THE SKIN The eyelid skin is composed of a thin dermis and contains little subcutaneous
fat.9 It is very elastic and is one of the thinnest of the body. It is loosely
adherent to the underlying orbicularis oculi muscle. The skin of the
upper eyelid is thinner than that of the lower eyelids. In later years, the
skin becomes redundant. The excess skin provides an excellent
source for skin grafting for eyelid reconstruction. There is a marked
transition from the thin eyelid skin to the thicker skin of the eyebrow
and cheek. The redundancy and elasticity of the eyelid skin and other
eyelid structures allows for primary closure of fairly large defects (up
to 30% of the eyelid in older adult patients). Upper eyelid skin
laxity increases with age. This is called dermatochalasis. Dermatochalasis
may progress and cause hooding, which results in mechanical ptosis
that constricts the superior visual fields, or cause a mechanical upper
eyelid entropion. Prominence of the lower eyelids may result from
prolapse of the orbital fat, malar bags, or hypertrophic or overriding
orbicularis oculi muscle. Excising excessive amounts of skin from either
the upper or lower eyelids can create lagophthalmos or frank ectropion. Transverse eyelid creases are present in both upper and lower eyelids, measuring 8 to 10 mm
above the upper eyelid margin and 4 to 5 mm below
the lower eyelid margin.3 The upper eyelid crease, more specifically called the superior palpebral
crease, represents the cutaneous insertion of fibers of the levator
aponeurosis into the preseptal orbicularis oculi, which is usually the
site of the eyelid fold. The upper eyelid fold refers to the roll of
skin overlying the eyelid crease. Absence of an eyelid crease implies
lack of LPS function, such as seen in congenital blepharoptosis. Dehiscence
of the levator aponeurosis from its insertion on the tarsus, as
seen in involutional ptosis, may also result in an elevated eyelid crease. Insertions
of the levator aponeurosis into the preseptal orbicularis
oculi and dermis are either weak or absent in the Asian eyelid. The
anatomy of the Asian eyelids is discussed later in the chapter. The
region between the upper eyelid crease and the superior orbital margin
is referred to as the superior orbital sulcus. Loss of orbital volume
following enucleation or atrophy of orbital fat as seen with aging may
result in a concavity of the superior sulcus skin and muscle. The lower eyelid has three creases (Fig. 5). The inferior palpebral crease is a true crease marking the inferior
edge of the tarsus and the insertions of the lower eyelid retractor muscles. The
other two creases are less well defined and are the nasojugal
crease inferomedially and the malar crease inferior to the lateral
canthus, which marks the junction of the orbicularis muscle and the malar
fat pad. These topographic landmarks demarcate the inferior border
of the lower eyelid. 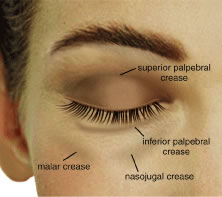 Fig. 5. Surface topography. Fig. 5. Surface topography.
|
EYELID MARGIN The upper and lower eyelid margins are composed of several identifiable
structures (Fig. 6). The lash line is the most anterior line found along the eyelid margin, which
is the site of origin of the cilia. Approximately 100 to 150 cilia
are found in the upper eyelid, and approximately 50 to 75 cilia
are in the lower eyelid. The eyelashes arise from hair follicles on the
anterior surface of the tarsus and project outward, anterior to the
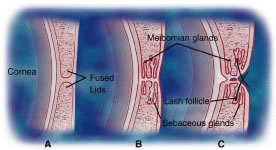
eyelid margin. Both meibomian glands and eyelashes differentiate during
the second month of gestation from a common pilosebaceous unit. Congenital
distichiasis can result from poor differentiation, because an extra
row of lashes arises from the meibomian orifices. A lash follicle
may also develop from a meibomian gland following trauma in certain disease
states, or with chronic irritation, leading to acquired distichiasis.10 Each hair follicle contains approximately two sebaceous glands called
glands of Zeis. Sweat glands, or glands of Moll, also lie near the cilia
and empty into the adjacent follicles. Glands of Moll and Zeis secrete
lipid that contributes to the superficial layer of the tear film and
slows evaporation.9 Posterior to the lash line and anterior to the tarsus on the eyelid margin
is the gray line. The gray line is also referred to as the muscle
of Riolan and represents the pretarsal orbicularis muscle on the eyelid
margin.11 An incision posterior to the gray line along the eyelid margin demarcates
the anterior lamella from the posterior lamella of the eyelid. The
meibomian glands and tarsus comprise the layer of the eyelid margin located
immediately posterior to the gray line and is part of the posterior
lamella. The meibomian glands are arranged vertically within the
tarsus with their orifices at the marginal surface. A mucocutaneous junction
is located posterior to the meibomian gland orifices on the eyelid
margin. The lacrimal puncta are also visible on all four eyelids near
the medial canthal angle. The puncta represent the opening from the
eyelid margin into the ampulla and canaliculi, which is the beginning
of the lacrimal drainage apparatus. The lacrimal drainage system is
described later in the chapter. 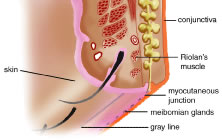 Fig. 6. Eyelid margin. Fig. 6. Eyelid margin.
|
THE ORBICULARIS OCULI MUSCLE The orbicularis oculi muscle is a thin sheet of concentrically arranged
muscle fibers covering the eyelids and periorbital region. It is the
main protractor of the eyelids and its primary function is narrowing of
the palpebral fissures and closure of the eyelids. It also plays a role
in the lacrimal pump system. The orbicularis oculi muscle is innervated
by the facial nerve, and although it is a skeletal muscle, it is
under voluntary, as well as reflex, control.12 The orbicularis oculi muscle is classically divided into three anatomic
parts13 (Fig. 7). The pretarsal orbicularis overlies the tarsus, the preseptal orbicularis
overlies the orbital septum, and the orbital portion lies beneath
the skin that surrounds the remaining orbital aperture (Fig. 8). The pretarsal and the preseptal orbicularis together are referred to
as the palpebral orbicularis. Voluntary squeezing of the orbital orbicularis
closes the palpebral fissure and protects the globe and orbit
from injury. Involuntary movements, such as blinking and the functioning
of the lacrimal pump, result mainly from contraction of the palpebral
portion of the orbicularis oculi muscle.10 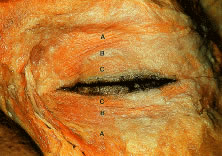 Fig. 7. Orbicularis oculi muscle, cadaver dissection. A. Orbital portion. B. Preseptal portion. C. Pretarsal portion. Fig. 7. Orbicularis oculi muscle, cadaver dissection. A. Orbital portion. B. Preseptal portion. C. Pretarsal portion.
|
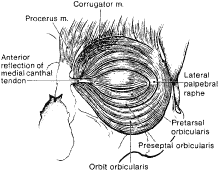 Fig. 8. Protractor muscles of eyelid closure with the skin layer removed. Fig. 8. Protractor muscles of eyelid closure with the skin layer removed.
|
The orbital portion of the orbicularis oculi muscle is the outermost and
the largest segment. It is responsible for forcible eyelid closure (squeezing) and
voluntary eyelid closure (winking). The orbital portion
of the orbicularis muscle originates from the periosteum of the frontal
and maxillary bones at the insertion of the medial canthal tendon (MCT). It
is a continuous muscle that encircles the orbit before reinserting
at the medial canthus inferiorly where it is attached to the periosteum
of the posterior lacrimal crest, the lacrimal fascia, and the
MCT.12 Superiorly, the orbital portion extends to the eyebrow and interdigitates
with the frontalis and the corrugator superioris muscles. Medially, attachments
extend from the supraorbital notch to the side of the nasal
bone. Inferiorly, the orbital portion arises from the anterior limb
of the MCT and the surrounding periosteum and extends to the infraorbital
foramen where it continues along the infraorbital margin. Laterally, the
orbital orbicularis portion courses over the zygoma and cheek
and over the temporal fascia. The preseptal portion of the orbicularis oculi muscle overlies the orbital
septum and functions in voluntary eyelid closure (winking) and involuntary
eyelid closure (blinking). The action of the superior preseptal
orbicularis muscle can also force the eyelid margin below the level
of its canthal attachments as seen in looking down.14 The preseptal portion has a superficial origin from the anterior limb
of the MCT and a deep origin, called the Jones muscle, which arises from
the posterior lacrimal crest and the fascia surrounding the lacrimal
crest.8 The orbital segment interdigitates with the preseptal orbicularis muscle
and courses laterally across the upper and lower eyelids. The two segments
fuse to form the lateral horizontal raphe, which overlies the
lateral canthal tendon (LCT) and the lateral orbital rim. The pretarsal portion of the orbicularis muscle is primarily responsible
for horizontal movement of the eyelid, which is important in the function
of the lacrimal pump. It also assists in eyelid closure mainly during
involuntary blinking. The lower one third of the pretarsal muscle
in the upper eyelid is adherent to the underlying tarsus, whereas the
upper two thirds of the muscle is adherent to the superficial insertion
of levator aponeurosis at the superior tarsal border.14 There are two medial insertions of the pretarsal portion of the orbicularis
muscle. The superficial head condenses to form the anterior limb
of the MCT and inserts into the anterior lacrimal crest. The deep head, called
the tensor tarsi muscle of Horner, shares the same site of origin
as the preseptal muscle. It inserts on fascia covering the lacrimal
sac fossa and on the periosteum of the posterior lacrimal crest. Here, it
is identified as the posterior limb of the MCT15 (Fig. 9). Contraction of the Horner's muscle draws the eyelids (especially
the lower) medially and posteriorly. The resulting lateral pull on the
lacrimal diaphragm creates a negative pressure in the lacrimal sac
and draws the tears from the canaliculi into the sac. The deep and superficial
heads of the pretarsal orbicularis oculi muscle encircle both
lacrimal canaliculi and along with the Jones muscle facilitate tear drainage. Contraction
shortens the ampullae of the canaliculi system and
facilitates the movement of tears into the sac. The lacrimal drainage
system is described in further detail later in the chapter. Laterally, the
upper and lower pretarsal muscles fuse to form the LCT and insert 3 to 4 mm
deep to the lateral palpebral raphe onto the lateral orbital
tubercle. The deep medial and lateral attachments of the pretarsal
orbicularis oculi muscle are important in maintaining eyelid to globe
apposition. 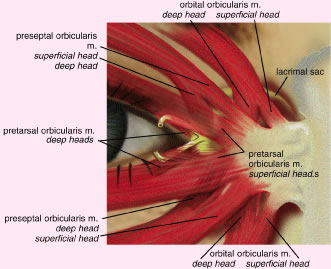 Fig. 9. Medial attachments of the orbicularis oculi muscle. Fig. 9. Medial attachments of the orbicularis oculi muscle.
