The choroid is a thin (0.2-mm), spongy, pigmented, vascular lamina. It
is located between the sclera and the retina, extending from the ora serrata
to the optic nerve (Fig. 15). Sensitive ultrasonographic techniques may detect increased thickness
of the choroid as manifested by inflammation and neoplasms, focal or
diffuse. Chromatophores are scattered within the choroid. The amount of
pigmentation of the choroid determines the color of the fundus. Because
the retina is transparent except for the blood vessels and the retinal
pigmented epithelium (RPE), the variation in pigment accumulation
within the choroid and retina determines the clinical picture of the
ocular fundus. A heavily pigmented (negroid) fundus has the characteristic
dark gray-green reflex, whereas a “blond” fundus has
relatively little pigment and the pink choroidal vessel pattern is easily
visible. Fluorescein and indocyanine green dye angiography readily
provides clinicians with a means of evaluating the vascular integrity
of the choroid and the changes that occur with disease. Also, the density
of the choroidal pigmentation may affect the degree of retinal burn
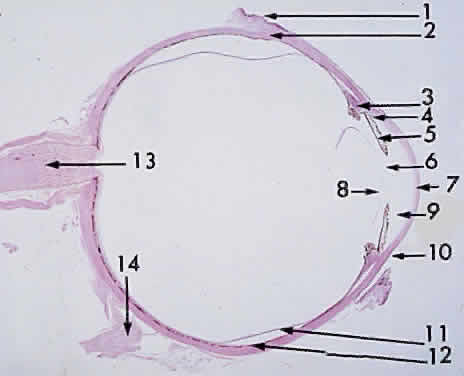
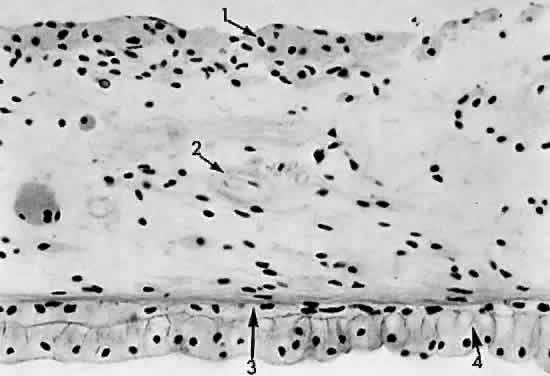
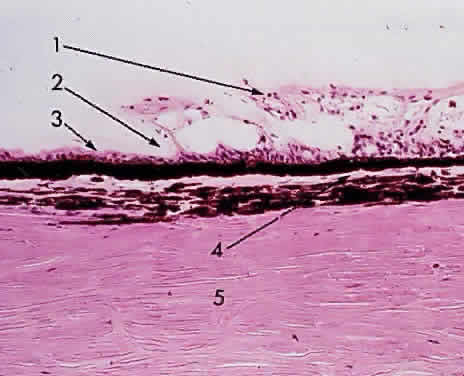
during photocoagulation of neovascular membranes.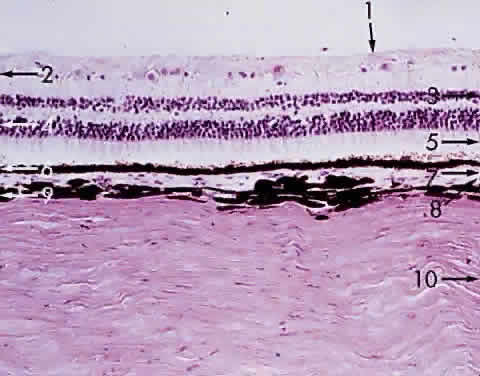 Fig. 15. Choroid wedged between the retina and sclera: 1, internal limiting membrane
of retina; 2, ganglion cell layer of retina; 3, bipolar cell layer
of retina; 4, nuclei of rods and cones; 5, rod and cone layer of retina; 6, pigment
epithelium of retina; 7, Bruch's membrane; 8, choriocapillaris; 9, large
blood vessel of choroid; 10, sclera (× 225, KEI 8982B). Fig. 15. Choroid wedged between the retina and sclera: 1, internal limiting membrane
of retina; 2, ganglion cell layer of retina; 3, bipolar cell layer
of retina; 4, nuclei of rods and cones; 5, rod and cone layer of retina; 6, pigment
epithelium of retina; 7, Bruch's membrane; 8, choriocapillaris; 9, large
blood vessel of choroid; 10, sclera (× 225, KEI 8982B).
