Crawford tubing system), can
be passed down through the lacrimal drainage system and identified
in the inferior meatus. The tubing can be extracted from the nostril, and
if necessary, the system can be reintubated.76 In an adult, the loose tubing usually can be visualized in the nares with
a head light and nasal speculum. Alternatively, an in office endoscope
may be utilized for visualization. The ends of the tubing can be
pulled out of the nostril with forceps, and a small silk suture can be
tied over a piece of scleral buckle sponge around the tube ends proximal
to the original knot, thereby shortening the loop and preventing the
knot from escaping up the nasolacrimal duct (Figs. 30 and 31). 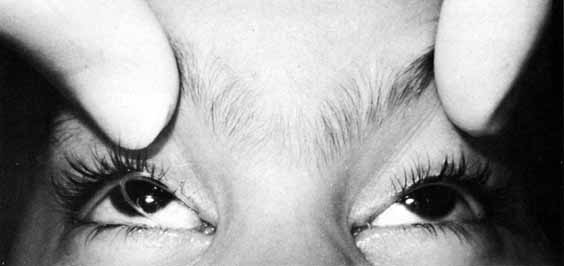 Fig. 28 Loop of tubing tied too loosely, enabling the child to pull knots of Silastic
into the lacrimal sac. Fig. 28 Loop of tubing tied too loosely, enabling the child to pull knots of Silastic
into the lacrimal sac.
|
 Fig. 29 Loop of tubing cut, and Bowman probe inserted into the lumen of Silastic
tubing from the upper canaliculus. The probe is then passed through
the nasolacrimal duct, and the tubing is retrieved from the floor of the
nose. Fig. 29 Loop of tubing cut, and Bowman probe inserted into the lumen of Silastic
tubing from the upper canaliculus. The probe is then passed through
the nasolacrimal duct, and the tubing is retrieved from the floor of the
nose.
|
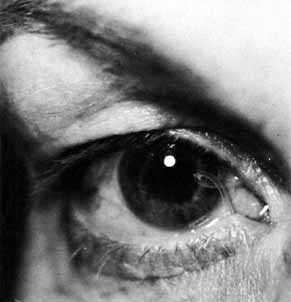 Fig. 30 Loop of tubing tied too loosely in an adult patient. Fig. 30 Loop of tubing tied too loosely in an adult patient.
|
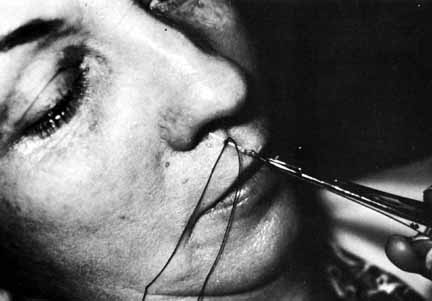 Fig. 31 Placement of a silk suture to tighten
the loop in the adult patient shown in Figure
30.
Fig. 31 Placement of a silk suture to tighten
the loop in the adult patient shown in Figure
30.
|
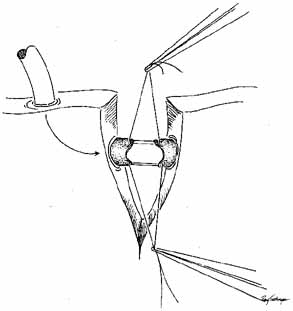
For bicanalicular stent fixation, in order to prevent these complications, we
prefer to pass each probe through a small piece of scleral buckle
sponge and then to tie square knots to secure the stent in the appropriate
position.75 The size of the loop is an important factor. It is important to allow
for 2 to 3 mm of lateral motion of the loop at the medial canthus when
it is pulled with a muscle hook after the sponge is positioned properly
underneath the inferior turbinate posteriorly. After the probes are
slipped through the sponge, they are removed, and multiple surgical knots
are tied so that the sponge cannot slip off of the ends of the Silastic
tubing. The ends should be cut so that they are not visible externally. An
alternative method is to secure the loop of Silastic inside
the nares with a 5–0 prolene suture again with the appropriate
amount of tension on the Silastic. POSTOPERATIVE MANAGEMENT. The postoperative management after Silastic intubation of the nasolacrimal
duct requires regular follow-up. There is little maintenance
required in these patients, but they should be monitored for potential
problems. With the use of monocanalicular stents, there is minimal
concern. The two problems in our experience with the monocanalicular system
are spontaneous extrusion in approximately 10% of patients
and corneal/conjuctival abrasions in less than 5%. In patients
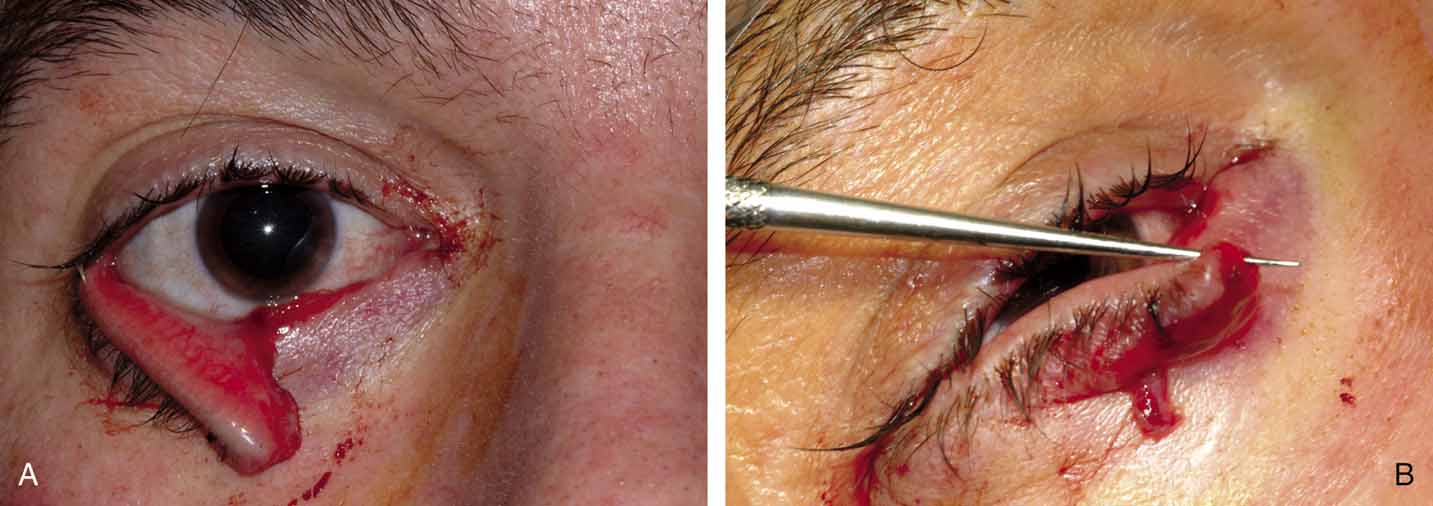
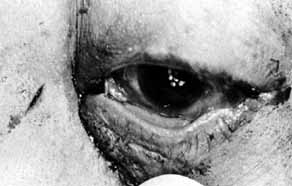
with bicanalicular stents, they should be watched for cheesewiring, or the slitting of the canaliculi and puncta (Fig. 32). This condition can occur if the loop is tied too tightly and is
also because of the rapid growth of young children. The loop has a fixed
length, so slitting of the canaliculi may occur as the face enlarges. A
slit of up to one half of the canalicular length does not seem to
cause any problems in the otherwise normal canaliculus, but nevertheless, it
is a complication that should be avoided if possible. The loop
should be tied loosely enough to allow for some growth, but not too
loosely such that the cornea could be irritated on adduction of the globe. Another
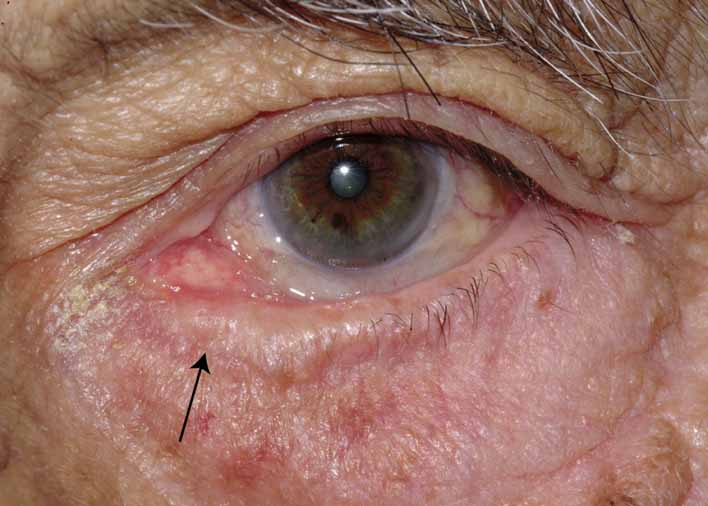
complication of Silastic intubation is the formation of a
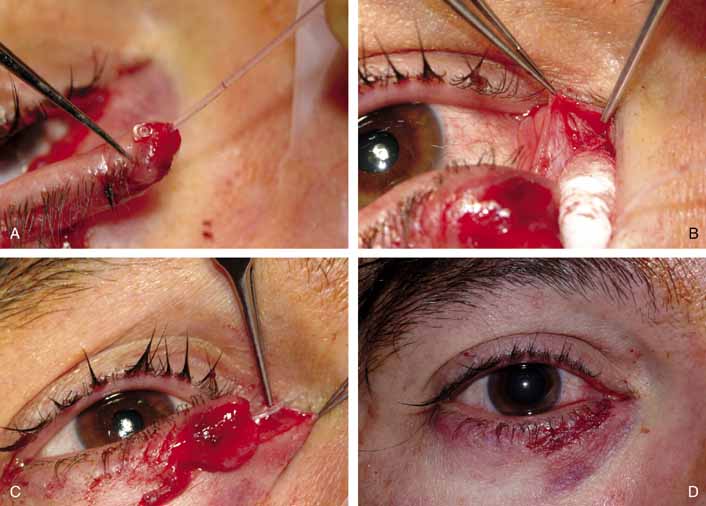
granuloma of the punctum or canaliculus (Fig. 33). This problem can be corrected by simple excision of the granuloma
and cautery of the base. 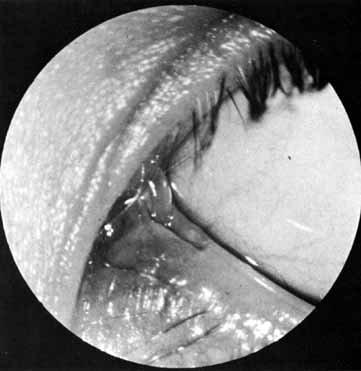 Fig. 32 Cheesewiring of the lower canaliculus secondary to the tubing loop being
tied to tightly. It also can occur if the tubing loop is left in place
for too long in a rapidly growing child. Fig. 32 Cheesewiring of the lower canaliculus secondary to the tubing loop being
tied to tightly. It also can occur if the tubing loop is left in place
for too long in a rapidly growing child.