|
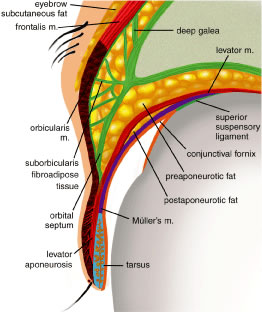
ORBITAL SEPTUM The orbital septum is a thin, fibrous, multilayered sheath that arises
from the anterior periorbita (periosteal lining of the orbit) at the arcus
marginalis (Fig. 10). The orbital septum separates the eyelids from the orbit and serves as
an important anatomic barrier to infection, hemorrhage, and edema. Inflammatory
or infectious processes anterior to the septum are considered
preseptal, whereas similar findings posterior to the orbital septum
are considered orbital. In older individuals, the septum can become
tenuous. Orbital or preaponeurotic fat is an important anatomic landmark
and herniation of orbital fat through the septum from trauma or surgery
indicates a disruption of the orbital septum.  Fig. 10. The multilayered orbital septum; the skin and orbicularis muscle are retracted. Fig. 10. The multilayered orbital septum; the skin and orbicularis muscle are retracted.
|
From the arcus marginalis, the septum spans the anterior orbit in a plane
deep to the orbicularis oculi muscle and ultimately fuses with the
eyelid retractors or tarsus (Fig. 11). In the upper eyelid, the orbital septum adjoins the posterior epimysium
of the orbicularis before it fuses with the levator aponeurosis approximately 2 to 3 mm
above the superior tarsal border and about 10 mm
above the lash line.16 In the lower eyelid, the septum arises from the inferior orbital rim as
a condensation of the periorbita and periosteum. It continues anteriorly
until it joins with the lower eyelid retractors as a single unit
at a point 4 to 5 mm below the inferior tarsus or it inserts on the lower
border of the tarsus. The septum travels medially with the pretarsal
orbicularis muscle and attaches to the posterior lacrimal crest with
a few fibers extending to the anterior lacrimal crest. Laterally, the
septum attaches to the deep insertion of the pretarsal segment of the
orbicularis muscle and inserts onto the lateral orbital tubercle.17  Fig. 11. The orbital septum. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 11. The orbital septum. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
In the Asian eyelids, the eyelid creases vary in position in relation to
the eyelid margin and may be lower than the occidental eyelid, or they
may be absent altogether. In the upper eyelid, the orbital septum may
fuse with the aponeurosis as is inserts into the tarsus. This insertion
can occur as high as the superior border of the tarsus or as low
as the lash line accounting for the lower or absent upper eyelid crease.3 There are also differences in the Asian lower eyelids. Epiblepharon is
a common finding in the Asian lower eyelid and is seen as an additional
fold of skin running horizontally below the lower eyelid margin. It
is often associated with a loss of the eyelid crease or with a crease
that rests very close to the eyelid margin of the lower eyelid. The absence
of an eyelid crease may be due to lack of deep anchoring of the
superficial skin to the preseptal portion of the orbicularis oculi muscle.18 An overriding orbicularis muscle is also associated with epiblepharon. The
weight of the skin fold and the orbicularis muscle may rotate the
lower eyelid margin inward, creating an entropion. FAT Fat within the orbit and eyelids serves as a protective cushion for the
globe and facilitates movement of the globe. There are three general
locations of fat that are described: eyelid, sub-brow, and orbital. In
the upper eyelid, there are two fat pads, medial and central (preaponeurotic) (Fig. 12). The central preaponeurotic fat is divided by the trochlea of the superior
oblique tendon and fascial strands of the medial retinaculum. These
divisions are arbitrary and the fascial planes can be variable, because
eyelid fat is interconnected and contiguous with deeper orbital
fat. The preaponeurotic fat pad is an important surgical landmark, because
it lies just posterior to the orbital septum and anterior to the
levator aponeurosis (Fig. 13). The central preaponeurotic fat pad tends to be yellow as compared with
the whiter more fibrous medial fat pad. The medial fat pad is derived
from orbital fat deep to the levator muscle. It is more vascular because
of the location of the palpebral arterial arcade that serpiginously
courses through the medial fat pad.19,20 The lacrimal gland occupies the temporal space lateral to the central
preaponeurotic fat pad. The lacrimal gland is a firm, pink, vascular, lobulated
structure that produces the aqueous component of the precorneal
tear film. (The anatomy and function of the lacrimal gland is described
later in the chapter.) Care must be taken to avoid excision of the
lacrimal gland while liposculpturing during blepharoplasty. Fine connective
tissue septa extend anteriorly from the capsule of preaponeurotic
fat to the orbital septum and posteriorly to the levator aponeurosis.17 The postaponeurotic or pretarsal space, located between the levator aponeurosis
anteriorly and the tarsus posteriorly, may contain a small amount
of peripheral orbital fat. The fibrous septa compartmentalizing
superficial and deep orbital fat and extensions of the fascial sheaths
of the orbital muscles and eyelid structures are of great importance
to the movement of the upper eyelid. 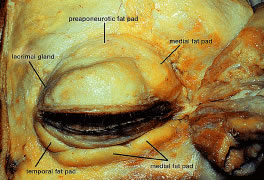 Fig. 12. Eyelid fat pads in a cadaver dissection. Fig. 12. Eyelid fat pads in a cadaver dissection.
|
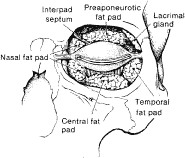 Fig. 13. Frontal view of the eyelids and orbit showing the fat pad distribution; the
skin and protractor muscles have been removed. Fig. 13. Frontal view of the eyelids and orbit showing the fat pad distribution; the
skin and protractor muscles have been removed.
|
Sub-brow fat can form a redundant upper eyelid skin fold and undergoes
gravitational descent during aging (Fig. 14). In females, the eyebrow is generally arched and above the level of the
supraorbital rim. The male eyebrow is flatter and at the level of the
supraorbital rim. The eyebrow fat pad is more prominent in the male, producing
a fuller appearance in the lateral brow area. The position
of the eyebrow can affect the height and excursion of the upper eyelid
and must be considered in a patient being evaluated for blepharoptosis
or for blepharoplasty. The retro-orbicularis oculus fat (ROOF) is defined
as the layer of fibrofatty tissue deep to the orbicularis oculus
muscle, superficial to the orbital septum and orbital rim, and extending
medially from the superior orbital nerve and laterally over the lateral
upper orbit.21 Resection of the ROOF in conjunction with aesthetic blepharoplasty can
soften and flatten heaviness and bulkiness in the lateral upper orbital
and brow region. A direct or indirect browplasty or browpexy may be
an alternative treatment for patients with brow ptosis.22 One must discern patients with eyebrow ptosis from those with eyelid ptosis
caused by levator muscle or Muller's muscle dysfunction.  Fig. 14. Sub-brow fat. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 14. Sub-brow fat. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
Some surgeons consider the lower eyelid to have three fat pads.9,23 A medial fat pad is subdivided by the origin of the inferior oblique muscle. Temporally, a
small fat pad lies inferior to the lateral canthus
and is separated from the main fat pad by a fibrous extension of connective
tissue from the orbital septum and periorbita that joins with
the capsulopalpebral fascia (CPF) and Lockwood's ligament.24 Because the eyelid fat pads are in direct communication with the deep
extraconal fat of the orbit, caution must be exercised when handling orbital
fat in the anterior orbit. Traction on the fat pad may injure deep
orbital vessels causing orbital hemorrhage and permanent loss of vision.19,25 The midfacial fat compartments include the suborbicularis oculi fat (SOOF) and
malar fat pads. These fat compartments are bound to the orbicularis
muscle by the superficial muscular aponeurotic system (SMAS) of
the cheek. The SOOF is composed of the deep subcutaneous fat and connective
tissue, which is located beneath the orbicularis muscle in the lower
eyelid and extends into the midface. In the aging lower eyelid, eyelid
tone decreases with associated skin and muscle laxity. Horizontal
laxity of the lower eyelid increases, and pseudoherniation of orbital
fat may result in contour irregularities. The SOOF can become apparent
with the gravitational descent seen in the midface with aging and contributes
to the aesthetic deformity of the lower lids. Malar bags may
also develop from descent of the malar fat pad. A SOOF lift is a technique
used for midfacial rejuvenation that includes subperiosteal dissection
of the SMAS to elevate the SOOF and redraping the orbicularis
muscle to reposition the midface.26 Recent emphasis has been placed on a SOOF lift for midfacial rejuvenation
surgery in conjunction with a lower eyelid blepharoplasty and lateral
canthoplasty.27 The SOOF lift has also been used for functional repair of lower eyelid
deformities seen in patients with significant involutional or cicatricial
ectropion. Asian eyelids characteristically have a fuller appearance than the eyelids
of Caucasian individuals. Upper eyelid fullness in the Asian person
is typically caused by extension of preaponeurotic fat and brow fat
into the upper eyelid. A recent study by Carter and colleagues28 used high-resolution magnetic resonance images to compare Asian and Caucasian
lower eyelids. The study revealed two major differences in the
lower eyelid anatomy: (1) there is more anterior projection of orbital
fat with respect to the orbital rim in the Asian eyelid as compared
with whites and (2) there is a more superior projection of orbital fat
that extends to the inferior border of the tarsus with poorly defined
eyelid creases in Asians. RETRACTORS The eyelid retractor muscles include the LPS muscle and the superior tarsal
muscle, called Muller's muscle, in the upper eyelid and the
CPF and the inferior tarsal muscle (ITM) in the lower eyelid (Fig. 15). The LPS is a skeletal muscle, whereas the ITM and Muller's muscle
are both composed of smooth muscle. The action of the LPS is to elevate
the upper eyelids. The oculomotor nerve (third cranial nerve) provides
the motor innervation of the LPS. The superior division of the oculomotor
nerve enters the orbit through the supraorbital fissure and
the annulus of Zinn. It passes around the medial border of the superior
rectus muscle to pierce the undersurface of the LPS at its posterior 1/3 anterior 2/3 junction, which is 12 to 13 mm from the orbital apex. Muller's
muscle and the ITM are sympathetically innervated and
also act to open the upper and lower eyelids. 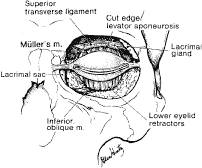 Fig. 15. Frontal view of the orbit with the skin muscle layer, septum, levator, and
fat pads removed. The retractor muscles are well visualized at this
plane. Fig. 15. Frontal view of the orbit with the skin muscle layer, septum, levator, and
fat pads removed. The retractor muscles are well visualized at this
plane.