|
During angiography, fluorescein dye is carried to the choriocapillaris
from the larger vessels. Because the choriocapillaris leaks fluorescein
easily, the dye diffuses rapidly throughout the choroid but cannot pass
anteriorly into the retina because of the tight junctions between
the RPE cells. Thus, clinically during an early phase of angiography, a
shaded diffuse glow of fluorescein appears in the choroid as a result
of the opaque RPE. If defects occur in the RPE (e.g., drusen, serous
detachment), fluorescein can leak into the retina from the choroid. With
the increasing incidence of age-related macular retinal degeneration, more
attention is being directed to the subfoveal choroidal anatomy
and changes in disease. Photodynamic therapy with verteporfin IV dye
and laser treatment may help to prevent visual loss secondary to subfoveal
choroidal neovascularization. New research into medication to inhibit
vascular endothelial growth factor offers potential treatment for
the future. The retinal vessels, in contrast to the choroidal vessels, do
not leak fluorescein normally. At the optic disc, the choroidal
vessels also leak fluorescein, so the perimeter of the disc “lights
up” in the late phase of angiography even though the disc vessels
proper do not normally leak fluorescein. The primary function of the choroid is to supply nutrition to the rod and
cone layer of the retina. Because of the erectile potential of the
vascular channels, the choroid may also have a role in regulating intraocular
pressure and in acting as a heat diffuser to protect the photoreceptors
from the heating effect of absorbed light, particularly at the
macula. At the optic disc, the choroidal circulation joins with the
short posterior ciliary vessels and the branches from the central retinal
artery to supply nutrition to the optic nerve. As a feature of glaucoma, choroidal
peripapillary atrophy is a frequent hallmark. Embryologically, the
choroid is derived from the mesoderm that surrounds the
posterior portion of the primitive cup. The choroidal bond of Bruch's
membrane to the pigmented epithelium of the retina is strong, so
in “true” retinal detachments the line of cleavage is between
the rod and cone layer and the pigmented epithelium. MACROSCOPIC FEATURES Grossly, the choroid represents a vascular bed formed by the junction of
