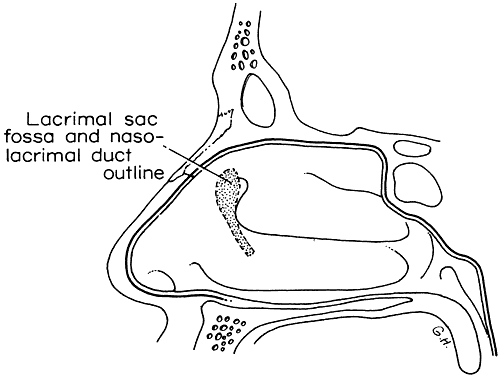
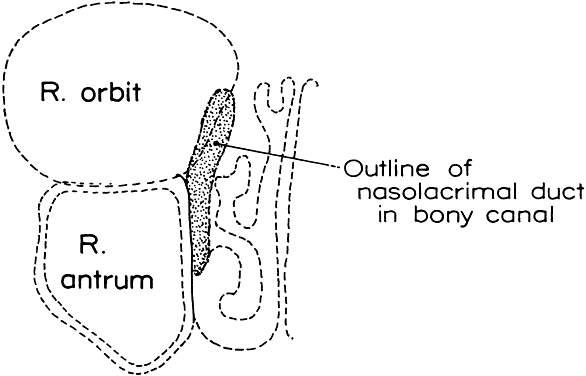
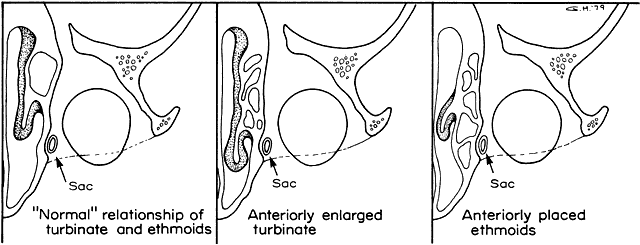
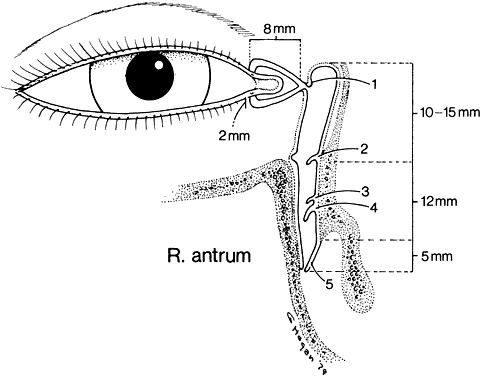
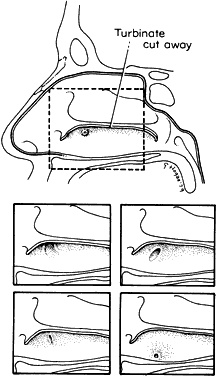
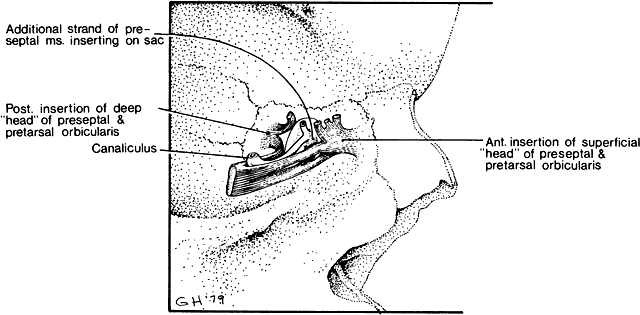
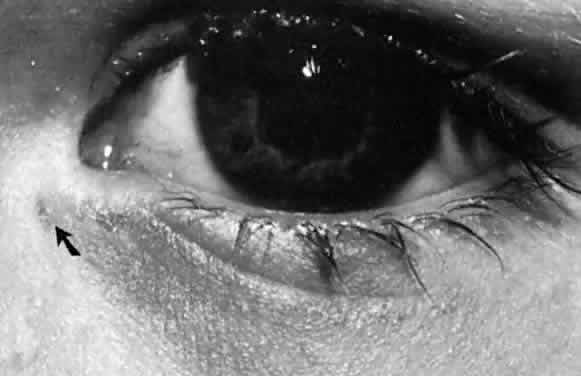
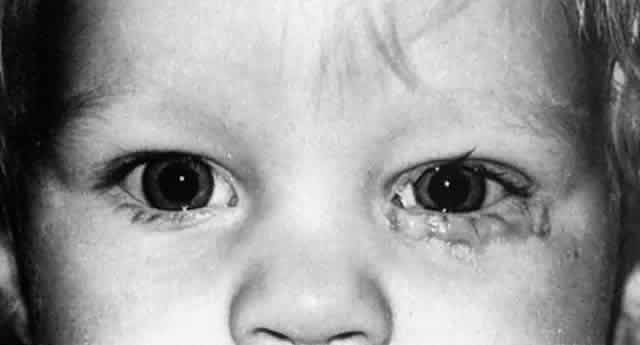
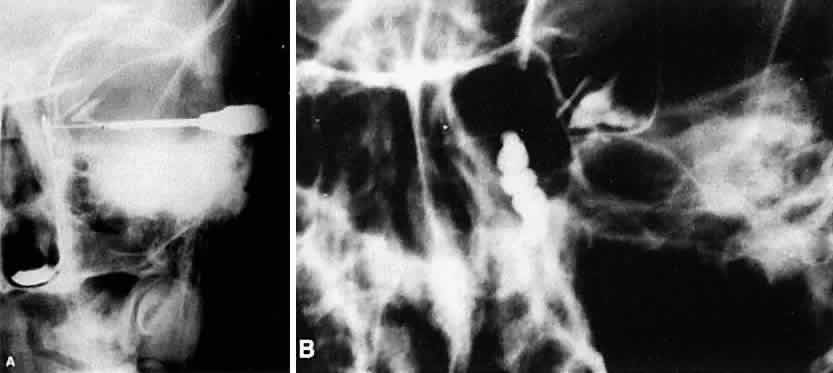
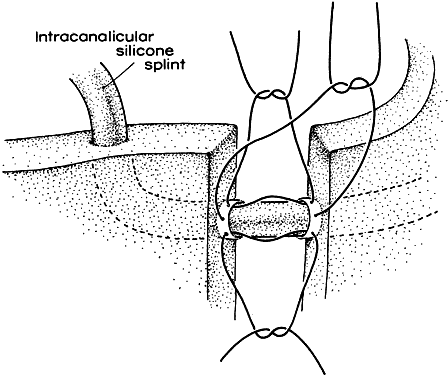
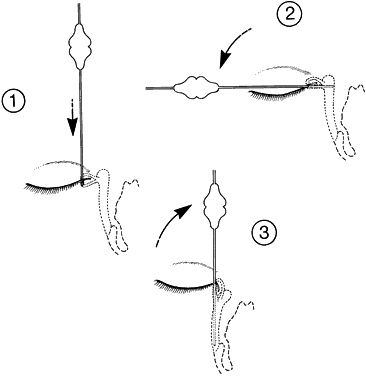
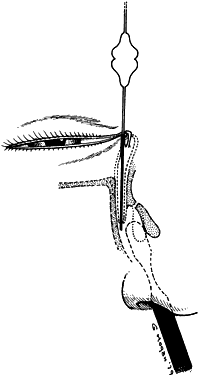
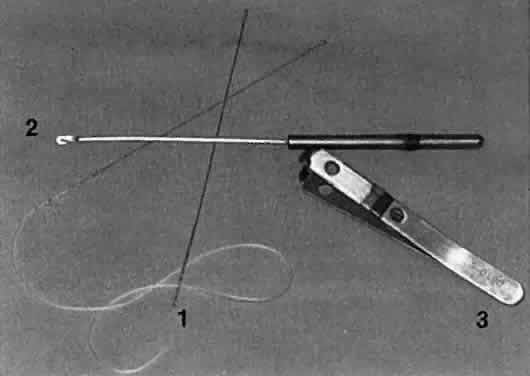
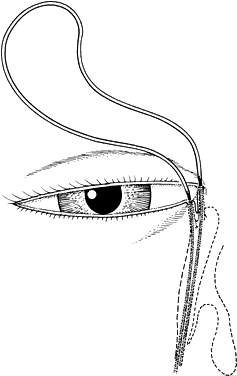
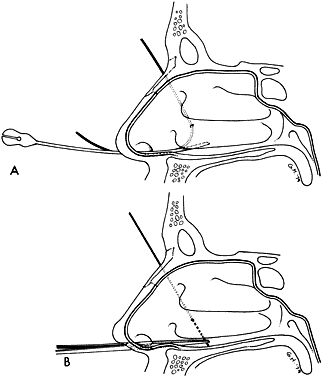
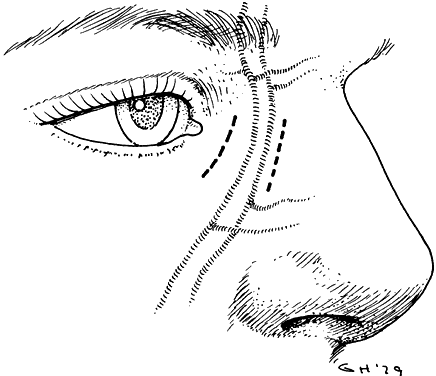
1. Blaylock WK, Moore CA, Lindberg JV: Anterior ethmoid anatomy facilitates dacryocystorhinostomy. Arch Ophthalmol 108:1774, 1990 2. Duke-Elder S: Textbook of Ophthalmology. Vol 1. St Louis: CV Mosby, 1940:238 3. Kestenbaum A: Applied Anatomy of the Eye. New York: Grune & Stratton, 1963:280–282 4. Jones LT: An anatomic approach to problems of the eyelids and lacrimal apparatus. Arch Ophthalmol 66:137, 1961 5. Doane MG: Blinking and the mechanics of lacrimal drainage system. Ophthalmology 88:844, 1981 6. Rosengren B: On lacrimal drainage. Ophthalmologica 164:409, 1972 7. Frieberg T: Wietere Untersuchungen uber die Mechanik Tranenableitung. Z Augenheilkd 39:266, 1918 8. Chavis RM, Welham RAW, Masey MW: Quantitative lacrimal scintillography. Arch Ophthalmol 96:2066, 1978 9. Maurice DM: The dynamics and drainage of tears. Int Ophthalmol Clin 13:103, 1973 10. Jones LT: Anatomy of the tear system. Int Ophthalmol Clin 13:3, 1973 11. White WL, Clover AT, Buckner AB et al: Relative canalicular tear flow as assessed by dacryoscintigraphy. Ophthalmotogy 96:167, 1989 12. Daubert J, Nik N, Chandeyssoun PA et al: Tear flow analysis through the upper and lower systems. Ophthalmol Plast Reconstr Surg 6:193, 1990 13. Linberg JV, Moore CA: Symptoms of canalicular obstruction. Ophthalmology 95:1077, 1988 14. Meyer D, Antonello A, Linberg J: Assessment of tear drainage after canalicular obstruction using fluorescein