|
In the upper eyelid, the LPS muscle begins to develop at about 10 weeks
of fetal life.14 The LPS muscle arises from the lesser wing of the sphenoid bone at the
orbital apex above the annulus of Zinn and superolateral to the optic
foramen. The mesenchymal origin of both the levator and the superior
rectus muscles is immediately below at the annulus of Zinn. The first 40 mm
of the LPS is the muscular portion, and the remaining anteriorly
projecting 14 to 20 mm becomes the levator aponeurosis.10 The levator muscle crosses over the superior transverse ligament of Whitnall (STL), which
is a condensation of horizontally oriented connective
tissue resting within the anterior aspect of the fibrous sheath surrounding
the body of the levator muscle.29 Whitnall's ligament marks the junction where the LPS changes from
a skeletal muscle to a fibrous sheath, the levator aponeurosis. STL, composed of collagen and elastic fibers, is seen as a white line traversing
horizontally across the eyelid, approximately 10 mm above the
superior border of the tarsus (Fig. 16). The STL medially attaches to the trochlear fascia and superior oblique
tendon and sends wisps of connective tissue to the medial retinaculum. It
attaches laterally to the fascia surrounding the orbital portion
of the lacrimal gland and at the frontozygomatic suture. The STL acts
to suspend the levator complex and also serves as a fulcrum, adding
a mechanical advantage to levator muscle action on the upper eyelid.3 Just posterior to Whitnall's ligament a dense intermuscular fascia
interconnects the undersurface of the levator aponeurosis and the superior
surface of the superior rectus muscle. A superior conjunctival
fornix suspensory ligament arises from the anterior surface of this intermuscular
membrane.30,31 The superior rectus muscle and the levator muscle are also joined by fibrous
attachments along their medial borders. 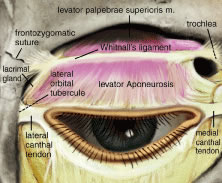 Fig. 16. Whitnall's ligament. The medial attachment is to the fascia of the
trochlea. The major lateral attachment is to the frontozygomatic suture, with
minor attachments to the lateral orbital tubercle. Fig. 16. Whitnall's ligament. The medial attachment is to the fascia of the
trochlea. The major lateral attachment is to the frontozygomatic suture, with
minor attachments to the lateral orbital tubercle.
|
The horns of the levator aponeurosis are broad fibrous condensations in
the lateral and medial edges and should be distinguished from the STL. The
lateral and medial horns of the levator aponeurosis and its osseous
insertions are located inferior to the STL. The lateral horn has more
strength and is more tendinous than the less dense medial horn. Laterally, it
courses through the lacrimal gland, dividing the gland into
the palpebral and orbital lobes. The lateral horn continues inferiorly
to join the lateral retinaculum and together they form a strong insertion
into the lateral orbital tubercle. The medial horn of the levator
aponeurosis passes over the superior oblique tendon where it has weak
attachments from the STL. The medial horn continues medially to join
the medial retinaculum to reach the MCT and posterior lacrimal crest. The
weaker attachments medially are thought to allow a greater mobility
of the medial upper eyelid.32 The lateral and medial posterior attachments are important in eyelid to
globe apposition. The levator aponeurosis continues anteriorly until it joins with fibers
of the orbital septum about 2 to 3 mm above the tarsal border. The orbital
septum fuses with the aponeurosis at or just above the eyelid crease. At
this point, additional fibers interdigitate with the orbicularis
oculi muscle. The eyelid crease may vary but is typically located
near the superior edge of the tarsal border at about 8 to 10 mm above
the eyelid margin.29 The actual eyelid crease is formed from the attachment of the levator
aponeurosis to the preseptal orbicularis muscle and subcutaneous tissue. The
levator aponeurosis sends connective tissue attachments to insert
on the lower one third of the anterior surface of the tarsus with its
strongest attachments 3 mm above the eyelid margin.33,34,35 These tarsal attachments are crucial for upper eyelid function. Attenuation of the levator muscle and dehiscence of its attachments may
demonstrate aging changes of the upper eyelid. This can result in elevation
of the eyelid crease as seen in involutional blepharoptosis.3 Congenital and acquired ptosis is a drooping of the upper eyelid usually
caused by poor elevating power of the upper eyelid. Congenital ptosis
is typically characterized by lagophthalmos and an absence of an eyelid
crease resulting from fibrofatty degeneration and poor function of
the LPS. There are several classifications of ptosis. (See Volume 5, Chapter 78.) Muller's muscle, also known as the superior tarsal muscle, is the
other retractor muscle of the upper eyelid (Fig. 17). It is a smooth, sympathetically innervated muscle that acts in concert
with the levator muscle to elevate the upper lid. Sympathetic innervation
is derived from nerve fibers, which travel along the peripheral
arterial arcade and other small arteries.29 Muller's muscle is highly vascularized and is often a source of bleeding
in surgery. It originates from the underside of the levator muscle
approximately 20 to 22 mm above the superior tarsal border at the
origin of the aponeurosis.24 High in the eyelid, Muller's muscle is loosely attached to the aponeurosis
anteriorly and to the conjunctiva posteriorly. It is more adherent
posteriorly to the conjunctiva as is nears the upper boarder of
the tarsus. The superior tarsal muscle inserts into the upper border
of the tarsal plate. Clinically, increased sympathetic stimulation as
seen in fright or Grave's ophthalmopathy can retract the upper lid 2 to 3 mm
above the normal resting position. Diminished tone as seen
in fatigue, paralysis, or Horner's syndrome may cause the lid to
drop as much as 2 mm. Topical administration of a short-term sympathomimetic
agent (i.e., phenylephrine) can be used to determine preoperatively
the effect of tarsal muscle surgery on ptosis.36,37 A positive result following topical administration of phenylephrine is
elevation the upper eyelid 2 to 3 mm, which suggests that Muller's
muscle resection may be beneficial. (See Volume 5, Chapter 78.) 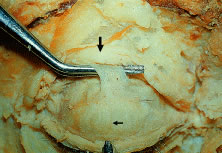 Fig. 17. Muller's muscle: A 10-mm strip of Muller's muscle is preserved
in this cadaver demonstrating its origin from the underside of the reflected
levator muscle (thick arrow) and its insertion onto the superior ridge of the tarsus. The tarsus is
seen with the vertically oriented meibomian glands (thin arrow). Fig. 17. Muller's muscle: A 10-mm strip of Muller's muscle is preserved
in this cadaver demonstrating its origin from the underside of the reflected
levator muscle (thick arrow) and its insertion onto the superior ridge of the tarsus. The tarsus is
seen with the vertically oriented meibomian glands (thin arrow).