|
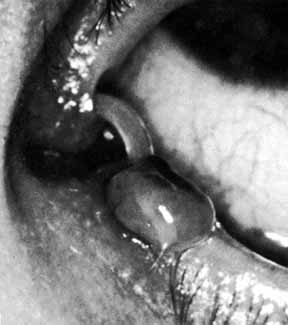 Fig. 33 Granuloma of the punctum secondary to the placement of Silastic tubing. Fig. 33 Granuloma of the punctum secondary to the placement of Silastic tubing.
|
Silastic intubation of the nasolacrimal system is done as an outpatient
day-surgery procedure. Patients or their parents should be cautioned
about postoperative bleeding from the nose. Frank hemorrhage is
uncommon but serious in a young child because of a relatively small total
blood volume. Afrin nasal spray, cold compresses, elevation of the
head in bed, and nasal packing can be used if necessary. If the hemorrhage
persists, thrombin in gelfoam or bipolar cautery may be required. Hospitalization
and possible assistance from an otorhinolaryngologist
may also be necessary. Fortunately, significant hemorrhage is a rare
complication. After Silastic intubation, the patient is routinely given
an antibiotic ointment such as polysporin or a combination antibiotic–steroid
eye drop to be used two to four times daily. Patients
should be seen several weeks postoperatively if there are no unusual
problems. Continued follow-up every 2 to 3 months is required
while the tubing is in place. REMOVAL OF TUBING. The time of tubing removal has increased from the original 6-week
concept to 6 months for children younger than 2 years, and up to 1 year
for older children.63,64,66 This timing allows the duct and its mucosal lining to respond to the stent. This
concept of a response is important; especially in a young child, rapid
growth is occurring, and in the same way that it is possible
to expand a microphthalmic orbit with tiny lid fissures with the use
of increasingly larger conformers, it is possible to expand the nasolacrimal
duct with the Silastic stent during the growth period. Removal of the monocanalicular stent is done in the office. Adult patients
will easily tolerate the removal, which is like placing a punctal
plug. Children can also tolerate the removal with the parent's assistance. The
child is held on the parent's lap and topical anesthetic
is placed in the cul-de-sac. Then with the parent
holding the child still, a Castroviejo needle holder is used to grab the
flat plate portion of the stent (anchor) out of the punctum. If
done quickly, the stent removal can be atraumatic. Removal of the
bicanalicular tubing usually requires general anesthesia by face mask
or laryngeal mask in children. Occasionally, in a very cooperative
child, removal can be done as an office procedure that is similar to removal
of tubing in the adult. Visualization of the inferior meatus through
a nasal speculum is aided by the use of 4% cocaine or Afrin
spray to shrink the nasal mucosa. Blowing the nose in the office and
suctioning in the operating room also may be helpful in placing the
tubing in an anterior location for removal. The loop is cut with a scissors
in the medial canthal area after a topical anesthetic is instilled. The
ends of the Silastic tubing are grasped with a hemostat or small
needle holder and pulled from the inferior meatus. A fiber-optic
head lamp or indirect ophthalmoscope is useful. The major advantage
of the monocanalicular tube system over the bicanalicular systems is
the ease of tubing removal in the office. Balloon Dacryoplasty Balloon dacryoplasty was initially developed from angioplasty technology.77 Subsequently, a lacrimal catheter was introduced to the market and its
efficacy has been proven by several groups in both children and adults.71,78,79 This technique utilizes a 2- or 3-mm diameter balloon inflated
to 8 atmospheres of pressure to dilate strictures or partial obstructions
in the nasolacrimal duct (Fig. 34). Balloon dacryoplasty is typically reserved for nasolacrimal obstruction
refractory to probing. Although some surgeons utilize this procedure
on as a primary probing technique in children, we typically reserve
it for patients who have persistent nasolacrimal duct obstruction
after primary probing and irrigation. 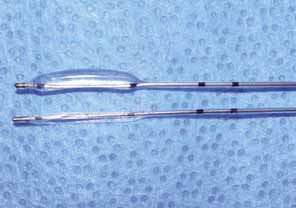 Fig. 34 Lacrimal balloon catheter in inflated and uninflated state. Fig. 34 Lacrimal balloon catheter in inflated and uninflated state.
|
Balloon dacryoplasty is performed just like a probing. A 2-mm balloon
is chosen for children under age 39 months and the 3-mm balloon
is used in those individual that are older. Both balloons have
two marks proximal to the balloon, one 5 mm proximal to the balloon and
the second 15 mm proximal. The procedure can be performed via the upper
or lower canaliculus. The punctum is dilated and the catheter is inserted
into the lacrimal system. Topical antibiotic ointment can be applied
to the end of the balloon to facilitate passage. The catheter is
fed down through the valve of Hasner similarly to passage of a Bowman
probe (Fig. 35). When the balloon is appropriately positioned, the mark 15 mm proximal
to the balloon is at or just inside the punctum. The balloon is
then inflated with a hand pump to 8 atmospheres of pressure for 90 seconds, deflated, and
then reinflated for 60 seconds. The balloon is then
deflated and the catheter is withdrawn 10 mm from the nasolacrimal
system using the 5 and 15 mm marks as a guide. The double-inflation
cycle is then repeated and the catheter is removed from the system. Care
should be taken when performing the second round of dilation in
the proximal portion of the nasolacrimal duct and sac not to accidentally
bring the end of the balloon through the common canaliculus. Inflation
in this area could damage the common canaliculus. 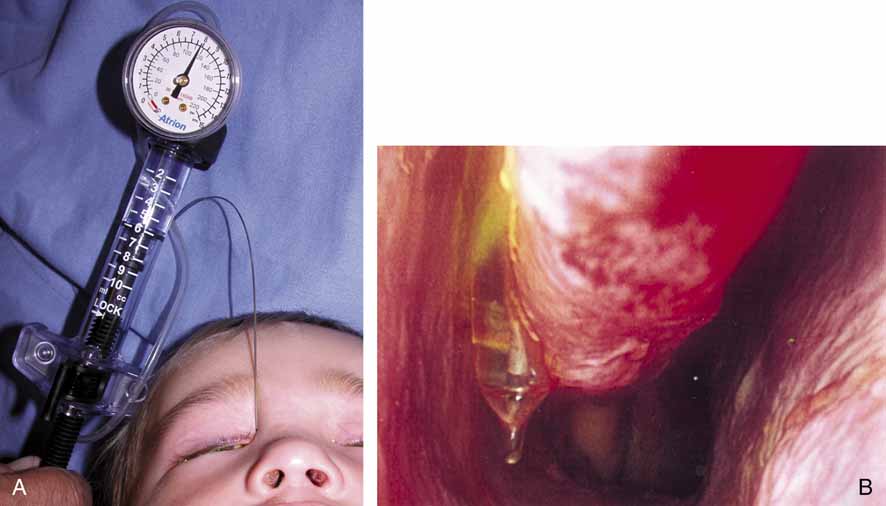 Fig. 35 A and B. Balloon catheter in use as well as endoscopic view in the inferior meatus
of the inflated balloon. Fig. 35 A and B. Balloon catheter in use as well as endoscopic view in the inferior meatus
of the inflated balloon.