dye disappearance. Ophthalmol 97:1370, 1990 15. Mann I: The development of the human eye. Br J Ophthalmol 260, 1949 16. Duke-Elder S: Textbook of Ophthalmology. Vol 5. St Louis: CV Mosby, 1952:5348–5358 17. Cassidy JV: Developmental anatomy of the nasolacrimal duct. Arch Ophthalmol 47:141, 1952 18. Jones LT, Wobig JL: Newer concepts of tear duct and eyelid anatomy and treatment. Trans Am Acad Ophthalmol Otolaryngol 82:603, 1977 19. Levitt JM, Kravitz D: Lacrimal anomalies. Arch Ophthalmol 61:9, 1959 20. Cassidy JV: Dacryocystitis of infancy. Am J Ophthalmol 21:773, 1948 21. Ffooks OO: Dacryocystitis in infancy. Br J Ophthalmol 46:422, 1962 22. Nelson LB, Calhoun JH, Menduke H: Medical management of congenital nasolacrimal duct obstruction. Ophthalmology 92:1187, 1985 23. Katowitz JA, Welsh MG: Timing of initial probing and irrigation in congenital nasolacrimal duct
obstruction. Ophthalmology 94:698, 1987 24. Bouzas A: Canalicular inflammation in ophthalmic cases of herpes zoster and simplex. Am J Ophthalmol 60:713, 1965 25. Harris G J, Hyndiuk RA, Fox MJ et al: Herpetic canalicular obstruction. Arch Ophthalmol 99:282, 1981 26. Harley RD, Stefanyszyn MA, Apt L et al: Herpetic canalicular obstruction. Ophthalmic Surg 18:367, 1987 27. Call NB, Welham RAN: Epiphora after irradiation of medial eyelid tumors. Am J Ophthalmol 92:842, 1981 28. Anderson DR: Unilateral epiphora caused by a papilloma of the lower canaliculus. Arch Ophthalmol 78:618, 1967 29. Wilson LA: Punctal occlusion in IDU toxicity. Personal communication, 1979 30. Ellis PP, Bausor SC, Fulmer JM: Streptothrix canaliculitis. Am J Ophthalmol 52:36, 1960 31. Wojno TH: Allergic lacrimal obstruction. Am J Ophthalmol 106:48, 1988 32. Jones LT: Tear sac foreign bodies. Am J Ophthalmol 60:111, 1965 33. Coleman SL, Brull S, Green WR: Sarcoid of the lacrimal sac and surrounding area. Arch Ophthalmol 88:645, 1972 34. Harris GJ, Williams GA, Clarke GP: Sarcoidosis of the lacrimal sac. Arch Ophthalmol 99:1198, 1981 35. Smith B, Tenzel RR, Buffam FV et at: Acute dacryocystic retention. Arch Ophthalmol 94:1903, 1976 36. Spaeth GL: Nasolacrimal duct obstruction caused by topical epinephrine. Arch Ophthalmol 77:355, 1967 37. Ashton N, Choyce DP, Fison LG: Carcinoma of the lacrimal sac. Br J Ophthalmol 35:366, 1951 38. Jones IS: Tumors of the lacrimal sac. Am J Ophthalmol 42:561, 1956 39. Radnot M, Gall J: Tumoran des Tranensackes. Ophthalmologica 151:2, 1966 40. Ryan SJ, Font RL: Primary epithelial neoplasms of the lacrimal sac. Am J Ophthalmol 76:73, 1973 41. Schenck NI, Ogura JH, Pratt LL: Cancer of the lacrimal sac: Presentation of five cases and review of the