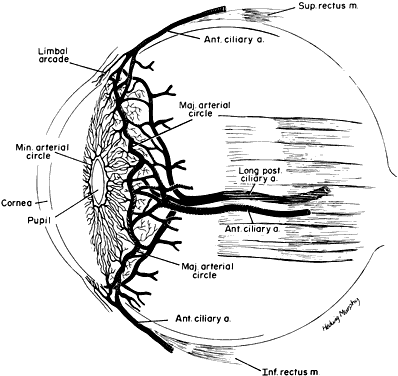
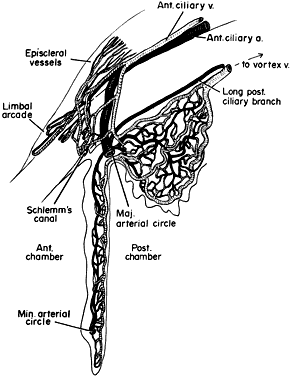
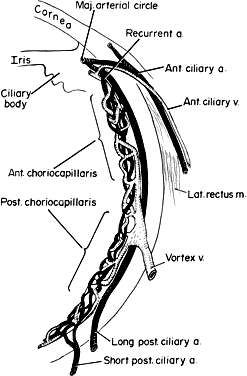
the anterior and posterior ciliary arteries (Fig. 16). The ciliary arteries (2 long posterior arteries, 20 short posterior
arteries, and posterior communications of the anterior ciliary arteries) pierce
the sclera to reach the choroid. They divide into vessels of
gradually smaller caliber and end as choriocapillaris, nestled adjacent
to Bruch's membrane. Thus, the choroid is an expandable vascular
plexus that supplies nutrition for the rod and cone layer of the retina
and up to 130 mm of the outer retina, particularly the macula. Thus, choroidal
vascular changes due to inflammation or degeneration lead
eventually to retinal pathology (e.g., subretinal neovascularization). The
venous drainage from the choroid is through the four vortex veins, one
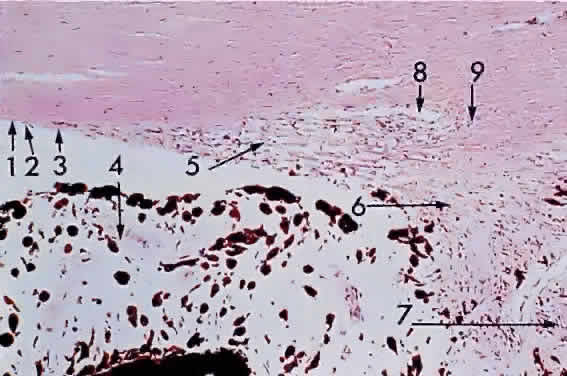
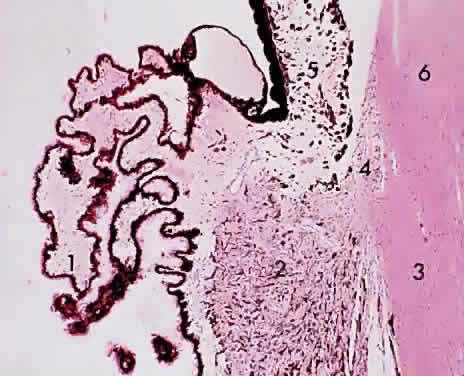
in each quadrant of the posterior sclera (Fig. 17). 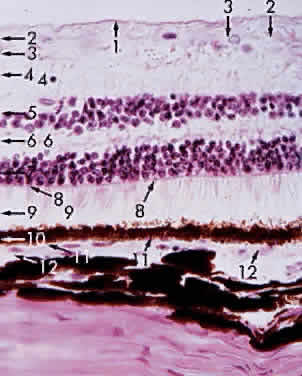 Fig. 16. Choroid at the macula: 1, internal limiting membrane of retina; 2, nerve
fiber layer of retina; 3, ganglion cell layer of retina (multicell thickness); 4, inner
plexiform layer; 5, bipolar cell layer; 6, outer plexiform
layer; 7, nuclei of rods and cones; 8, outer limiting membrane; 9, cone (and
rod) layer; 10, pigment epithelium of retina; 11, Bruch's
membrane; 12, choriocapillaris (× 520, KEI 8982B). Fig. 16. Choroid at the macula: 1, internal limiting membrane of retina; 2, nerve
fiber layer of retina; 3, ganglion cell layer of retina (multicell thickness); 4, inner
plexiform layer; 5, bipolar cell layer; 6, outer plexiform
layer; 7, nuclei of rods and cones; 8, outer limiting membrane; 9, cone (and
rod) layer; 10, pigment epithelium of retina; 11, Bruch's
membrane; 12, choriocapillaris (× 520, KEI 8982B).
|
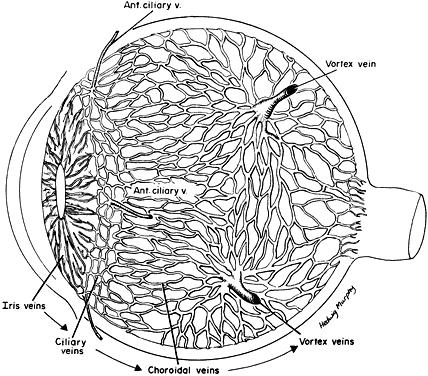 Fig. 17. Venous drainage of the choroid. Fig. 17. Venous drainage of the choroid.
|
In choroidal detachment, the vortex veins still adhere to the sclera, inducing
loculations or grapelike mounds of fluid compartments in the edematous
choroid. MICROSCOPIC FEATURES The choroid may be classified in layers, from the external to the internal