|
The postoperative care after balloon dilatation is the same as after probing
and irrigation. A topical antibiotic–steroid ophthalmic solution
is used four times daily for 1 week. Dacryocystorhinostomy STANDARD TECHNIQUE. If the more conservative procedures described above are unsuccessful, or
if there is obvious bony obstruction below the lacrimal sac, a DCR must
be performed.62 Local anesthesia can be used in adults by blocking the infratrochlear
nerve, which can be accomplished by injecting several milliliters of a
local anesthetic mixed with epinephrine in a ratio of 1:100,000. The
effects can be enhanced by controlled sedation, which is of particular
value in the elderly patient. Controlled hypotension is beneficial as
well. The middle meatus should be packed with quarter-inch gauze
tape or one-half × 3 inch patties soaked with oxymetazoline
or 4% cocaine. This treatment will shrink the nasal mucosa
and reduce hemorrhage as well. A reverse Trendelenberg position with
the table tilted to 30 degrees (head up and feet down) and
injection of a local vasoconstrictive agent are useful adjuncts for controlling
bleeding. Positive pressure applied to the respiratory bag during
general anesthesia also will reduce venous flow in the operative
area. Controlled hypotension anesthesia provides the best hemostasis. Some
surgeons use a small amount of diluted methylene blue instilled
in the lacrimal sac through the punctum and canaliculus. This stain will
outline the sac mucosa. However, if not sufficiently diluted, the stain
can leak from the sac when cut, and cause confusion in differentiating
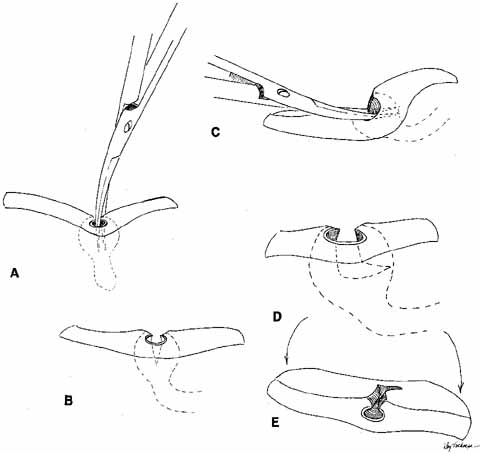
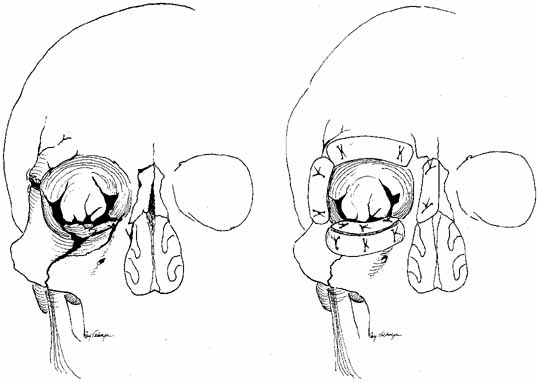
anatomic layers (see Fig. 5). Usually, a straight vertical incision is made through the skin only over
the nasal bridge, approximately 10 mm medial to the canthal angle. Caution
is exercised to avoid the angular vessels that are located approximately 8 mm
medial to the medial canthus. A medial lower lid crease
incision or an extended subciliary blepharoplasty incision can be utilized
if there are any cosmetic concerns. The vertical incision is preferable
as it facilitates excellent visualization of the common internal
punctum when the lacrimal sac is opened. Using the standard approach
on the nasal bridge, the superior end of the incision should be placed
just below the level of the medial canthal tendon. A straight incision
on the nose reduces the chances of a postoperative bowstring scar, although
several variations and modifications of this incision have been
designed to eliminate this concern.80 With a number 15 Bard-Parker scalpel blade, the orbicularis muscle
is then carefully incised down to the periosteum (Fig. 36). The angular vessels are retracted laterally with the orbicularis
muscle by small rake retractors or a self-retaining retractor. If
these vessels are cut, they should be cauterized or ligated to ensure
visualization of the surgical field. The periosteum is incised with
a scalpel blade and reflected laterally with a sharp periosteal elevator
to the anterior lacrimal crest. The medial canthal tendon can be
disinserted with the periosteum if wider exposure is needed later. Caution
is required as the periosteum is lifted over the anterior lacrimal
crest, where it is very adherent, so that the elevator does not tear
through and enter the lacrimal sac. In the child, this crest is flat
and the lacrimal fossa very shallow. Once over the periosteal attachment, at
the crest, the lacrimal fossa is easily stripped free of periosteum. The
periosteum is then retracted laterally to visualize the medial
lacrimal sac wall. 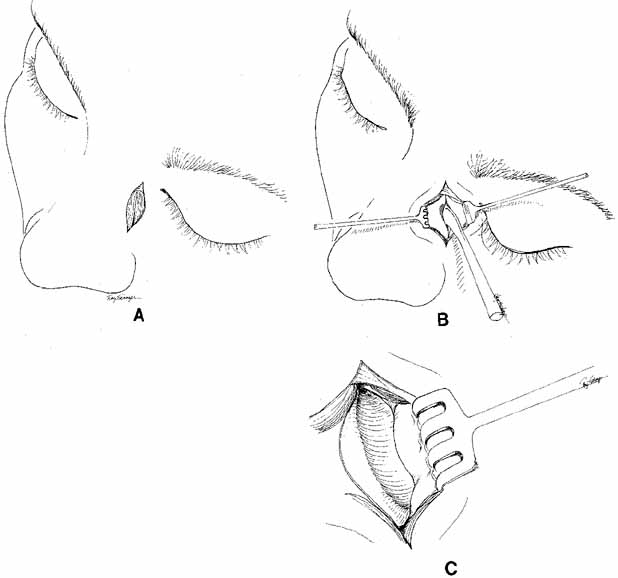 Fig. 36 Dacryocystorhinostomy (DCR) technique. A. A vertical incision, 15 mm long, is made 10 to 11 mm medial to the canthal
angle. B. Periosteum is incised and reflected laterally from the bone with a periosteal
elevator. C. The lacrimal fossa is exposed; the lacrimal sac lies behind the periosteum, which
is reflected laterally. Fig. 36 Dacryocystorhinostomy (DCR) technique. A. A vertical incision, 15 mm long, is made 10 to 11 mm medial to the canthal
angle. B. Periosteum is incised and reflected laterally from the bone with a periosteal
elevator. C. The lacrimal fossa is exposed; the lacrimal sac lies behind the periosteum, which
is reflected laterally.
|
A hand trephine of 10 mm diameter (Arruga, Dixey Co., London, England) can
be used to drill down to the nasal mucosa (Fig. 37). There are two trephines, one with a sharp central point and coarser
teeth to engage the bone and create the initial cut (Fig. 38), and a finer toothed trephine that can be worked more gently down
to the level of nasal mucosa. The surgeon cuts down perpendicularly
to a sufficient depth and uses a sense of feel to rock the hand trephine
back and forth, thereby fracturing the bone. The circle of bone can
be removed with an Adson or other heavy forceps. It is peeled off of
the nasal mucosa gently, with the use of a Freer periosteal elevator. A
Stryker saw attachment, curved hemostat, Hall air drill, or mallet and
osteotome are alternatives for gaining entrance through the bone to
the nasal mucosa. The advantage of the hand trephine over the other methods
is a controlled entrance to the nasal mucosa, which produces less
chance of mucosal disruption and allows a sufficient opening for the
use of bone punches. 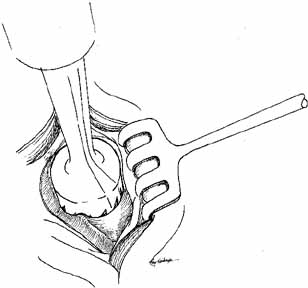 Fig. 37 An Arruga trephine (Dixey Co., London, England) is used to open
the bone down to the nasal mucosa. Fig. 37 An Arruga trephine (Dixey Co., London, England) is used to open
the bone down to the nasal mucosa.
|
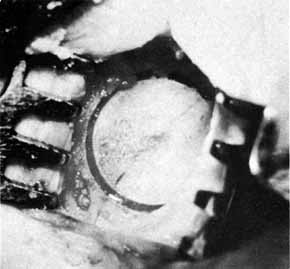 Fig. 38 Marking by a coarse Arruga trephine (Dixey Co., London, England) with
a central pin. Fig. 38 Marking by a coarse Arruga trephine (Dixey Co., London, England) with
a central pin.
|
The periosteum anterior to the bony opening should be elevated from bone
with a periosteal elevator for 2 to 3 mm to allow later suturing of
the lateral edge of the periosteum with the medial canthal tendon back
to its normal anatomic position. The nasal packing is removed to relax
the mucosa, and the bony opening extended to about 15 mm in diameter, approximately
thumbnail size (Fig. 39). The lacrimal sac is identified beneath the periosteal layer, which
has been reflected laterally, by placing a blunt right-angle
probe (Werb, Dixey Co., London, England)) or Bowman probe
into one canaliculus to point up under the periosteum. The sac can
be entered with a scalpel blade, by cutting directly over the probe, or
preferably by cutting the sac with right-angle scissors (Werb). Cutting
also can be done at the entrance of the sac to
the nasolacrimal duct (Fig. 40). This step is facilitated by prior removal of the medial wall of
the nasolacrimal duct. 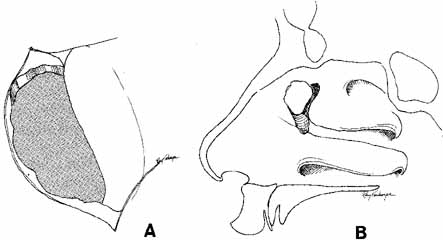 Fig. 39 A. The bony ostium has been enlarged with a bone punch. The ostium should
be approximately 15 mm in diameter. The posterior lacrimal crest and
the medial portion of the osseous lacrimal duct should be removed. B. Sagittal section showing the relationship of the ostium to the nasolacrimal
sac and duct. Fig. 39 A. The bony ostium has been enlarged with a bone punch. The ostium should
be approximately 15 mm in diameter. The posterior lacrimal crest and
the medial portion of the osseous lacrimal duct should be removed. B. Sagittal section showing the relationship of the ostium to the nasolacrimal
sac and duct.