literature. Ann Otol Rhinol Laryngol 82:153, 1973 42. Stokes DP, Flanagan JC: Dacryocystectomy for tumors of the lacrimal sac. Ophthalmic Surg 8:85, 1977 43. Jakobiec FA, Iwamoto I, Knowles DM: Ocular adnexal lymphoid tumors: Correlative ultrastructural and immunologic
marker studies. Arch Ophthalmol 100:84, 1982 44. Farkas RG, Lamberson RE: Malignant melanoma of the lacrimal sac. Am J Ophthalmol 66:45, 1968 45. Peretz WL, Ettinghausen SE, Gray GF: Oncocytic adenocarcinoma of the lacrimal sac. Am J Ophthalmol 66:45, 1968 46. Sen DK, Mohan H, Chatterjee PK: Neurilemmoma of the lacrimal sac. Eye Ear Nose Throat Monthly 50:56, 1971 47. Singh K, Mersol V, Mastny V et al: Adenoacanthoma of the lacrimal sac. Ann Ophthalmol 9:1027, 1977 48. Gurney N, Chakley T, O'Grady R: Lacrimal sac hemangiopericytoma. Am J Ophthalmol 71:757, 1971 49. Cole SH, Ferry AP: Fibrous histiocytoma of the lacrimal sac. Arch Ophthalmol 96:1647, 1978 50. Linberg JV, McCormick SA: Primary acquired nasolacrimal duct obstruction: A clinicopathologic report
and biopsy technique. Ophthalmology 93:1055, 1986 51. Harris GJ, Furste FH: Lacrimal intubation in the primary repair of mid facial fractures. Ophthalmology 94:242, 1987 52. Cies WA, Baylis HI: Epiphora following rhinoplasty and Caldwell-Luc procedures. Ophthalmic Surg 7:77, 1976 53. Colvard DM, Waller RR, Neault RW et al: Nasolacrimal duct obstruction following transantral-ethmoidal orbital decompression. Ophthalmic Surg 10:25, 1979 54. Seiff SR, Shorr N: Nasolacrimal drainage system obstruction after orbital decompression. Am J Ophthalmol 160:204, 1988 55. Gaynon IE: Lacrimal insufficiency: Keratoconjunctivitis sicca and malfunction of the
inferior turbinate. Am J Ophthalmol 53:614, 1960 56. Jones LT, Linn ML: The diagnosis of the causes of epiphora. Am J Ophthalmol 67:751, 1969 57. Zappia RJ, Milder B: Lacrimal drainage function, Part I. The Jones fluorescein test. Am J Ophthalmol 74:154, 1972 58. Hornblass A: A simple taste test for lacrimal obstruction. Arch Ophthalmol 90:435, 1973 59. Jones LT, Wobig JL: Surgery of the Eyelids and Lacrimal System, pp 141, 221. Birmingham, AL: Aesculapius, 1976 60. Zappia RJ, Milder B: Lacrimal drainage function. Part II: The fluorescein dye disappearance