surface. The suprachoroid is the space between the inner pigmented
sclera (lamina fusca) and the large vessels of the choroid. The large-vessel
layer (Haller's layer) is the outermost layer of the choroid. It
is characterized by wide-caliber veins and arteries. Many melanocytes
and ciliary nerve fibers are scattered throughout the vessel layers. The
melanocytes and the Schwann cells encasing the nerve fibers, along
with an occasional cluster of nevus cells, may be the precursors
of the dreaded malignant melanoma of the choroid. The medium-vessel
layer (Sattler's layer) is composed of medium-sized blood vessels
and is located in the center of the choroid. The choriocapillaris is
a layer of very large fenestrated capillaries (40 to 60 μm in diameter) lying
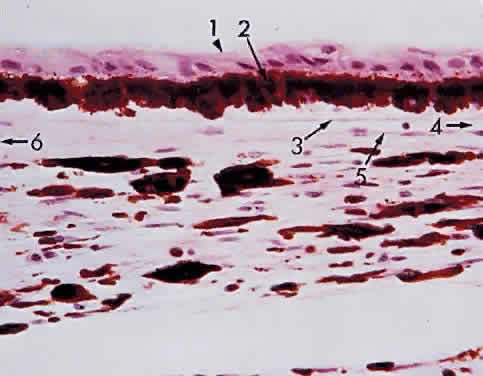
in a single plane external to Bruch's membrane (Fig. 18; no fenestration of the choriocapillaris is visible at this level of magnification). The
capillary lumina are large enough to pass several red
blood cells simultaneously (Fig. 19). Unlike the larger vessels in the choroid, the fenestrated choriocapillaris
lacks an internal elastic lamina and therefore leaks fluorescein
during clinical angiography. Pericytes are found on the outer wall of
the capillaries. Postmortem injection studies suggest that the choriocapillaris
is continuous, but the choriocapillaris functions clinically
like an end arteriole network. At the posterior pole, the choriocapillaris
has a lobular pattern with a central precapillary arteriole and
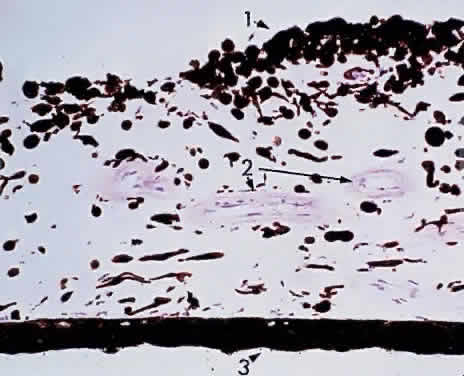
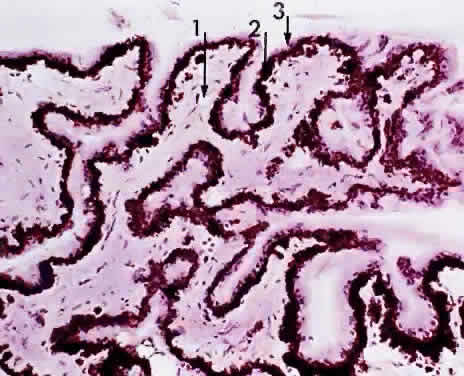
a peripheral postcapillary venule. 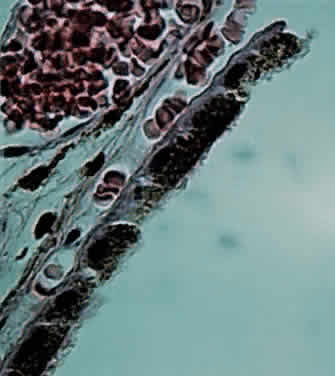 Fig. 18. Choriocapillaris at macula under Bruch's membrane and the retinal
pigment epithelium (retina is detached) (× 800, KEI 71125). Fig. 18. Choriocapillaris at macula under Bruch's membrane and the retinal
pigment epithelium (retina is detached) (× 800, KEI 71125).
|
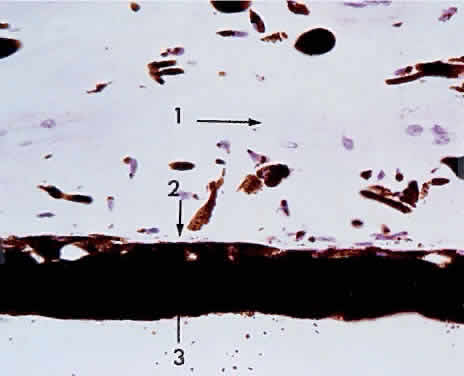
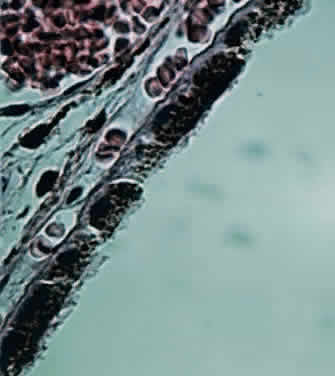 Fig. 19. Choriocapillaris at macula shows red blood cells in rouleaux pattern (retina
is detached) (× 800, KEI 71125). Fig. 19. Choriocapillaris at macula shows red blood cells in rouleaux pattern (retina
is detached) (× 800, KEI 71125).