|
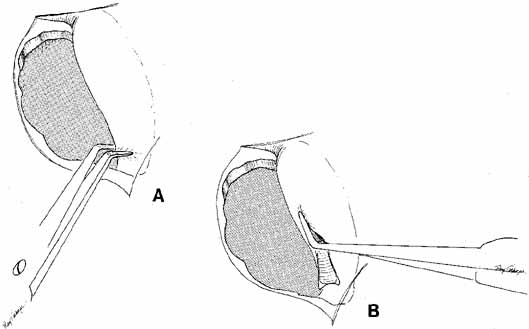 Fig. 40 A. The lacrimal sac is opened by cutting the sac at its entrance to the nasolacrimal
duct with right-angle Werb (Kaiser, Bibra Lake, Western
Australia) scissors. B. One blade is passed into the sac, and the anterior and posterior flaps
are fashioned. Fig. 40 A. The lacrimal sac is opened by cutting the sac at its entrance to the nasolacrimal
duct with right-angle Werb (Kaiser, Bibra Lake, Western
Australia) scissors. B. One blade is passed into the sac, and the anterior and posterior flaps
are fashioned.
|
One blade of the scissors is passed into the opening created at the top
of the duct, and the sac is divided from an inferior to a superior direction. The
most important flaps are the anterior flaps from the sac
and the nasal mucosa because the posterior flaps will fall together. The
only concern with large posterior flaps is the creation of a posterior
sac or nasal flap that is too large and might move out of position
postoperatively occluding the common internal punctum where the canaliculi
enter the sac. This problem is avoided with the use of short posterior
flaps. Before incising the nasal mucosa, the surgeon removes the
nasal packing and passes a small curved hemostat up to the nose to
indent the mucosa and confirm that the space to be entered is actually
in the nose and not in an anteriorly placed ethmoidal air cell. Nasal mucosal flaps are fashioned after incision of the mucosa from the
inferior to the superior end of the bony opening. The width of the anterior
flap is important; there must be an adequate flap to suture to
the anterior flap of the lacrimal sac if this type of technique is used. The
posterior flaps can be closed with interrupted 4–0 or 5–0 absorbable
sutures on a semicircle needle, or they can be cauterized
together.81,82 Closure of the posterior flaps usually is not necessary if the flaps are short and lie in near apposition. In this position, the flaps will
not be able to occlude the internal common punctum postoperatively. An alternative method recommended by McCord6 is to create a large posterior flap of nasal mucosa by incising the nasal
mucosa high and folding it down to meet a short posterior flap of
lacrimal sac. The anterior flap is formed entirely of lacrimal sac. The
incision into the lacrimal sac is made very posterior so that a large
anterior mucosal flap is created; the flap can be sutured to the remaining
anterior nasal mucosa or to periosteum at the end of the bony opening (Fig. 41). This method also is useful if the nasal mucosal flap has been damaged
or inadvertently cut near the top of the bony ostium. The DCR also
has been described without forming any flaps but we do not recommend
this for the external approach.83 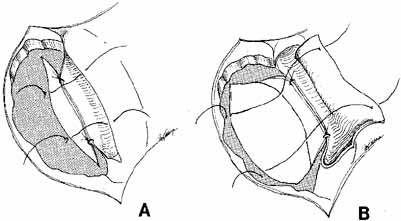 Fig. 41 A. The mucous membrane of the nose can be sutured to that of the lacrimal
sac with interrupted 4–0 absorbable sutures, or as an alternative, they
can be cauterized together. B. An alternative method of creating flaps can be used, particularly if the
nasal mucosa is opened too high. In this instance, the entire mucosal
lining of the nose can be used as a posterior flap, and the entire
lacrimal sac used as an anterior flap.24 The anterior flap of the lacrimal sac can be sutured to the periosteum
at the anterior bony opening. Fig. 41 A. The mucous membrane of the nose can be sutured to that of the lacrimal
sac with interrupted 4–0 absorbable sutures, or as an alternative, they
can be cauterized together. B. An alternative method of creating flaps can be used, particularly if the
nasal mucosa is opened too high. In this instance, the entire mucosal
lining of the nose can be used as a posterior flap, and the entire
lacrimal sac used as an anterior flap.24 The anterior flap of the lacrimal sac can be sutured to the periosteum
at the anterior bony opening.
|
The common internal punctum should be examined carefully by passing blunt
probes through this structure through each canaliculus (Fig. 42). Most patients have a true shared internal entrance of the canaliculi, but
occasionally, there may be separate, but normal, internal puncta.43 Any scars or obstructions of this area must be excised with scissors, and
a new common opening created by intubating the canaliculi with Silastic
material (Fig. 43). 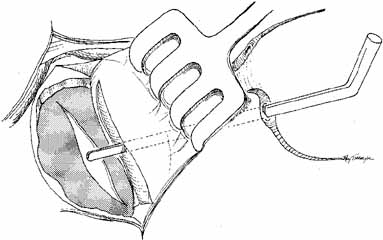 Fig. 42 A blunt right-angle Werb (Kaiser, Bibra Lake, Western Australia) probe
is introduced into the sac through each canaliculus. The probes
emerge through a common internal punctum in most instances. Any scar
tissue present over the internal punctum should be excised, and the canalicular
system intubated with Silastic tubing. Fig. 42 A blunt right-angle Werb (Kaiser, Bibra Lake, Western Australia) probe
is introduced into the sac through each canaliculus. The probes
emerge through a common internal punctum in most instances. Any scar
tissue present over the internal punctum should be excised, and the canalicular
system intubated with Silastic tubing.
|
 Fig. 43 A probe in position, running through the common opening after scar tissue
has been excised. The nasal mucosa is opened to create a posterior
flap matching that of the lacrimal sac. Fig. 43 A probe in position, running through the common opening after scar tissue
has been excised. The nasal mucosa is opened to create a posterior
flap matching that of the lacrimal sac.
|
If Silastic intubation is not done as part of the DCR procedure, separation
of the mucosal flaps must be maintained by some other means for some
time during the postoperative period. The posterior flaps can be held
in place and separated from the anterior flaps by the placement of
a large catheter through the DCR ostium and into the nose.62 Silastic is superior to rubber for this purpose because it cannot shed
pieces that might potentially be aspirated. The wider end of the catheter
is held in position, separating the nasal and lacrimal sac mucosal
flaps, by a single 4–0 absorbable suture placed superiorly in
soft tissue. The lower end of the catheter can be cut short at the external
nares. This catheter should be removed 5 to 7 days postoperatively
by placing one blade of a hemostat into the lower opening, clamping
it, and twisting it clockwise until the suture holding the superior end
pulls free and the catheter can be removed easily. Another method of
separating the flaps is to pack the sac and nose with one-quarter- or
one-half–inch gauze. Plain Vaseline gauze (Chesebrough-Ponds, Greenwich, CT) can be used and is
preferable to iodoform types, which may be irritating. This type of
packing can be removed 5 to 7 days postoperatively. However, the packing
material may be sutured inadvertently during the anterior flap closure. The
packing can adhere to the nasal mucosa and cause bleeding on
removal. The packing could also act as a potential source of infection
or even produce a toxic-shock–type syndrome. If Silastic
intubation is not performed with the DCR, we prefer to use a rubber catheter, or
if there is concern for postoperative hemorrhage, a Gelfoam (Upjohn, Kalamazoo, MI) thrombin stent placed between the
flaps.79 SILASTIC INTUBATION COMBINED WITH DACRYOCYSTORHINOSTOMY Silastic intubation is recommended as a standard portion of DCR surgery. Silastic
tubing bonded to a probe is passed through each punctum and
canaliculus to emerge from the internal common punctum (Fig. 44). The Guibor tubing system is useful. Either a groove director or
a hemostat can be used to pull the probes through the opened lacrimal
sac and out the external wound. After the two ends of the Silastic stent
is recovered from the wound, each end is passed through a 1-cm
length of Silastic tubing of slightly larger internal diameter, pulling
the thinner Silastic tubing through. The metal probes are pulled
free from the ends of the tubing. A triple tie is placed in the ends
of the Silastic tubing and pulled tightly enough to estimate the appropriate
tension of the loop of the medial canthal area. The Silastic cuff
and tubing should be placed through the bony ostium before completion
of the surgical knots, and the ends are pulled out of the nose to estimate
this tension properly. The cuff also functions in stenting the
system. A small curved hemostat passed up the nose from the external
nares is useful for grasping the ends of the Silastic tubing to pull them
inferiorly. It is useful to place the ends of the tubing in a needle
holder and pass them into the open clamps of the hemostat. As was previously
described for Silastic intubation in dacryostenosis, approximately 2 to 3 mm
of lateral movement in the tube should be permitted. This
movement can be estimated by pulling the loop with a muscle hook
at the medial canthus. 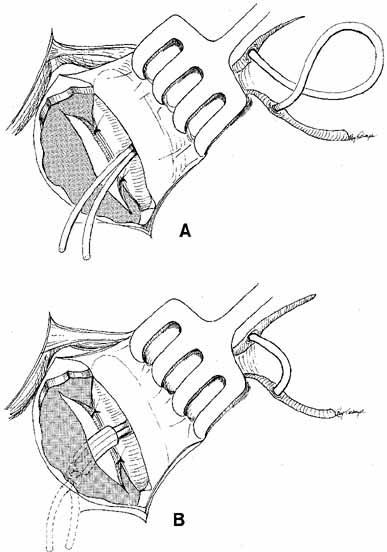 Fig. 44 A. Silastic intubation is required after excision of scars in the area of
the common internal punctum and for moderate degrees of canaliculostenosis
or obstruction. The probes are placed through the upper and lower
canaliculi and through the common internal punctum. Any scar tissue
over this area is excised. B. The Silastic is brought down through the dacryocystorhinostomy (DCR) window
and cuffed as described earlier. This type of tubing with
a cuff will suffice as a stent in these instances, and will maintain
separation of the anterior and posterior mucosal flaps. Use of this
tubing eliminates the need for a catheter or for packing to act as a
stent. Fig. 44 A. Silastic intubation is required after excision of scars in the area of
the common internal punctum and for moderate degrees of canaliculostenosis
or obstruction. The probes are placed through the upper and lower
canaliculi and through the common internal punctum. Any scar tissue
over this area is excised. B. The Silastic is brought down through the dacryocystorhinostomy (DCR) window
and cuffed as described earlier. This type of tubing with
a cuff will suffice as a stent in these instances, and will maintain
separation of the anterior and posterior mucosal flaps. Use of this
tubing eliminates the need for a catheter or for packing to act as a
stent.