|
The retractor muscles of the lower eyelids include the inferior tarsal
muscle and the CPF. The CPF arises from the inferior rectus muscle sheath
just posterior to the inferior oblique muscle (Fig. 18). The CPF is analogous to the levator aponeurosis of the upper eyelid, and
they are also embryologically related.3 The CPF has no distinct innervation, but its action mirrors the action
of the inferior rectus muscle, which is innervated by sympathetic postganglionic
fibers running with the inferior branch of the oculomotor
nerve. The ITM lies posterior to the CPF and arises from the CPF extending
from the sheath of the inferior rectus muscle. The ITM is adherent
to the overlying CPF and to the underlying conjunctiva38 (Fig. 19). The ITM is analogous to the Muller's muscle of the upper eyelid
as a retractor muscle for the eyelids, and it is also sympathetically
innervated. In Horner's syndrome, the atonic sympathetic ITM may
allow the lower lid to elevate as much as 1 mm. The fascial sheaths of
the CPF and the ITM divide and surround the inferior oblique muscle
and reunite before inserting into the anterior aspect of the inferior
tarsus. 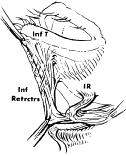 Fig. 18. The inferior eyelid with septum removed. The lower forceps grasp the capsulopalpebral
head just as it comes off the inferior rectus muscle (IR) and
inserts onto the lower boarder of the tarsus (T) and the conjunctival
fornix. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 18. The inferior eyelid with septum removed. The lower forceps grasp the capsulopalpebral
head just as it comes off the inferior rectus muscle (IR) and
inserts onto the lower boarder of the tarsus (T) and the conjunctival
fornix. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
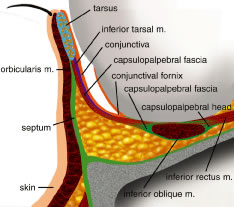 Fig. 19. Cross section of the lower eyelid. Fig. 19. Cross section of the lower eyelid.
|
Anterior to the inferior oblique muscle, the two portions of the capsulopalpebral
head join to form Lockwood's suspensory ligament. Lockwood's
ligament acts as a suspensory hammock for the globe.9 It is composed of intramuscular septa and check ligaments, thickened Tenon's
capsule, and fibers from the inferior rectus sheath and lower
eyelid retractors. Its attachments are to the medial orbital wall
posterior to the posterior lacrimal crest, to the medial retinaculum, and
at the lateral orbital tubercle through the lateral retinaculum. Anterior
projections from Lockwood's ligament send strands into the
conjunctival fornix, forming the suspensory ligament of the fornix. The
fibers of the CPF and the ITM fuse with the orbital septum 4 to 5 mm
below the inferior tarsus and insert as a single layer onto the inferior
border of the inferior tarsus.35,39 The lower eyelid opens passively by traction from the inferior rectus
muscle through the CPF.9 Excursion from full depression of the upper eyelid to full elevation is
about 15 to 20 mm, which is primarily the action of the LPS. Muller's
muscle is responsible for approximately 2 mm of upper eyelid elevation. The
muscles of the forehead and brow also play a role in assessing
the elevating power of the eyelid. The primary muscle of the forehead
is the frontalis muscle innervated by the seventh cranial nerve. It
is a muscle of facial expression, whose primary action is elevation
of the forehead and brow. Other muscles of the forehead play a role in
eyebrow function. The corrugator muscle draws the head of the eyebrows
to the nose. It is responsible for vertical furrows on the bridge of
the nose.10 Depression of the head of the eyebrow is a result of contraction of the
procerus muscle, which can result in horizontal furrows in the skin
of the glabellar region of the forehead overlying the bridge of the nose. In
examination of patients for ptosis, it is important to distinguish
recruitment of the forehead muscles to elevate the eyelids and the
eyebrows from the use of eyelid retractor muscles. The forehead should
be in a completely relaxed position toaccurately measure the severity
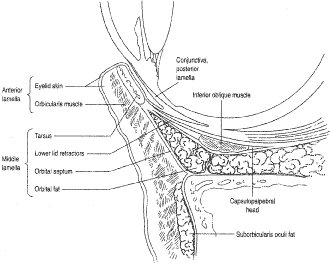
of blepharoptosis. (See Volume 5, Chapter 78.) TARSUS The tarsal plate is part of the posterior lamellae of the eyelid and provides
the structural framework of the eyelid. It is composed of condensed
fibrous and elastic tissue but contains no cartilage (Fig. 20). It extends along the entire length of the upper and lower eyelids measuring
approximately 25 mm horizontally and 1 mm in width. It extends
horizontally in a convex curve tapering medially and laterally. The superior
tarsal plate is approximately 9 to 10 mm in vertical height at
its highest point just medial to the pupil.14 The inferior tarsal plate of the lower eyelid measures 4 to 5 mm in central
vertical height and tapers medially and laterally in a convex curve.40 Both upper and lower tarsal plates are anchored to the orbital bones by
their connections to the medial and LCTs. On the anterior superior tarsal
surface are the attachments of the septum and retractor muscles. The
upper tarsus contains approximately 30 meibomian glands, and the
lower tarsus contains approximately 20. The oil-secreting glands are aligned
vertically, and their orifices are seen at the eyelid margin just
posterior to the gray line and anterior to the mucocutaneous junction. The
posterior surface of both tarsal plates is covered by conjunctiva. Only 4 to 5 mm
of tarsus is needed for upper eyelid stability, when
the tarsus is used in eyelid reconstruction. Aging changes in the tarsus
and surrounding eyelid structures, including atrophy of the tarsus, laxity
of its medial and lateral attachments, and decreased orbicularis
tone, contribute to the loss of horizontal structural stability
in the eyelid.41 In addition, weakness of elastin fibers in the tarsus has been found in
patients with floppy eyelid syndrome. These patients demonstrate significant
horizontal eyelid laxity.  Fig. 20. The upper and lower tarsal plates. Fig. 20. The upper and lower tarsal plates.
|
CONJUNCTIVA The conjunctiva is composed of nonkeratinizing stratified squamous epithelium
and forms the posterior layer of the eyelids. It is a transparent
mucous membrane lining the eye socket from the eyelid margin to the
corneal scleral limbus. The bulbar conjunctiva loosely attaches to the
globe, whereas the palpebral conjunctiva adheres tightly to the eyelids. The
conjunctiva contains mucous-secreting goblet cells and aqueous-producing
glands of Krause and Wolfring. The glands of Krause and Wolfring
are accessory lacrimal glands and are histologically identical
to the structure of the main lacrimal gland. These glands are mainly localized
in the subconjunctival tissue in the upper eyelid between the
superior tarsal border and the fornix. Few glands are found in the lower
eyelid at the inferior fornix. Mucin-secreting goblet cells are scattered
throughout the conjunctiva and are concentrated in the crypts
of Henle just above the tarsal border. Mucin is an essential component
of the basic secretion of the tear film. Medially, the conjunctiva forms
the semilunar fold, a vestige of the nictitating membrane of some
animals. A small, fleshy body of transitional tissue, called the caruncle, lies
at the medial commissure and contains multiple sebaceous glands
and hair follicles.9 LATERAL CANTHAL TENDON The LCT, often called the lateral canthal ligament, is a broad band of
dense fibrous connective tissue, which serves as the upper and lower crus
of the lateral border of the upper and lower tarsus3 (Fig. 21). The true anatomic origin of the LCT remains controversial. The LCT fuses
at the lateral border of the tarsal plates to join with the lateral
retinaculum, a sheath of connective tissue, which is a condensation
of several structures that insert onto the lateral orbital tubercle of
Whitnall. The lateral retinaculum consists of fibers from the LCT, the
lateral horn of the levator aponeurosis, the inferior suspensory ligament
of Lockwood, the STL (of Whitnall), the check ligament of the lateral
rectus muscle (before its insertion 1.5 mm posterior to the lateral
orbital rim onto the lateral orbital tubercle of Whitnall), and deep
fibers of the pretarsal orbicularis muscle. The lateral orbital tubercle
of Whitnall is located 2 to 4 mm posterior to the lateral orbital
rim at the level of the lateral commissure. In a space between the
anteriorly placed orbital septum and the lateral retinaculum is sometimes
found a small fat pad, called Eisler's pocket.36 The insertion of the LCT is approximately 3 mm superior to the MCT insertion. This
gives a slightly upward slope of the eyelids from medial
to lateral. The connection of the check ligament of the lateral rectus
muscle with LCT plays another important role in mobility of the lateral
canthal angle on far lateral gaze. Gioia and colleagues42 were the first to describe that on far lateral gaze, the lateral canthal
angle is displaced 2 mm laterally, which in part increases the peripheral
visual field. 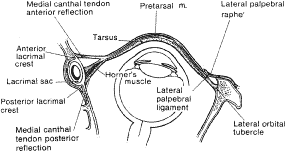 Fig. 21. Axial view of the eyelids and orbit as seen from above. The anterior reflection
of the medial canthal tendon and its position anterior to the
lacrimal sac are shown in the medial orbit. The normal direction of the
tarsoligamentous sling insertion is posterior. This posterior attachment
is at the level of the posterior lacrimal crest. The lateral palpebral
ligament or tendon inserts inside the edge of the lateral orbital
rim at the tubercle and not at the anterior edge of the rim. Fig. 21. Axial view of the eyelids and orbit as seen from above. The anterior reflection
of the medial canthal tendon and its position anterior to the
lacrimal sac are shown in the medial orbit. The normal direction of the
tarsoligamentous sling insertion is posterior. This posterior attachment
is at the level of the posterior lacrimal crest. The lateral palpebral
ligament or tendon inserts inside the edge of the lateral orbital
rim at the tubercle and not at the anterior edge of the rim.