test. Am J Ophthalmol 74:160, 1972 61. Jordan A, Baum J: Basic tear flow: Does it exist? Ophthalmology 87:920, 1980 62. Clinch TE, Benedetto DA, Felberg NT et at: Schirmer's test: A closer look. Arch Ophthalmol 101:1383, 1983 63. Milder B, Demorest BH: Dacryocystography: The normal lacrimal apparatus. Arch Ophthalmol 51:180, 1954 64. Trokel SL, Potter GD: Kinetic dacryocystography: The normal lacrimal apparatus. Arch Ophthalmol 51:180, 1954 65. Hurwitz JJ, Welham RAN, Maisey MN: Intubation macrodacryocystography and quantitative scintillography: The “complete” lacrimal assessment. Trans Am Acad Ophthamol Otolaryngology 81:575, 1976 66. Rossomondo RM, Carlton WH, Trueblood JH et al: A new method of evaluating lacrimal drainage. Arch Ophthalmol 88:523, 1972 67. Chavis RM, Welham RAN, Maisey M: Quantitative lacrimal scintillography. Arch Ophthalmol 96:2066, 1978 68. Tenzel RR, Buffam FV, Miller GR: The use of the “lateral canthal sling” in ectropion repair. Can J Ophthalmol 12:199, 1977 69. Anderson RL, Gordy DD: The tarsal strip procedure. Arch Ophthalmol 97:2192, 1979 70. Nowinski TS, Anderson RL: The medial spindle procedure for involutional medial ectropion. Arch Ophthalmol 103:1750, 1985 71. Tse DT: Surgical correction of punctal malposition. Am J Ophthalmol 100:339, 1985 72. Hughes WL: Conjunctivochalasis. Am J Ophthalmol 25:48, 1942 73. Serrano F, Mora LM: Conjunctival chalasis: A surgical technique. Ophthalmic Surg 20:883, 1989 74. Bosniak SL, Smith BC: Conjunctivochalasis. Adv Ophthalmic Plast Reconstr Surg 3:153, 1984 75. Liu D: Conjunctivochalasis: A cause of tearing and its management. Ophthalmic Plast Reconstr Surg 2:25, 1986 76. Jones LT: Anatomic approach to problems of the eyelids and lacrimal apparatus. Arch Ophthalmol 66:111, 1961 77. Hanselmayer H: Prognosis of injured canaliculi in relation to elapsed time until primary
operation. Ophthalmologica 166:175, 1973 78. Crawford JS: Intubation of obstructions in the lacrimal system. Can J Ophthalmol 12:289, 1977 79. Hawes MJ, Segrest DR: Effectiveness of bicanalicular silicone intubation in the repair of canalicular
lacerations. Ophthalmic Plast Reconstr Surg 1:185, 1985 80. Kuchar A, Huber E, Steinkogler FJ: Monoka-Silikonintubation der Tranenwege nach retrograder Dilation. Ophthalmologe 92:43, 1995 81. McLeish WM, Bowman B, Anderson RL: The pigtail probe protected by silicone intubation: A combined approach