|
Once the appropriate tension is achieved, multiple surgical knots that
are large enough to prevent inferior displacement and loss of the Silastic
cuff should be tied. The Silastic tubing is then stretched from the
nose under moderate tension and cut so that the ends remain long enough
to avoid untying, but short enough that when cut, they will retract
high enough to be invisible externally. Aside from a slight sense of
nasal stuffiness, the presence of the tubing presents no postoperative
problem to normal nasal breathing. The anterior flaps of the lacrimal
sac and nasal mucosa are joined with interrupted 4–0 or 5–0 absorbable
sutures (Fig. 45). We prefer to use 4–0 Vicryl suture on a small half circle (Ethicon
P2 cutting needle, J503, Ethicon, Inc., Somerville, NJ) needle. If
the nasal mucosa has been damaged and is insufficient
for closure, a larger anterior flap of the lacrimal sac should be fashioned
and the mucosa sutured directly to the periosteum at the medial
edge of the bony ostium (see Fig. 41B). If the anterior flap of nasal mucosa is too large and there is
risk of it causing obstruction, it should be trimmed conservatively. The
lateral periosteum, which may include the medial canthal tendon if
a wider dissection was done, is rejoined to the medial edge of the periosteum
with interrupted 4–0 absorbable sutures. The muscle and
subcutaneous tissues are closed with interrupted 6–0 absorbable
sutures. The skin is typically closed with a running subcuticular 5–0 nylon
suture (Fig. 46). Fixation of the suture can be accomplished by tying a loop at each
end. This method is particularly useful in children. This suture should
not be pulled too tightly, however, because the knots can migrate
subcutaneously during the postoperative period. An alternative is to
apply tincture of benzoin to the wound and a steri-strip over
the shortened sutured ends. With either method, a mild pressure dressing
should be applied for 12 to 24 hours. 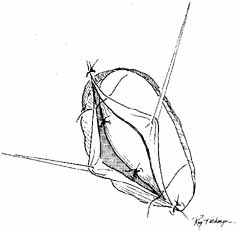 Fig. 45 The anterior flaps of the lacrimal sac and nasal mucosa are closed with
interrupted absorbable sutures. The periosteum is closed in a similar
fashion. A Silastic catheter and nasal packing can be used to separate
the anterior and posterior flaps, but the Silastic tubing with the cuff
in place has advantages; it can remain in place almost indefinitely
in an adult, and it is cosmetically invisible. Fig. 45 The anterior flaps of the lacrimal sac and nasal mucosa are closed with
interrupted absorbable sutures. The periosteum is closed in a similar
fashion. A Silastic catheter and nasal packing can be used to separate
the anterior and posterior flaps, but the Silastic tubing with the cuff
in place has advantages; it can remain in place almost indefinitely
in an adult, and it is cosmetically invisible.
|
 Fig. 46 The orbicularis muscle and subcutaneous tissues are closed with interrupted 6–0 absorbable
sutures. The skin is closed with a running subcuticular
suture of 5–0 nylon. Fig. 46 The orbicularis muscle and subcutaneous tissues are closed with interrupted 6–0 absorbable
sutures. The skin is closed with a running subcuticular
suture of 5–0 nylon.
|
POSTOPERATIVE CARE. Unless there are extenuating circumstances, DCRs are usually done as an
outpatient surgical procedure. If Silastic tubing has been used as a
stent, the postoperative course is more pleasant. There is minimal edema
with the DCR technique described above, regardless of the stent used. The
small pressure dressing usually is left in place only until the
next morning. In a pediatric patient, the dressing can be removed as
soon as the child awakens from anesthesia. Antibiotic ointment or drops
usually are applied to the conjunctival cul-de-sac at
bedtime only during the first week postoperatively. Patients with nasal
packing or a fixed catheter, which applies to those patients without
a stent, need to have the packing or catheter removed 5 to 7 days after
surgery. Again, the use of these materials can increase the risk of
infection or result in bleeding when removed. Thus it is less common
for these to be currently used and are discussed only for completeness. Finally, appropriate
systemic antibiotic, such as a first-generation
cephalosporin can be given intravenously during the procedure
and continued orally for 3 to 5 days postoperatively. However, some believe
that one or the other is adequate and that both intraoperative antibiotics
and postoperative antibiotics are not necessary.84–87 The Silastic tubing can be left in place for long periods in the adult
as it is so well tolerated. The stent is typically removed 6 to 12 weeks
postoperatively if there are no associated canalicular or common internal
punctal problems noted at the time of surgery. The nylon subcuticular
suture can be removed 5 to 7 days postoperatively. Although granulomas
at the ostium have been noted after DCR with Silastic tubes, many
patients who are free of symptoms are reluctant to have the tubing
removed. We have seen adult patients retain Silastic tubing without any
problems for as long as 10 years. Endoscopic Dacryocystorhinostomy Endoscopic DCR has become popular over the last decade because minimally
invasive surgical techniques have come into vogue. As early as 1893, the
internal approach to DCR was described,88 and subsequently it was developed to approach success rates similar to
those for the external approach to DCR. However, the internal approach
was not commonly in use until the development of the endonasal laser
DCR in 1990,89 utilizing an internal DCR technique with the use of fiber-optic
and laser technology. An argon blue–green laser is coupled to a
fiberoptic laser catheter and used endoscopically to remove nasal mucosa
and bone. The reported advantages of endoscopic laser surgery include
decreased tissue damage, absence of cutaneous scar, excellent hemostasis, and
decreased perioperative morbidity. More recently, endoscopic DCR utilizing functional endoscopic sinus equipment
has increased the popularity and success rate of the endoscpopic
approach. A variety of techniques are described in the literature.90–96 These range from endonasal drilling and trephination, to the use of a
ronguer and/or adjunctive use of mitomycin C. One approach is the
following: the nasal mucosa is anesthetized with 4% cocaine and
local 1% lidocaine with epinephrine injection into the nasal mucosa
just anterior to the middle turbinate. The mucosa in this area is
incised with a sickle knife and removed with front biting cutting endoscopic
instruments. Once the bone is exposed, a burr is used to remove
the wall separating the nasal cavity form the lacrimal sac. With the
sac exposed it is incised vertically and exposed. The system is then
stented with bicanalicular Silastic stents and secured in place (Fig 47A, 47B, and 47C). This technique is efficient with minimal morbidity and no external
scar. However, because the lacrimal sac is not sewn to the nasal mucosa, the
ultimate size of the fistula reduces to that of the Silastic
tubing diameter. Some individuals have tried treating the fistula site
with topical mitomycin C to prevent the fistula opening from shrinking
with varied results. Overall, in experienced hands the endoscopic
approaches are efficient and effective with success rates well in the 90% range
similar to external DCR. 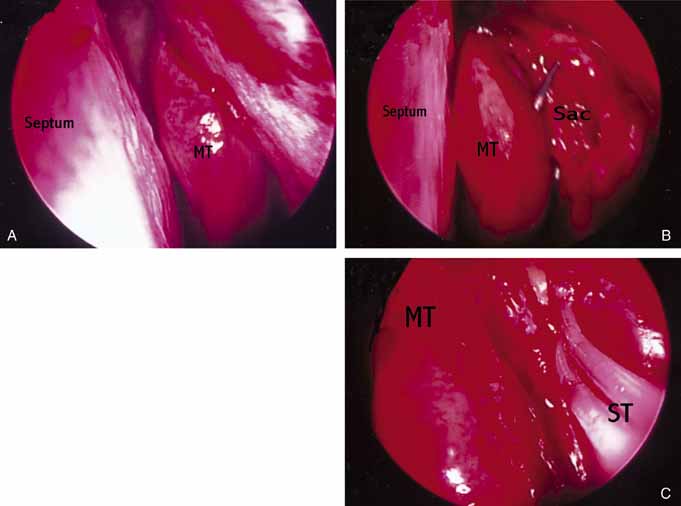 Fig. 47 Left endoscopic dacryocystorhinostomy (DCR). A. Endoscopic view of nasal cavity anterior to middle turbinate (MT). B. Lacrimal sac opened with Bowman probe in common canaliculus. C. Silastic stents (ST) in palce at end of operation. Fig. 47 Left endoscopic dacryocystorhinostomy (DCR). A. Endoscopic view of nasal cavity anterior to middle turbinate (MT). B. Lacrimal sac opened with Bowman probe in common canaliculus. C. Silastic stents (ST) in palce at end of operation.