|
The LCT functions to fixate the lateral canthal angle and upper and lower
tarsus to the lateral orbital tubercle. This enables the eyelids to
be properly opposed to the globe laterally. LCT dehiscence or laxity
is a common involutional change that appears as a rounding of the lateral
canthal angle or as lower eyelid laxity. Severing the inferior crus
of the LCT as performed in a lateral canthotomy or cantholysis provides
an access to the orbit in decompression or reconstruction. A lateral
canthoplasty is a commonly used procedure to reestablish lower eyelid
stability and support of the globe. The periosteum overlying the lateral
orbital tubercle is an excellent surgical anchor for lateral canthal
angle fixation in surgery. MEDIAL CANTHAL TENDON The MCT or ligament provides eyelid support and aids in the proper functioning
of the lacrimal pump.43 The MCT has two components: the anterior limb and a posterior limb (Fig. 22). The anterior limb is a broad fibrous structure attaching the eyelids
to the frontal process of the maxillary bone and to the anterior lacrimal
crest. It is the origin of the superficial head of the pretarsal
and preseptal orbicularis muscles. The posterior limb of the medial canthal
ligament inserts on the posterior lacrimal crest and the lacrimal
fossa. The posterior limb and deep heads of the pretarsal and preseptal
orbicularis muscles draw the medial portion of the eyelids posteriorly
for good apposition of the eyelids to the globe.16 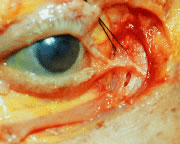 Fig. 22. The medial canthal tendon. Orbicularis oculi muscles are dissected away. The
lacrimal sac is exposed (arrow) through a small tear below the tendon. A suture is placed around the
fascia superior to the tendon, known as the vertical component of the
medial canthal tendon complex. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 22. The medial canthal tendon. Orbicularis oculi muscles are dissected away. The
lacrimal sac is exposed (arrow) through a small tear below the tendon. A suture is placed around the
fascia superior to the tendon, known as the vertical component of the
medial canthal tendon complex. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
LACRIMAL SYSTEM The lacrimal system has a dual function: a secretory component contributing
to the formation of tears and an excretory component that provides
a conduit though which tears drain from the eye into the nose9 (Fig. 23). Three types of glands comprise the basic secretors that produce the
tear film (Fig. 24). The first group consists of conjunctival tarsal and limbal mucin-secreting
goblet cells, which produce the inner mucoprotein layer of the
tear film. The second group consists of the main lacrimal gland and accessory
lacrimal exocrine glands of Kraus and Wolfring in the subconjunctival
tissues. These glands produce the middle aqueous layer of the
tear film. The third group is the oil-producing meibomian glands in the
tarsus and the palpebral glands of Zeiss and Moll. These produce the
superficial lipid layer, which is essential in slowing evaporation and
stabilizing the tear film.44 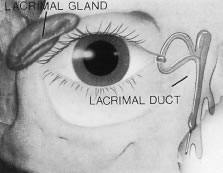 Fig. 23. The lacrimal apparatus divided into two components: the secretory and the
excretory systems. Fig. 23. The lacrimal apparatus divided into two components: the secretory and the
excretory systems.
|
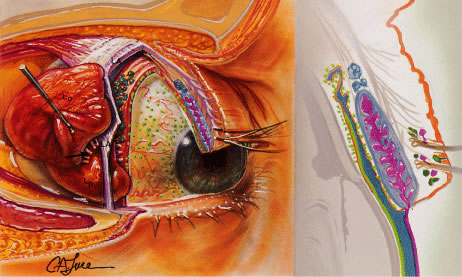 Fig. 24. The basic and reflex secretors of the lacrimal system. The first set of
basic secretors includes the conjunctival, tarsal, and limbal mucin-secreting
goblet cells, which produce a mucoprotein layer covering the
corneal epithelium (green). This is the inner layer of the precorneal tear film. The set of basic
secretors consists of the accessory lacrimal exocrine glands of Krause
and Wolfring in the subconjunctival tissue (blue). They produce an intermediate aqueous layer of the precorneal tear film. The
third group of basic secretors is the oil producing meibomian
glands and the palpebral glands of Zeis and Moll (pink), which produce the outermost layer of the tear film. The reflex stimulated
lacrimal gland is divided into two portions by the lateral horn
of the levator palpebrae superioris (LA). The orbital lobe of the gland (Lo) is
larger than the palpebral portion (Lp). The superior surface
of the gland is connected to the frontal bone by weak trabeculae. The
tear ducts (arrow) from the orbital portion traverse the palpebral portion, which empties
its contents into 6 to 12 tear ductules onto the superior lateral conjunctival
fornix. Removal of the palpebral portion will, thus, block
orbital lobe secretion. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 24. The basic and reflex secretors of the lacrimal system. The first set of
basic secretors includes the conjunctival, tarsal, and limbal mucin-secreting
goblet cells, which produce a mucoprotein layer covering the
corneal epithelium (green). This is the inner layer of the precorneal tear film. The set of basic
secretors consists of the accessory lacrimal exocrine glands of Krause
and Wolfring in the subconjunctival tissue (blue). They produce an intermediate aqueous layer of the precorneal tear film. The
third group of basic secretors is the oil producing meibomian
glands and the palpebral glands of Zeis and Moll (pink), which produce the outermost layer of the tear film. The reflex stimulated
lacrimal gland is divided into two portions by the lateral horn
of the levator palpebrae superioris (LA). The orbital lobe of the gland (Lo) is
larger than the palpebral portion (Lp). The superior surface
of the gland is connected to the frontal bone by weak trabeculae. The
tear ducts (arrow) from the orbital portion traverse the palpebral portion, which empties
its contents into 6 to 12 tear ductules onto the superior lateral conjunctival
fornix. Removal of the palpebral portion will, thus, block
orbital lobe secretion. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
The main lacrimal gland is an almond shaped structure located in the superior
temporal bony orbit. The main lacrimal gland secretion contributes
to the aqueous layer of the tear film. It contains the reflex secretors, and
the tears it produces are mainly triggered by peripheral sensory
or emotional stimuli. The lacrimal gland is surrounded by fibrous
tissue that is superiorly attached to the periosteum of the frontal
bone and inferiorly to the orbital portion of the zygomatic bone. The
lateral horn of the levator aponeurosis divides the lacrimal gland into
a larger superior orbital lobe and a smaller inferior palpebral lobe. The
orbital lobe comprises 70% of the gland measuring approximately 20 mm × 5 mm × 12 mm.3 The palpebral lobe represents approximately 30% of the gland and lies
in the subaponeurotic space. It extends anteriorly beyond the orbital
rim and is the visible portion through the conjunctiva (Fig. 25). The lacrimal gland has approximately 12 secretory ducts, 2 to 5 originate
in the orbital lobe, and 6 to 8 are from the palpebral lobe.45 The ductules from the orbital portion pass through the palpebral lobe
before exiting into the superotemporal portion of the conjunctival fornix. 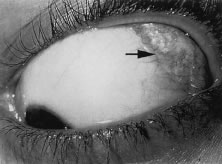 Fig. 25. The palpebral lobe (arrow) is seen through the conjunctiva when the eye is elevated (left eye). Fig. 25. The palpebral lobe (arrow) is seen through the conjunctiva when the eye is elevated (left eye).
|
The accessory lacrimal exocrine glands of Wolfring and Krause structurally
are similar to but much smaller than the main lacrimal gland. They
mainly are located in the superior conjunctival fornix and above the
tarsus, and fewer lie in the inferior conjunctival fornix. These are called
the basal secretors because they lack direct secretory motor fibers. Other
basal secretors include sebaceous glands (meibomian and Zeis) and
mucous glands (goblet cells). The accessory lacrimal glands provide
the tears for daily corneal hydration and secrete the aqueous layer
of the tear film. Drainage of tears begins with the lacrimal puncta located medially on upper
and lower eyelid margins. The punctal orifice is directed posteriorly
towards the lacrimal lake where it accepts the tears. Lacrimal papillae
are seen as a fibrous ring on the eyelid margin surface surrounding
the lacrimal puncta. The puncta is the opening to the lacrimal drainage
system and empties into the ampulla. The ampulla is oriented 1 to 2 mm
vertically and is surrounded by a portion of the pretarsal orbicularis
muscle and opens into the canaliculus. The canaliculus is a horizontal
structure, which is directed between the plica semilunaris and