to canalicular reconstruction. Ophthalmic Surg 23:281, 1992 82. Morrison FD: An aid to repair of lacerated tear ducts. Arch Ophthalmol 72:341, 1964 83. Demant E, Hurwitz JJ: Canaliculitis: Review of 12 cases. Can J Ophthalmol 15:73, 1980 84. Birchansky LD, Nerad JA, Kersten RC et al: Management of congenital lacrimal sac fistula. Arch Ophthalmol 108:388, 1990 85. Putterman AM: Dacryocystography with occluded common canaliculus. Am J Ophthalmol 76:1010, 1973 86. Crigler LW: The treatment of congenital dacryocystitis. JAMA 81:23, 1923 87. Kassoff J, Meyer DR: Early office-based versus late hospital-based nasolacrimal duct probing. Arch Ophthalmol 113:1168, 1995 88. Kuschner BJ: Early office-based versus late hospital-based nasolacrimal duct probing [editorial]. Arch Ophthalmol 113:1103, 1995 89. Stager D, Baker JD, Frey T et al: Office probing of congenital nasolacrimal duct obstruction. Ophthalmic Surg 23:482, 1992 90. EI-Mansoury J, Calhoun JH, Nelson LB et al: Results of late probing for congenital nasolacrimal duct obstruction. Ophthalmology 93:1052, 1986 91. Dortzbach RK, France TD, Kushner BJ et al: Silicone intubation for obstruction of the nasolacrimal duct in children. Am J Ophthalmol 94:585, 1982 92. Leone CR, Van Gemert JV: The success rate of silicone intubation in congenital lacrimal obstruction. Ophthalmic Surg 21:90, 1990 93. Becker BB, Berry FD, Koller H: Balloon catheter dilation for treatment of congenital nasolacrimal duct
obstruction. Am J Ophthalmol 121:304, 1996 94. Crawford JS: Lacrimal intubation set with suture in the lumen. Ophthalmic Plast Reconstr Surg 4:249, 1988 95. Quickert MH, Dryden RM: Probes for intubation in lacrimal drainage. Trans Am Acad Ophthalmol Otolaryngology 74:431, 1970 96. Hunter LR: Crawford tubes (with suture) secured with absorbable suture. J Pediatr Ophthalmol Strabismus 32:197, 1995 97. Welsh M, Katowitz J: Timing of Silastic tubing removal after intubation for congenital nasolacrimal
duct obstruction. Ophthalmic Plast Reconstr Surg 5:43, 1989 98. Kathuria SS, Harvey JT: Transcanalicular removal of silastic nasolacrimal tubes. Ophthalmic Plast Reconstr Surg 11:221, 1995 99. Snead JW, Rathbun JE, Crawford JB: Effects of the silicone tube on the canaliculus: An animal experiment. Ophthalmology 87:1031, 1980 100. Jordan DR, Nerad JA: An active inflammatory reaction to silicone stents. Ophthalmic Plast Reconstr Surg 3:147, 1987 101. Angrist RC, Dortzbach RK: Silicone intubation for partial and total nasolacrimal duct obstruction