|
Attempts to streamline the technique have included utilizing a vitrectomy
fiber-optic light pipe to assist with surgery. The light pipe
is fed into the canaliculus and into the lacrimal sac. The light then
visualizes where a small mucosal incision is made and the bone can be
removed under direct visualization with ronguers followed by stenting. 97 The most recent technique described by Dolman87 involves placing a large Bowman probe into the lacrimal sac and simply
puncturing through into the nasal cavity. After the probe is identified
in the nasal cavity, the opening is expanded under direct visualization
with a ronguer and stented. This proves to be quick, efficient, and
as effective as standard DCR surgery. Finally, a new large 9-mm balloon catheter was recently introduced
as an additional alternative. In this instance multiple passes are
made with a large Bowman probe to create a Swiss cheese-like pattern
in the bone between the lacrimal sac and the nasal cavity. The balloon
is fed into the lacrimal sac in retrograde fashion, straddling
the bone and inflated. The authord have no experience with this technique. With these advantages, many still consider the external approach the gold
standard. The success of the traditional external approach and its
low morbidity are well documented. Yet with the improved technology of
the last 10 years along with exposure to endonasal surgery and experience, the
endoscopic approach is a viable alternative, especially to the
patient who does not wish to have an external scar. In fact, in experienced
hands, the endoscopic success rates are approaching that of the
traditional approach. Dacryocystorhinostomy Revision Previously, many methods to revise a failed DCR have been described.62–64,98–100 These include dilation with a lacrimal probe, intranasal dilation with
a muscle hook, and enlarging the passageway with a knife, trephine or
laser. Intranasal rongeuring, directly or endoscopically, of obstructive
tissue has also been described. In 1989, Becker and Berry101 described dilation with a balloon catheter for revision of failed DCR (Fig. 48). Although balloon dacryoplasty is useful in congenital nasolacrimal
duct obstruction, we have had limited success with the procedure in
adult DCR revisions. 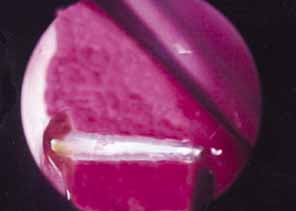 Fig. 48 Balloon dacryocystorhinostomy (DCR) revision of scarred DCR ostium. Fig. 48 Balloon dacryocystorhinostomy (DCR) revision of scarred DCR ostium.
|
The endoscopic approach is useful in patients who need revision of DCR. The
nasal ostium is easily identified and work is accomplished in a retrograde
fashion. The ostium can easily be expanded with functional endoscopic
sinus surgery instruments or a rongeur. Debris obstructing the
fistula can also be identified and removed (Fig. 49). 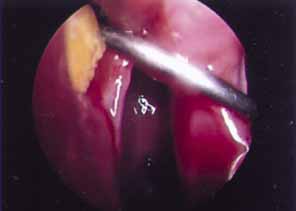 Fig. 49 Endoscopic view of dacryolith blocking ostium of previous dacryocystorhinostomy (DCR). Fig. 49 Endoscopic view of dacryolith blocking ostium of previous dacryocystorhinostomy (DCR).
|
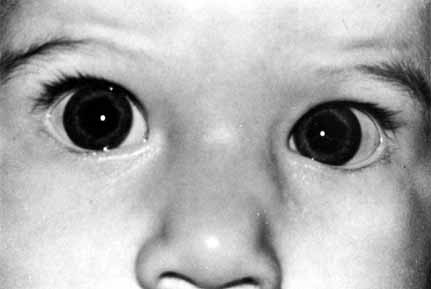
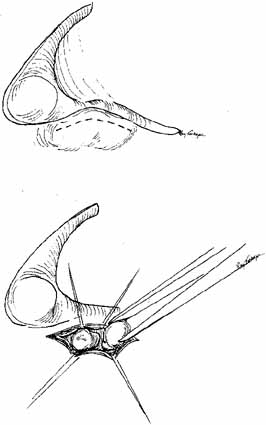
ABNORMALITIES OF THE PUNCTA AND CANALICULI (UPPER SYSTEM BLOCKS) Surgery of the punctum should be as conservative as possible. For simple
congenital veils, gentle puncture followed by dilation and irrigation
often will suffice. For greater degrees of atresia, however, injection
of methylene blue into the lacrimal sac through the skin may be of
value, particularly when the surgeon suspects that the canaliculus may
be involved (Figs. 50 and 51). The surgeon can apply pressure over the lacrimal sac area and look
for retrograde flow of dye through the lid margin. If the dye is not
visible, an incision in the lid margin can be made in the area of the
lacrimal papilla, if present. If the punctum is not present, an incision
can be made approximately 8 mm lateral to the medial canthal angle (Fig. 52). If dye is visible, the channel is normal (Fig. 53). If suction of the nose reveals dye, the sac and duct are clearly
patent, and a stent of Silastic tubing can be placed throughout the
nasolacrimal system, as is done for dacryostenosis (Fig. 54). However, if there is obstruction in the lower system, a DCR will
be necessary. Silastic tubing can be run through the entire upper and
lower canaliculus and through a DCR opening. 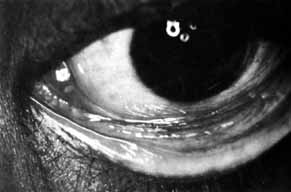 Fig. 50 Both puncta are absent in the left eye. Fig. 50 Both puncta are absent in the left eye.
|
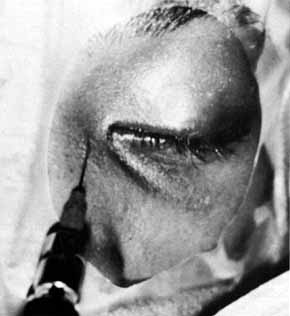 Fig. 51 Methylene blue injected through the medial canthal tendon to the area of
the assumed lacrimal sac. Fig. 51 Methylene blue injected through the medial canthal tendon to the area of
the assumed lacrimal sac.
|
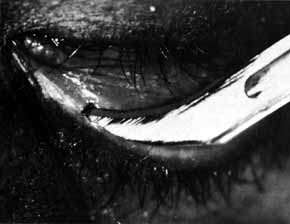 Fig. 52 If pressure placed on the sac does not show dye on the lid margin, a cutdown
is done over the lacrimal papilla, or approximately 8 mm lateral
to the canthal angle. Fig. 52 If pressure placed on the sac does not show dye on the lid margin, a cutdown
is done over the lacrimal papilla, or approximately 8 mm lateral
to the canthal angle.
|
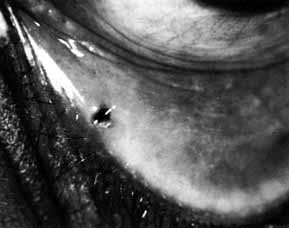 Fig. 53 Probing of the cutdown shows a canaliculus that is patent to the lacrimal
sac, as evidenced by methylene blue being seen in the cutdown site
when pressure is placed on the sac. Intubation through the canaliculi
and nasolacrimal duct is done as described earlier for dacryostenosis. Fig. 53 Probing of the cutdown shows a canaliculus that is patent to the lacrimal
sac, as evidenced by methylene blue being seen in the cutdown site
when pressure is placed on the sac. Intubation through the canaliculi
and nasolacrimal duct is done as described earlier for dacryostenosis.
|
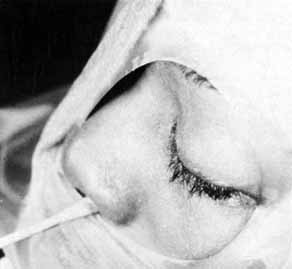 Fig. 54 The lower system is open as shown by methylene blue collected from the
nose. When the lower system is open, only Silastic intubation of the system
through the nasolacrimal duct is required. Fig. 54 The lower system is open as shown by methylene blue collected from the
nose. When the lower system is open, only Silastic intubation of the system
through the nasolacrimal duct is required.