the caruncle. The canaliculi are surrounded by thick pretarsal orbicularis
oculi muscle fibers. The upper canaliculus in the upper eyelid
is approximately 8 mm long, and the lower canaliculus is approximately 10 mm
long in the lower eyelid. The blinking action of the muscles of the eyelid helps direct tears medially
toward the puncta46 (Fig. 26). Capillary attraction allows tears to enter the punctum into the ampulla
and canaliculus. On eyelid closure, the ampulla collapses, while the
canaliculus shortens. The action of the lacrimal pump is then to draw
tears through the canaliculi into the lacrimal sac, which is approximately 10 mm
long. Gravity forces fluid through the elastic nasolacrimal
duct, measuring approximately 12 mm, into an opening or ostium at
the inferior meatus of the nose and into the nasopharynx. 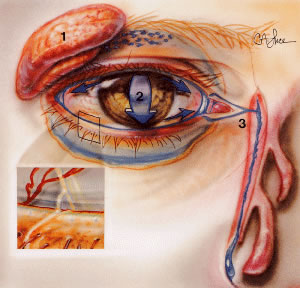 Fig. 26. Tears are produced by accessory and main lacrimal glands (1). The distribution
of these tears over the surface of the eye is achieved by movements
of the eyelids (2) that spread the marginal tear bead (inset) shown here in optical cross section by a slit lamp beam. The passage
of tears into the nose occurs via the lacrimal drainage system. (From Zide BM, Jelkes G. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 26. Tears are produced by accessory and main lacrimal glands (1). The distribution
of these tears over the surface of the eye is achieved by movements
of the eyelids (2) that spread the marginal tear bead (inset) shown here in optical cross section by a slit lamp beam. The passage
of tears into the nose occurs via the lacrimal drainage system. (From Zide BM, Jelkes G. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
Valve-like folds of epithelium line the nasolacrimal duct preventing the
retrograde flow of tears and air. The valve of Rosenmüller is located
at the junction of the common canaliculus as it enters the nasolacrimal
sac. This fold prevents reflux of tears back into the sac from
the canaliculi. An incompetent valve may allow reflux of purulent material
from the sac into the eye in nasal lacrimal duct obstruction. The
valve of Hasner is located on the distal end of the duct. This is often
imperforate at birth in neonates and is a major cause of epiphora
in infants. It usually undergoes perforation within 6 months after birth
spontaneously or after manual massage of the sac. VASCULAR SUPPLY A network of vessels derived from two major sources, the internal and the
external carotid arteries, richly vascularizes the eyelids (Fig. 27). The internal carotid artery supplies the deep or intraorbital vascular
system including the ophthalmic artery, whose terminal branches primarily
supply the upper eyelid. The external carotid artery supplies the
superficial arterial system namely facial and angular arteries, which
principally supply the lower eyelid. Collateralization between the
internal and external systems contributes to the rapid wound healing and
the low incidence of infection following eyelid surgery. As the vessels
approach the eyelids, branches of the ophthalmic artery from the
internal carotid artery and branches of the lacrimal arteries off of the
maxillary branch of the external carotid artery form the marginal and
peripheral vascular arcades of the eyelids. 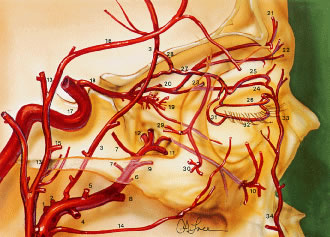 Fig. 27. Lateral view demonstrating the relationship of the internal and the external
carotid arterial systems to the orbit. Internal maxillary artery (O): (1) deep
auricular; (2) anterior tympanic; (3) middle meningeal; (4) inferior
alveolar; (5) masseteric; (6) pterygoid; (7) deep temporal; (8) buccal; (9) posterior superior alveolar; (10) infraorbital; (11) sphenopalatine; (12) artery of the pterygoid canal; (13) superficial
temporal artery; (14) transverse facial; (15) zygomatico-orbital; (16) frontal
branch; (17) internal carotid artery; (18) ophthalmic; (19) intraconal
branches of ophthalmic artery; (20) posterior ethmoidal branch
of ophthalmic; (21) supraorbital artery; (22) supratrochlear; (23) anterior
ethmoidal branch of ophthalmic; (24) infratrochlear; (25) peripheral
arcade (superior); (26) marginal arcade (superior); (27) lacrimal; (28) recurrent
meningeal; (29) zygomaticotemporal; (30) zygomaticofacial; (31) lateral
palpebral; (32) inferior marginal arcade; (33) angular; (34) facial; (35) central retinal; (36) lateral posterior ciliary; (37) muscular
branches to superior rectus, to levator palpebrae, and
to superior oblique; (38) medial posterior ciliary; (39) short ciliary; (40) long
ciliary; (41) anterior ciliary; (42) greater circle
of iris; (43) lesser circle of iris; (44) episcleral; (45) subconjunctival; (46) conjunctival; (47) marginal arcade; (48) vortex vein; (49) medial
palpebral; (50) dorsal nasal. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 27. Lateral view demonstrating the relationship of the internal and the external
carotid arterial systems to the orbit. Internal maxillary artery (O): (1) deep
auricular; (2) anterior tympanic; (3) middle meningeal; (4) inferior
alveolar; (5) masseteric; (6) pterygoid; (7) deep temporal; (8) buccal; (9) posterior superior alveolar; (10) infraorbital; (11) sphenopalatine; (12) artery of the pterygoid canal; (13) superficial
temporal artery; (14) transverse facial; (15) zygomatico-orbital; (16) frontal
branch; (17) internal carotid artery; (18) ophthalmic; (19) intraconal
branches of ophthalmic artery; (20) posterior ethmoidal branch
of ophthalmic; (21) supraorbital artery; (22) supratrochlear; (23) anterior
ethmoidal branch of ophthalmic; (24) infratrochlear; (25) peripheral
arcade (superior); (26) marginal arcade (superior); (27) lacrimal; (28) recurrent
meningeal; (29) zygomaticotemporal; (30) zygomaticofacial; (31) lateral
palpebral; (32) inferior marginal arcade; (33) angular; (34) facial; (35) central retinal; (36) lateral posterior ciliary; (37) muscular
branches to superior rectus, to levator palpebrae, and
to superior oblique; (38) medial posterior ciliary; (39) short ciliary; (40) long
ciliary; (41) anterior ciliary; (42) greater circle
of iris; (43) lesser circle of iris; (44) episcleral; (45) subconjunctival; (46) conjunctival; (47) marginal arcade; (48) vortex vein; (49) medial
palpebral; (50) dorsal nasal. (From Zide BM, Jelkes GW. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
At the orbital apex, the ophthalmic artery, a branch of the internal carotid
artery, enters the orbit through the optic canal lateral to the
optic nerve (Fig. 28). As the ophthalmic artery passes over the optic nerve and continues supermedially
within the orbit, four terminal branches pierce the orbital
septum to supply the upper eyelid. The four branches include the lacrimal
artery, the supraorbital artery, the supratrochlear (frontal) artery, and
the dorsal nasal artery.47,48 The lacrimal artery runs temporally along the upper border of the lateral
rectus muscle along with the lacrimal nerve and terminates as the
lateral palpebral artery. This is the blood supply to the lacrimal gland, the
conjunctiva and the lateral aspect of the upper eyelids. The supraorbital
artery branches off the ophthalmic artery as it courses over
the optic nerve and travels forward between the levator muscle and
the periorbita of the roof. It accompanies the supraorbital nerve through
the supraorbital foramen to supply the upper eyelid, scalp, forehead, levator
muscle, periorbita, and diploë of the frontal bone.3 The supratrochlear (frontal) artery accompanies the supratrochlear nerve
to supply the skin of the superior medial aspect of the orbit, the
forehead, and scalp. The ophthalmic artery pierces the orbital septum
dorsonasally to become the dorsal nasal artery. This supplies the skin
of the bridge of the nose and the lacrimal sac, terminating as the medial
palpebral artery. The medial palpebral artery and the lateral palpebral
artery anastomose to form the vascular arcades of the upper eyelids. The
marginal palpebral arcade lies on the anterior tarsal surface 2 to 3 mm
from the eyelid margin. The peripheral palpebral arcade courses
above and parallel to the superior border of the tarsus, between
the levator aponeurosis and Muller's muscle in the upper eyelid. Medially, the
arcades run a tortuous course throughout the medial fat
pad. The medial fat pad is often a site of bleeding during blepharoplasty.20 The deep peripheral palpebral arcade anastomoses with the anterior ciliary
arteries near the corneal scleral limbus and supplies the superior
conjunctival fornix.3 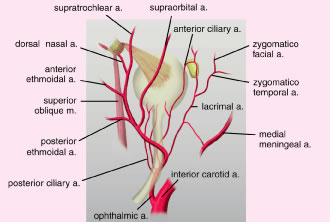 Fig. 28. Deep arterial circulation of the eyelid. Fig. 28. Deep arterial circulation of the eyelid.