|
Scanning electron microscopy reveals three patterns of vascular structures
in the choriocapillaris. At the posterior pole, the capillaries assume
a lobular pattern. This architecture of the macular areas choriocapillaris
makes it ideal for photodynamic therapy for “wet” macular
retinal degeneration. At the equator, the pattern is spindle-shaped, and
at the periphery, the capillaries have a ladder pattern. This
structure explains the various degrees of flushing of fluorescein
during angiography. In the foveal zone, the sole vascular supply to the retina is the choriocapillaris. In
central retinal artery occlusion, a major portion of the
retinal circulation is blocked, and ischemia results. The cherry-red
spot represents the intact choriocapillaris providing continued nutrition
to the overlying area of macular photoreceptors. If a cilioretinal
artery is present, this tiny vessel may be adequate to save the eye
from blindness after a central retinal artery occlusion. The choriocapillaris
circulation also acts as a heat diffuser to protect the macula
from the heat generated by light rays striking the retina. This protection
may be diminished by aging or disease, resulting in a reduction
of central vision by heat phototoxicity, and may be a factor in age-related
maculopathy. Bruch's membrane (lamina vitrea) is a composite multilaminar hyaline
PAS-positive layer featuring primarily an outer elastic lamina (derived
from the mesodermal choroid) and an inner cuticular layer (basement
membrane of the RPE neuroectoderm). it is usually difficult to demonstrate
the multilaminar structure of Bruch's membrane, but hyaline
nodular thickenings of the cuticular or basement membrane layer form
the various drusen bodies (Figs. 20 and 21) seen frequently with advancing age on funduscopic examination. At the
optic disc, Bruch's membrane forms the outer boundary of the disc. In
high myopia, the scleral crescent denotes the stretched, distorted
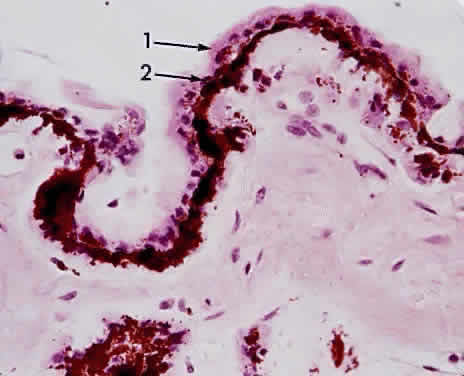
end of Bruch's membrane. 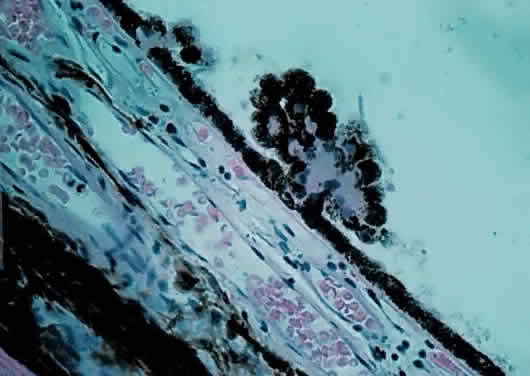 Fig. 20. Choriocapillaris near macula with small drusen on Bruch's membrane
elevating retinal pigment epithelium (retina is detached) (× 800, KEI 71125). Fig. 20. Choriocapillaris near macula with small drusen on Bruch's membrane
elevating retinal pigment epithelium (retina is detached) (× 800, KEI 71125).
|
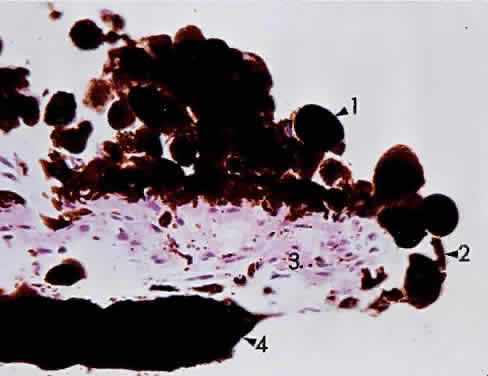
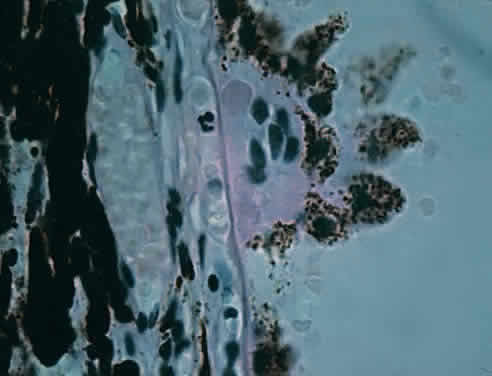 Fig. 21. Choriocapillaris with large drusen elevating retinal pigment epithelium
and displacing tight retinal pigment epithelium junctions (retina is
detached) (× 800, KEI 71125). Fig. 21. Choriocapillaris with large drusen elevating retinal pigment epithelium
and displacing tight retinal pigment epithelium junctions (retina is
detached) (× 800, KEI 71125).
|
ULTRASTRUCTURE OF BRUCH'S MEMBRANE Electron microscopy shows that Bruch's membrane is formed of five
elements: - Basal lamina of the choriocapillaris
- Outer collagenous layer
- Porous band of elastic fibers
- Collagenous inner layer
- Basal lamina of the RPE
Bruch's membrane thus is permeable to fluorescein. Diseases that affect
the choroid and RPE may therefore lead to subretinal neovascularization. Fluorescein
may record the defect during angiography, thereby
facilitating the site for photocoagulation to abort or minimize the degenerative
process. |