|
Punctal stenosis in the adult population is a common cause of epiphora
as previously discussed. Again, before proceeding with surgery, the surgeon
should be especially cautious of the older patient with a concominant
dry eye as increased tear outflow may further exacerbate the reflex
tearing. Several techniques are available to treat punctal stenosis. The
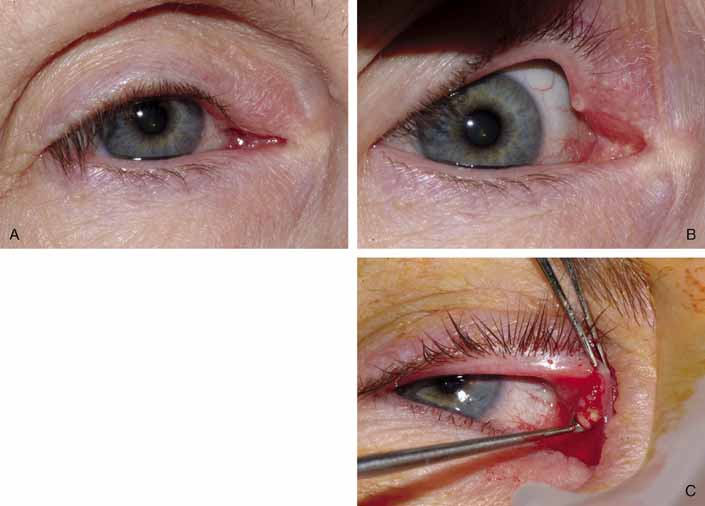
two recommended techniques are a punctoplasty and Silastic stenting. The
punctoplasty allows for controlled opening of the punctum. Typically
a two-snip procedure is performed. This is performed in
one of two ways. The first is to remove a V-shaped wedge from
the vertical portion of the puncta and canaliculus on the conjunctival
surface. This opens the most proximal portion of the system with out
risking damage or scarring to the remainder of the canaliculus. The second
two-snip procedure consists of a single vertical cut that
is created in the puncta and canaliculus followed by a single horizontal
cut along the proximal portion of the canaliculus. The concern with
these techniques is the potential damage to the canaliculus and its
function. If too much is cut, the canaliculus may not function properly. Also
there is a greater chance for scarring within the canaliculus
as it heals. To counterbalance the potential for scarring after the snip
procedure, a Silastic stent can be placed. The stent may also serve
the function of dilating and holding open the puncta and canaliculus
as the system heals. Overall punctoplasty and stenting can be quite helpful. If
the patient does not respond, however, consideration should
be given to some of the other functional problems such as the flaccid
canalicular syndrome. It may be useful to obtain dacryoscintigraphy in
evaluating the tear outflow function of the problem patient. For more extensive blocks in the canalicular system, a bypass procedure
is usually required. Until recently, the use of a bypass tube, such as
the Jones Pyrex tube (Gunther Weiss, Beaverton, OR), was considered
impractical for the pediatric age group. Greater experience, as
well as the use of a suture to hold the tube in place, has made this
approach more practical for pediatric patients with canalicular atresia
or severe obstruction.102 The Cox Pyrex glass tube set (Cox Ocular Prosthetics, Birmingham, AL) is
also useful because of the suture eye in its collar, which
enables fixation to adjacent tissue. Furthermore, the Cox set includes
a trocar for insertion and a measuring device to determine the tube
lengths (Cox tubes).103 Although a DCR with Silastic intubation is preferable to Pyrex bypass tubes, this
has limited success in patients with more extensive blockage
of the canalicular passages, despite earlier optimistic reports.104 An alternative to the bypass approach is a microsurgical anastomosis of
the involved canaliculus directly to the nasolacrimal sac.105 Such techniques, although promising, are technically difficult and cannot
correct extensive blocks in the canalicular system. Therefore, the
bypass procedure remains one of the most important procedures for controlling
canalicular causes of epiphora. The Jones Pyrex tube is useful because of its excellent capillarity. The
tube is available in a variety of lengths and angles. Tubes can be manufactured
with a suture eye in the collar of the tube. This type of
tube is particularly useful for the pediatric age group as the postoperative
period is more difficult in children. If the tube is not held securely
with a nonabsorbable suture, sneezing or blowing the nose can
cause loss of the tube. Even without an eye in the collar, the tube can
be secured by tying a suture around the cuff to the adjacent tissue
for stability. Most recently Porex (Fairburn, GA) introduced
a Pyrex tube with a porous polyethylene coating that allows the tube
to be fibrovascularly integrated into the soft tissue as another means
of securing the tube. Several other materials, including Silastic and
polytetrafluoroethylene have been suggested for bypass tubes.106,107 The Silastic tubes often require cutting the collar to fit, which could
produce rough and potentially irritating edges. None of the alternative
materials, including venous or mucous membrane grafts, have the excellent
capillarity of Pyrex. Therefore, use of alternate materials is
less successful as the tear flow is reduced. In addition, synthetic materials
allow more rapid accumulation of protein deposits postoperatively. The bypass technique was well described by Jones108,109 as a conjunctivodacryocystorhinostomy. This technique is required not
only for true congenital atresia of the canaliculi but also for obstructions
larger than 2 mm, particularly when previous attempts at reconstruction
with Silastic intubation or anastomosis of the canaliculi to
the sac have been unsuccessful. This technique is useful in instances
in which massive damage has occurred to the medial canthal region as a
result of trauma, radiation, or prior surgical reconstruction. DCR is
essential for the best placement and function of the tube. Placement
without mucosal flaps reduces movement with blinking and allows for potential
future dacryocystitis if the sac is not opened directly into the
nose. If a DCR has been done in a prior surgical effort, the glass
tube can be inserted by a closed approach (Fig. 55). When there has been no previous DCR, the steps described earlier
for DCR are followed to the point at which flaps are formed in the nasolacrimal
sac mucosa, which is an open approach for placement of the
Pyrex tube (Fig. 56). A small portion of the caruncle may be excised to accommodate the
tube. A sharp 23-gauge trocar or guide needle 30-mm long
is passed at a 45-degree angle in an inferior and somewhat
anterior direction through the lateral lacrimal sac wall and bony DCR
ostium, and into the nose. If the path is blocked by the middle turbinate, a
portion can be resected. Any severe deviations of the nasal septum
should have been diagnosed by proper nasal examination before this
surgical procedure. This may be facilitated by endoscopic examination. A 2-mm
trephine can be slipped down the guide needle to cut a
track into the nose at the proper angle. The maneuver will allow the
tube to be slipped into place, but held tightly enough that it will not
easily slip out of position during the postoperative period. The Luer-Lok
portion of the needle is snapped off and the trephine is
removed. A number 69 Beaver blade passed into the nose, adjacent to the
trocar, can be used instead of the trephine. An appropriate length of
Jones Pyrex tube is slipped over a probe and through the opening, into
position. The tube should project 2 mm into the nose, but should not
touch the nasal septum (Fig. 57). A single 6–0 nylon suture is passed through the hole in the
tube collar or slipped around the neck of the tube, and tied tightly. The
needle is passed behind the lid margin, deep into the posterior
palpebral tissues at the medial canthus and tied. The DCR is then completed
as previously described. 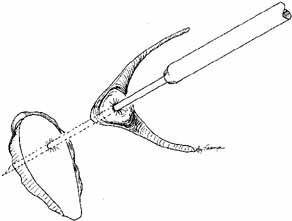 Fig. 55 Closed bypass tube method. This method is used when a dacryocystorhinostomy (DCR) was
completed as an earlier procedure. A 2-mm
trephine is slipped down over a guide needle that is placed at an
appropriate angle running through the previous DCR ostium into the nose. A
portion of the caruncle can be removed to facilitate placement. Fig. 55 Closed bypass tube method. This method is used when a dacryocystorhinostomy (DCR) was
completed as an earlier procedure. A 2-mm
trephine is slipped down over a guide needle that is placed at an
appropriate angle running through the previous DCR ostium into the nose. A
portion of the caruncle can be removed to facilitate placement.
|
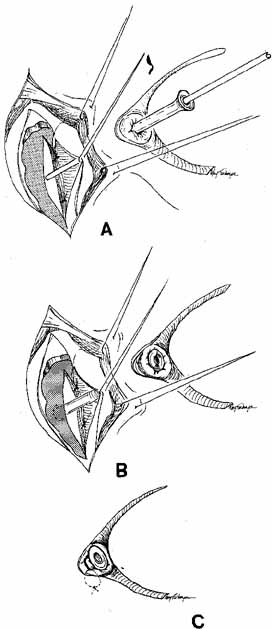 Fig. 56 Open bypass tube method. A. Surgery is the same as for a routine dacryocystorhinostomy (DCR) until
the formation of mucosal flaps. B. A portion of the caruncle can be excised to provide more room for the
Jones tube collar. The needle is removed, and the Jones tube inserted
over a straight probe. C. A 6–0 nonabsorbable suture is tied around the collar to the lid
on the palpebral surface. Fig. 56 Open bypass tube method. A. Surgery is the same as for a routine dacryocystorhinostomy (DCR) until
the formation of mucosal flaps. B. A portion of the caruncle can be excised to provide more room for the
Jones tube collar. The needle is removed, and the Jones tube inserted
over a straight probe. C. A 6–0 nonabsorbable suture is tied around the collar to the lid
on the palpebral surface.