|
In the lower eyelid, a marginal arcade arises from the medial and lateral
palpebral branches running horizontally approximately 3 mm inferior
to the lower eyelid margin and anterior to the lower eyelid tarsus.35 The lower eyelid does not have a peripheral arcade like the upper eyelid. Lateral
anastomoses from the zygomatico-orbital branch of the superficial
temporal artery also feed into these branches. SUPERFICIAL BLOOD SUPPLY From the external carotid artery, three branches of the facial vascular
system ultimately supply the eyelid: the facial artery, the superficial
temporal artery, and the infraorbital artery (Fig. 29). The facial artery crosses the mandible anterior to the masseter muscle, coursing
diagonally to the nasolabial fold. Within the orbicularis
muscle it travels as the angular artery 6 to 8 mm medial to the medial
canthus and 5 mm anterior to the lacrimal sac. The angular artery perforates
the orbital septum above the medial canthal ligament to anastomose
with the dorsal nasal branch of the ophthalmic artery. The superficial
temporal artery is a terminal branch of the external carotid artery. It
arises from within the parotid gland and ascends superiorly in
the preauricular region to cross the zygomatic process of the temporal
bone, approximately 1 cm anterior to the tragus. The superficial temporal
artery gives off three branches to supply the eyelids: the frontal
branch, zygomatico-orbital branch, and the transverse facial branch.20 The frontal branch courses upward across the temple to the frontalis muscle
and the orbicularis oculi muscle anastomosing with the lacrimal
and supraorbital arteries. The zygomatico-orbital branch travels along
the upper border of the zygoma and supplies the upper eyelid and anterior
orbit. The transverse facial branch courses below the zygoma to supply
the malar region and the lower lateral eyelid and anastomoses with
the lacrimal and infraorbital arteries. The infraorbital artery is
a branch of the internal maxillary artery and enters the orbit from the
pterygopalatine fossa, passing through the posterior end of the infraorbital
fissure through the infraorbital canal. It exits the orbit through
the infraorbital foramen to supply the lower eyelid. 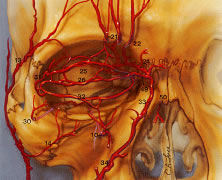 Fig. 29. Superficial blood supply to the eyelids. From the external carotid artery, three
branches of the facial vascular system ultimately supply the
eyelid: the facial artery, the superficial temporal artery, and the infraorbital
artery. The main points here are the following: First, most
branches of the ophthalmic artery system from the posterior third of
the orbit travel forward. Second, the ophthalmic artery is tethered to
the medial orbital wall by the ethmoid arteries. Third, the collateralization
that occurs among internal carotid artery (ICA) (17), the recurrent
meningeal (28), and the facial arterial tree33,34 accounts for the reversal of flow when the ICA is obstructed. Finally, the
central retinal artery enters the optic nerve in the posterior third. Internal
maxillary artery (O): (1) deep auricular; (2) anterior tympanic; (3) middle
meningeal; (4) inferior alveolar; (5) masseteric; (6) pterygoid; (7) deep
temporal; (8) buccal; (9) posterior superior alveolar; (10) infraorbital; (11) sphenopalatine; (12) artery of the pterygoid
canal; (13) superficial temporal artery; (14) transverse facial; (15) zygomatico-orbital; (16) frontal branch; (17) internal carotid
artery; (18) ophthalmic; (19) intraconal branches of' ophthalmic artery; (20) posterior
ethmoidal branch of ophthalmic; (21) supraorbital artery; (22) supratrochlear; (23) anterior ethmoidal branch of ophthalmic; (24) infratrochlear; (25) peripheral arcade (superior); (26) marginal
arcade (superior); (27) lacrimal; (28) recurrent meningeal; (29) zygomaticotemporal; (30) zygomaticofacial; (31) lateral palpebral; (32) inferior
marginal arcade; (33) angular; (34) facial; (35) central retinal; (36) lateral
posterior ciliary; (37) muscular branches to superior
rectus, to levator palpebrae, and to superior oblique; (38) medial
posterior ciliary; (39) short ciliary; (40) long ciliary; (41) anterior
ciliary; (42) greater circle of iris; (43) lesser circle of iris; (44) episcleral; (45) subconjunctival; (46) conjunctival; (47) marginal
arcade; (48) vortex vein; (49) medial palpebral; (50) dorsal nasal. (From Zide BM, Jelkes G. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 29. Superficial blood supply to the eyelids. From the external carotid artery, three
branches of the facial vascular system ultimately supply the
eyelid: the facial artery, the superficial temporal artery, and the infraorbital
artery. The main points here are the following: First, most
branches of the ophthalmic artery system from the posterior third of
the orbit travel forward. Second, the ophthalmic artery is tethered to
the medial orbital wall by the ethmoid arteries. Third, the collateralization
that occurs among internal carotid artery (ICA) (17), the recurrent
meningeal (28), and the facial arterial tree33,34 accounts for the reversal of flow when the ICA is obstructed. Finally, the
central retinal artery enters the optic nerve in the posterior third. Internal
maxillary artery (O): (1) deep auricular; (2) anterior tympanic; (3) middle
meningeal; (4) inferior alveolar; (5) masseteric; (6) pterygoid; (7) deep
temporal; (8) buccal; (9) posterior superior alveolar; (10) infraorbital; (11) sphenopalatine; (12) artery of the pterygoid
canal; (13) superficial temporal artery; (14) transverse facial; (15) zygomatico-orbital; (16) frontal branch; (17) internal carotid
artery; (18) ophthalmic; (19) intraconal branches of' ophthalmic artery; (20) posterior
ethmoidal branch of ophthalmic; (21) supraorbital artery; (22) supratrochlear; (23) anterior ethmoidal branch of ophthalmic; (24) infratrochlear; (25) peripheral arcade (superior); (26) marginal
arcade (superior); (27) lacrimal; (28) recurrent meningeal; (29) zygomaticotemporal; (30) zygomaticofacial; (31) lateral palpebral; (32) inferior
marginal arcade; (33) angular; (34) facial; (35) central retinal; (36) lateral
posterior ciliary; (37) muscular branches to superior
rectus, to levator palpebrae, and to superior oblique; (38) medial
posterior ciliary; (39) short ciliary; (40) long ciliary; (41) anterior
ciliary; (42) greater circle of iris; (43) lesser circle of iris; (44) episcleral; (45) subconjunctival; (46) conjunctival; (47) marginal
arcade; (48) vortex vein; (49) medial palpebral; (50) dorsal nasal. (From Zide BM, Jelkes G. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
VENOUS SYSTEM The venous drainage system of the eyelids is through the tributaries of
the ophthalmic vein and superficially through the angular and superficial
temporal veins12 (Fig. 30). The junction of the superficial frontal vein and the supraorbital vein
from the orbit forms the angular vein. The angular vein has a dual
drainage: posteriorly into the deep venous system by the superior ophthalmic
vein and superficially and inferiorly into the anterior facial
vein. The angular vein then empties into the common facial vein, which
empties into the internal jugular vein. Superiorly and laterally, venous
blood from the forehead, eyebrow, and eyelid drain from the supraorbital
vein into the superficial temporal vein to drain into the external
jugular vein.24 Anteriorly, the angular vein anastomoses with the orbital venous system. 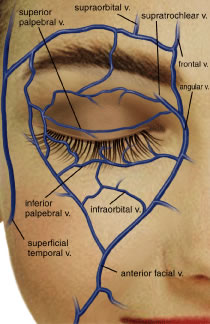 Fig. 30. Superficial venous system. Fig. 30. Superficial venous system.
|
The deep venous system draining the forehead, eyebrow, and upper eyelid
is via the supraorbital vein coursing into the frontal vein and into
the superior ophthalmic vein. The union of the angular and supraorbital
veins forms the superior ophthalmic vein at the supranasal aspect of
the orbit. The superior ophthalmic vein is formed at the supranasal orbit
by union of the supraorbital and angular veins. It is in close proximity
to the ophthalmic artery as it travels posterolaterally in the
orbit. The superior ophthalmic vein penetrates the muscle cone and receives
drainage from the superior vortex veins of the globe. The superior
ophthalmic vein also receives drainage from the ethmoidal, lacrimal, central
retinal, and ciliary veins. It is joined at the orbital apex
by the inferior ophthalmic vein. It leaves the orbit through the superior
orbital fissure and enters the cavernous sinus. The inferior ophthalmic
vein is part of a venous plexus at the anterior orbital floor
that is a tributary for the lower eyelid, lacrimal sac, inferior rectus
muscle, inferior oblique, and two inferior vortex veins. At the orbital
apex, one branch of the inferior orbital vein feeds into the superior
ophthalmic vein and enters the cavernous sinus and another branch
drains through the inferior orbital fissure to the pterygoid plexus. LYMPHATIC SYSTEM The lymphatic system of the eyelids is divided into a superficial and a
deep system. The superficial system drains skin and orbicularis, whereas
the deep system drains the tarsi and the conjunctiva.49 The upper eyelid, lateral ½ of the lower eyelid, and the lateral
canthus empty into the preauricular and deep parotid nodes. The skin
and orbicularis oculi muscles drain into the deep cervical nodes near
the internal jugular vein. The medial portion of the upper and lower
eyelids, the medial canthus, and the conjunctiva drain into the submandibular
nodes. Lymphadenopathy may correlate to inflammation or infection
of the affected anatomic eyelid structure. NERVES Three of the 12 cranial nerves essentially supply the eyelids: the oculomotor
nerve (CN-3), the trigeminal (CN-5), and the facial nerve (CN-7). The
eyelids also receive sympathetic innervation. CN-3: The oculomotor nerve provides motor innervation to several extraocular
muscles and the upper eyelids. The oculomotor nerve originates from
the midbrain and passes into the interpeduncular fossa to enter the
middle cranial fossa piercing the dura and enters the lateral wall of
the cavernous sinus. As it travels along the lateral wall of the cavernous
sinus, it divides into the superior and inferior divisions before
entering the orbit through the superior orbital fissure into the annulus
of Zinn.3,7 The superior division courses 1 mm anterior to the annulus of Zinn and
innervates the superior rectus muscle. The nerve courses medially where
it innervates the LPS. The inferior division courses beneath the optic
nerve branching to innervate the medial rectus, inferior rectus, and
inferior oblique muscles. Parasympathetic fibers run with the oculomotor
nerve and synapse in the ciliary ganglion. CN-5: The trigeminal nerve divides into the ophthalmic, maxillary, and
mandibular branches and is the major sensory nerve of the eyelids and
face (Fig. 31). It arises from the pons from the sensory, motor, and mesencephalic roots. These