|
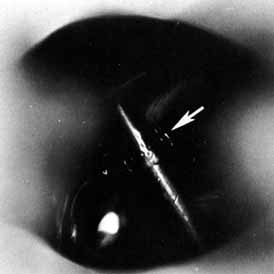 Fig. 57 The Jones tube resting on the guiding probe is seen protruding 2 mm into
the nose through the dacryocystorhinostomy (DCR) ostium. Fig. 57 The Jones tube resting on the guiding probe is seen protruding 2 mm into
the nose through the dacryocystorhinostomy (DCR) ostium.
|
Postoperative management of the Pyrex tube is difficult in the pediatric
age group but not impossible. Topical antibiotic drops are used four
times per day for 2 weeks. In an adult, periodic syringing is easily
done as an office procedure. In the pediatric age group, however, examination
under anesthesia may be required to syringe adequately through
mucus obstructions. The use of a contact lens wetting solution once daily
is helpful in both pediatric and adult patients to reduce mucus formation. It
is also helpful to instruct the patient to do the Valsalva
maneuver by a quick inspiration with closure of the mouth and pinching
both nostrils. In some instances, 10% N-acetylcysteine drops can be given several times daily. The mucolytic
action of this agent sometimes will clear the tube. It is not harmful
to the globe or adnexae, and obviously should be tried in the pediatric
age group before subjecting a child to a general anesthetic. Patients should be instructed to close their eyes or hold a finger over
the medial canthal region when they sneeze or blow their nose. Otherwise, the
tube can dislodge, even when secured with a suture. If a tube
is lost, it should be replaced as soon as possible. The channel can be
dilated with gold dilators (Weiss, Gunther Weiss), and a new
tube of appropriate length slipped in over a probe. A suture can be
used for fixation, as previously described. ABNORMALITIES OF THE LACRIMAL SAC Early probing for mucoceles (amniotoceles) and for neonatal dacryocystitis
was discussed earlier. These entities usually are seen at
birth or in the immediate postpartum period.33,110 The anlage or supernumerary duct (Fig. 58) is another congenital abnormality of the sac. With a supernumery
duct, tears drain directly onto the face if the upper portion of the
nasolacrimal system is open. Repair of a supernumerary duct can be accomplished
by first injecting a small amount of methylene blue through
a lacrimal cannula into the duct to outline the mucosal lining down to
the lacrimal sac. An appropriately sized probe is placed into the supernumerary
duct. An ellipse of skin is resected conservatively around
the probe. The duct is traced down to the lacrimal sac, where it is ligated
and cut (Fig. 59). A layered closure of muscle and skin is done. This type of simple
closure will prove to be sufficient if there is no lower block to tear
flow in the nasolacrimal duct. If there is a lower system block, however, Silastic
intubation of the nasal duct or DCR will be required
to eliminate this problem.  Fig. 58 A supernumerary duct with associated absence of punctum and canaliculus. Fig. 58 A supernumerary duct with associated absence of punctum and canaliculus.
|
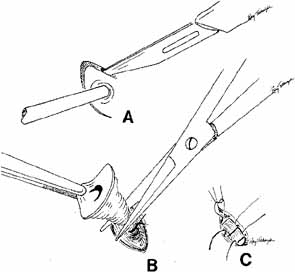 Fig. 59 Surgical repair of the supernumerary duct. A. Methylene blue is injected into the duct with the lacrimal cannula; an
ellipse is cut through the skin and muscle. Using a probe as a guide, careful
dissection is completed to the connection of the duct to the
sac. B. The supernumerary duct is ligated at its junction at the sac with a 5–0 absorbable
suture, and cut. C. The subcutaneous tissue and skin are closed with appropriate interrupted
sutures. Fig. 59 Surgical repair of the supernumerary duct. A. Methylene blue is injected into the duct with the lacrimal cannula; an
ellipse is cut through the skin and muscle. Using a probe as a guide, careful
dissection is completed to the connection of the duct to the
sac. B. The supernumerary duct is ligated at its junction at the sac with a 5–0 absorbable
suture, and cut. C. The subcutaneous tissue and skin are closed with appropriate interrupted
sutures.
|
Neoplasms of the lacrimal sac can occur, and may present as functional
blocks. Dacryocystography may be helpful in diagnosing these masses preoperatively. A
frozen section is mandatory. Malignancy of the sac is
one of the few indications for a dacryocystectomy. The nasopharynx should
not be opened because of the potential risk of spread of a malignant
tumor. Congenital entropion, distichiasis, or trichiasis can stimulate
reflex tearing and cause epiphora. Appropriate steps should be taken
to identify and surgically correct these problems. Ectropion of the
punctum or lid is rare as a congenital problem. Medial canthal or lacrimal sac tumors are best excised with careful margin
and base controls.6,47,48 No attempt should be made initially to violate the bone or nasal mucosa. Canaliculoplasty
to the lacrimal sac or Silastic intubation through
the nasolacrimal duct can be considered. A DCR or Jones bypass tube should
be avoided until at least 1 year has elapsed without clinical or
radiographic evidence of tumor recurrence. This waiting period is mandatory
to avoid seeding malignant cells into the nasopharynx. It is better
for the patient to experience epiphora than to risk tumor seeding, which
may have life-threatening consequences. Dacryocystectomy should be
reserved for treatment of lacrimal sac tumors.112 It is not an appropriate technique for the relief of uncomplicated epiphora
that results from lower system block. If necessary, bypass surgery
can be done later, regardless of whether flaps, skin grafts, or granulation
had been used to cover the dissected area in the medial canthal
region. MAJOR CONGENITAL CRANIOFACIAL DEFORMITIES Any of the lacrimal abnormalities described above can occur singly or in
combination with craniofacial anomalies. Repair for each entity is identical
to that previously described for routine lacrimal problems. However, the
timing of repair is different.111 Deformities such as telecanthus, epicanthal folds, orbital volume problems, and
many canthal deformities can be managed during the initial craniofacial
reconstruction. Specific nasolacrimal problems, however, except
for simple dacryostenosis or acute dacryocystitis, are best treated
after major bone shifts or on lay bone grafts in the midface region
have been completed. Unfortunately, good results are too easily undone
by subsequent surgery. Repeat lacrimal procedures are particularly
difficult and have significantly less potential for success (Figs. 60 and 61).  Fig. 60 Craniofacial deformity with encephalocele, right upper lid coloboma, and
nasolacrimal obstruction. Fig. 60 Craniofacial deformity with encephalocele, right upper lid coloboma, and
nasolacrimal obstruction.
|
 Fig. 61 Postoperative appearance of the patient
shown in Figure 40.
The patient underwent reconstruction of deformities with conjunctivodacryocystorhinostomy
with a Jones Pyrex tube.
Fig. 61 Postoperative appearance of the patient
shown in Figure 40.
The patient underwent reconstruction of deformities with conjunctivodacryocystorhinostomy
with a Jones Pyrex tube.
|
RECONSTRUCTION OF THE LACRIMAL DRAINAGE SYSTEM AFTER TUMOR EXCISION Lid tumors involving the punctum or canaliculus require careful excision
under meticulous frozen section control or Mohs-type excision. However, a
lid defect with a remaining proximal portion of the canaliculus
can be repaired with a Silastic stent in a fashion similar to that
described for canalicular lacerations (Figs. 62, 63, 64). Silastic tubing is passed through the normal punctum and canaliculus
and brought out through the nasolacrimal duct. The other end, with
the probe attached, is threaded into the remaining canalicular remnant. The
lid is sutured into position, leaving a loop of Silastic that
will form a pathway from the palpebral surface to the mucosa of the canaliculus. The
tubing loop must be relatively loose to minimize the greater
chances of cheesewiring that are present here.  Fig. 62 Defect after excision of basal cell carcinoma involving the punctum and
right lower lid. Fig. 62 Defect after excision of basal cell carcinoma involving the punctum and
right lower lid.
|
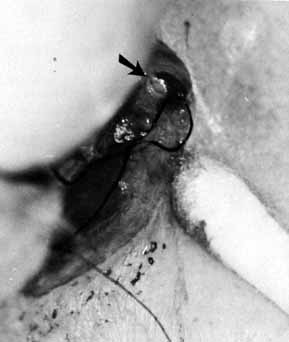 Fig. 63 Proximal end of the punctum visible through a microscope (arrow). The entire nasolacrimal system can be intubated with Silastic tubing, and
the lid supported to the canthal tendon. Fig. 63 Proximal end of the punctum visible through a microscope (arrow). The entire nasolacrimal system can be intubated with Silastic tubing, and
the lid supported to the canthal tendon.
|
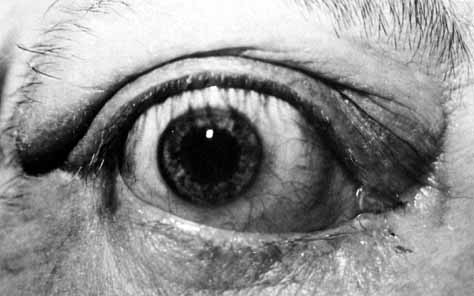 Fig. 64 Postoperative result 6 months later, with lid repaired and Silastic tubing
in place. The lower lid will form a channel where the lateral aspect
is joined to the canalicular remnant. The channel will epithialize
if the stent is left in place for a minimum of 6 months. Fig. 64 Postoperative result 6 months later, with lid repaired and Silastic tubing
in place. The lower lid will form a channel where the lateral aspect
is joined to the canalicular remnant. The channel will epithialize
if the stent is left in place for a minimum of 6 months.
|
|