roots meet at the gasserian ganglion at the petrous bone. The
first two branches continue in a path to provide sensory supply to
the eyelids, coursing through the middle cranial fossa and piercing the
dura to enter the lateral wall of the cavernous sinus. The first ophthalmic
division enters the orbit through the supraorbital foramen where
it subdivides into the lacrimal, frontal, and nasociliary nerves. 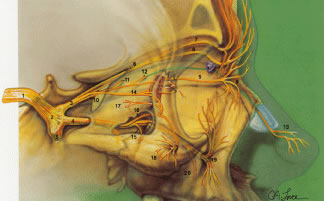 Fig. 31. Sensory innervation to the orbit. Sensory nerves. (1) fifth cranial nerve; (2) trigeminal
ganglion; (3) ophthalmic division of trigeminal nerve
V1; (4) maxillary division of trigeminal nerve V2; (5) mandibular division of trigeminal nerve V3; (6) frontal nerve; (7) supraorbital nerve; (8) supratrochlear nerve (trochlea
noted by purple); (9) infratrochlear nerve; (10) nasociliary
nerve; (11) posterior ethmoidal nerve; (12) anterior ethmoidal nerve; (13) external
or dorsal nasal nerve; (14) lacrimal nerve; (15) posterior
superior alveolar nerve; (16) zygomatic nerve; (17) zygomatico-temporal
nerve; (18) zygomaticofacial nerve; (19) infraorbital nerve; and (20) anterior
superior alveolar nerve. (21) ciliary ganglion; (22) nerve
to inferior oblique; (23) sensory root of ciliary ganglion; (24) long
ciliary nerves; (25) short ciliary nerves. (From Zide BM, Jelkes G. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 31. Sensory innervation to the orbit. Sensory nerves. (1) fifth cranial nerve; (2) trigeminal
ganglion; (3) ophthalmic division of trigeminal nerve
V1; (4) maxillary division of trigeminal nerve V2; (5) mandibular division of trigeminal nerve V3; (6) frontal nerve; (7) supraorbital nerve; (8) supratrochlear nerve (trochlea
noted by purple); (9) infratrochlear nerve; (10) nasociliary
nerve; (11) posterior ethmoidal nerve; (12) anterior ethmoidal nerve; (13) external
or dorsal nasal nerve; (14) lacrimal nerve; (15) posterior
superior alveolar nerve; (16) zygomatic nerve; (17) zygomatico-temporal
nerve; (18) zygomaticofacial nerve; (19) infraorbital nerve; and (20) anterior
superior alveolar nerve. (21) ciliary ganglion; (22) nerve
to inferior oblique; (23) sensory root of ciliary ganglion; (24) long
ciliary nerves; (25) short ciliary nerves. (From Zide BM, Jelkes G. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
The lacrimal nerve enters the orbit above the annulus of Zinn and gives
sensory innervation to the lacrimal gland, the lateral aspect of the
eyelid, and the forehead. A superior branch sends sensory fibers to the
lacrimal gland and skin as the lateral palpebral nerve. An inferior
branch of the lacrimal nerve innervates the lacrimal gland and anastomoses
with the fibers from the zygomaticotemporal branch of the maxillary
division of V2, which carries parasympathetic secretory fibers to the lacrimal gland
from CN-7. The frontal nerve enters the orbit outside the annulus of Zinn
and courses anteriorly to branch into the supraorbital and supratrochlear
nerves (Fig. 32). The supraorbital nerve leaves the orbit through the supraorbital foramen
or supraorbital notch and innervates the skin of the upper eyelid, forehead, and
scalp. The supratrochlear nerve passes above the trochlea
and pierces the septum at the superonasal aspect of the orbit giving
sensory innervation to the medial commissure, the skin of the root
of the nose, the middle forehead, and the lacrimal drainage structures. It
also sends a branch to an infratrochlear twig of the nasociliary
nerve.50 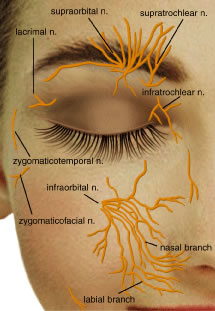 Fig. 32. The first division of the fifth cranial nerve (V1) provides sensory innervation to the upper eyelid, whereas the second
division V2 provides sensory innervation to the lower eyelid. Fig. 32. The first division of the fifth cranial nerve (V1) provides sensory innervation to the upper eyelid, whereas the second
division V2 provides sensory innervation to the lower eyelid.
|
The nasociliary nerve enters the orbit laterally within the annulus of
Zinn, courses over the optic nerve along the medial orbit and gives off
several branches. Branches of the nasociliary nerve include: (1) long
sensory root of the ciliary ganglion; (2) long ciliary nerves to supply
the iris, ciliary body, and cornea; (3) infratrochlear nerve supplying
the medial canthus, conjunctiva, lacrimal sac, canaliculus, and caruncle; and (4) posterior
ethmoidal nerve supplying the ethmoidal air
cells and sphenoid sinus.49 Patients with herpes zoster ophthalmicus who have skin lesions along a
V1 dermatome and a lesion on the tip of the nose with associated kerato-uveitis (Hutchison's
sign) typically have nasociliary nerve (branch
of V1) involvement (Fig. 33). 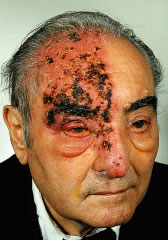 Fig. 33. Herpes zoster ophthalmicus, demonstrating the distribution of the ophthalmic
division of the fifth cranial nerve (V1). Fig. 33. Herpes zoster ophthalmicus, demonstrating the distribution of the ophthalmic
division of the fifth cranial nerve (V1).
|
The maxillary nerve branch or V2 division proceeds from the gasserian ganglion to enter the inferior cavernous
sinus. It leaves the middle cranial fossa from the foramen rotundum
and crosses the pterygopalatine fossa. It then enters the orbit
through the inferior orbital fissure where it becomes the infraorbital
nerve branch. The infraorbital nerve passes through the infraorbital
canal and exits through the infraorbital foramen 4 to 8 mm below the infraorbital
rim (Fig. 34). It supplies the skin and conjunctiva of the lower eyelid, the medial
and lateral canthi, the ala of the nose, and the superior lip.3,7 This nerve is often involved in orbital floor fractures, resulting in
paraesthesia of the cheek, lip, teeth, and gums. The maxillary nerve branches
into the zygomatic nerve before it enters into the infraorbital
canal. The zygomatic nerve divides into the zygomaticotemporal nerve, which
communicates with the lacrimal nerve and the zygomaticofacial
nerve, giving sensation to the cheek. The third branch of CN-5 or mandibular
division is the motor supply for the muscles of mastication, with
additional sensory fibers giving sensation to the jaw and lower lip.  Fig. 34. The infraorbital nerve as it exits the infraorbital foramen, approximately 7 to 10 mm
below the infraorbital margin. (From Zide BM, Jelkes G. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.) Fig. 34. The infraorbital nerve as it exits the infraorbital foramen, approximately 7 to 10 mm
below the infraorbital margin. (From Zide BM, Jelkes G. Surgical Anatomy of the Orbit. New York: Raven
Press, 1985.)
|
CN-7: The facial nerve supplies most of the muscles of facial expression
and motor function of the eyelids. It has both motor and parasympathetic
secretomotor elements. The facial nerve arises from the brain stem
ventrally at the pons-medullary junction near the cerebellum. The nerve
enters the temporal bone at the internal auditory meatus along with
the sensory intermedius nerve and the acoustic nerve (CN-8). As the
fibers reach the geniculate ganglion, they branch into motor fibers or
parasympathetic secretomotor fibers. The motor fibers exit the facial
canal of the temporal bone through the stylomastoid foramen and pass
anteriorly through the parotid gland. The nerve divides into five branches
within the gland: temporal, zygomatic, buccal, mandibular, and cervical. These
branches serve as the muscles of facial expression. The
temporal, zygomatic, and buccal divisions supply the orbicularis oculi, the
procerus, and corrugator muscles.9 PARASYMPATHETIC NERVE SUPPLY The facial nerve also contains a parasympathetic secretomotor component. The
parasympathetic secretomotor fibers pass through the geniculate
ganglion as preganglionic parasympathetic sensory fibers and course into
the middle cranial fossa as the great superficial petrosal nerve. These
fibers join with the great deep petrosal sympathetic nerve to form
the vidian nerve. Fibers then synapse at the pterygopalatine or sphenopalatine
ganglion. Postganglionic parasympathetic fibers travel along
with the zygomaticotemporal and lacrimal branches of CN-5 to supply
parasympathetic secretory function to the lacrimal gland. Parasympathetic
fibers that control pupillary constriction and accommodation of the
eye run with the oculomotor nerve in the middle cranial fossa, cavernous
sinus, and superior orbital fissure to enter the orbit. They branch
off the nerve to the inferior oblique muscle and synapse in the ciliary
ganglion to supply the constrictor pupillae and ciliary muscles of
the eye. SYMPATHETIC NERVE SUPPLY Sympathetic nerves to the orbit provide vasoconstriction, smooth muscle
function, pupillary dilation, hidrosis, eyelid retraction, and pilomotor
and sweat gland function of the skin and face. The sympathetic pathway
begins in the hypothalamus and descends the brain stem as uncrossed
fibers through the pons and mesencephalon. The fibers come together
in the lateral medulla oblongata traveling as first-order neurons to
terminate in the intermediolateral cell column at the level of the eighth
cervical to second thoracic vertebrae of the spinal cord. The fibers
exit the spinal cord and enter the cervical sympathetic chain at the
superior portion of the stellate ganglion as second-order neurons. They
ascend the chain and synapse in the superior cervical ganglion. Postganglionic, or
third-order neuron fibers travel with the internal carotid
artery to form a sympathetic nerve plexus surrounding the intracavernous
carotid artery, then branch at the orbital apex. The branches
supplying the pupil are thought to enter the orbit through the superior
orbital fissure traveling along the ophthalmic branch of CN-5.10 Nerve fibers course along the nasociliary nerve, pass through the ciliary
body, pass through the long ciliary nerves, and terminate in the dilator
muscles of the iris. Sympathetic innervation to the superior and
inferior tarsal muscles of the eyelids remains controversial.51,52 The branches of the ophthalmic artery and nerves in the orbit are thought
to carry sympathetic fibers.7 In the eyelids, it is thought that sympathetic fibers travel along the
peripheral arcades to supply the superior and inferior tarsal muscles. Interruption
of the sympathetic nerve fibers anywhere along the sympathetic
chain may result in Horner's syndrome with ptosis, anhidrosis, miosis, vascular
dilation, and heterochromia. (See Chapter 78.) A thorough understanding of embryology, anatomy, and function of the structures
of the eyelid is integral in the management of eyelid disorders. The
eyelids can be subject to a variety of conditions including congenital, infectious, inflammatory, neoplastic, traumatic, degenerative, and
involutional. Knowledge of anatomy will enable the surgeon to make
informed decisions in surgical planning and to optimize the outcome
in eyelid reconstruction, both functionally and aesthetically. |