in adults. Adv Ophthalmic Plast Reconstr Surg 1:51, 1985 102. Hallus AV (trans): Procédé Plastique de dacryocystorhinostomie et ses résultats. Ann Ocul 158:241, 1921 103. Hallum AV: The Dupuy-Dutemps dacryocystorhinostomy. Trans Am Ophthalmol Soc 46:243, 1948 104. Patrinely JR, Anderson RL: A review of lacrimal drainage surgery. Ophthalmic Plast Reconstr Surg 2:97, 1986 105. Linberg JV, Anderson RL, Bumstead RM et al: Study of intranasal ostium external dacryocystorhinostomy. Arch Ophthalmol 100:1758, 1982 106. Viers ER: The use of cautery in external dacryocystorhinostomy. Am J Ophthalmol 72:679, 1971 107. Pico G: A modified technique of external dacryocystorhinostomy. Am J Ophthalmol 72:679, 1971 108. Iliff CD: A simplified dacryocystorhinostomy. Arch Ophthalmol 85:586, 1971 109. Older JJ: Routine use of a silicone stent in a dacryocystorhinostomy. Ophthalmic Surg 13:911, 1982 110. Blaylock WK, Moore CA, Lindberg JV: Anterior ethmoid anatomy facilitates dacryocystorhinostomy. Arch Ophthalmol 108:1774, 1990 111. Cruz O, Patrinely J, Reyna G et al: Urine drug screening for cocaine after lacrimal surgery. Am J Ophthalmol 111:703, 1991 112. Massaro BM, Gonnering RS, Harris GJ: Endonasal laser dacryocystorhinostomy: A new approach to nasolacrimal duct
obstruction. Arch Ophthalmol 108:1172, 1990 113. Gonnering RS, Lyon DB, Fisher JC: Endoscopic laser-assisted lacrimal surgery. Am J Ophthalmol 111:152, 1991 114. Kong YT, Kim TI, Kong BW: A report of 131 cases of endoscopic lacrimal surgery. Ophthalmol 101:1793, 1994 115. Woog JJ, Metson R, Puliafito CA: Holmium:YAG endonasal laser dacryocystorhinostomy. Am J Ophthalmol 116:1, 1993 116. Bartley GB: The pros and cons of laser dacryocystorhinostomy. Am J Ophthalmol 117:103, 1994 117. Tarbet KJ, Custer PL: External dacryocystorhinostomy: Surgical success, patient satisfaction, and
economic cost. Ophthalmology 102:1065, 1995 118. Mannor GE, Millman AL: The prognostic value of preoperative dacryocystography in endoscopic intranasal
dacryocystorhinostomy. Am J Ophthalmol 113:134, 1992 119. Boush GA, Lemke BN, Dortzbach RK: Results of endonasal laser-assisted dacryocystorhinostomy. Ophthalmology 101:955, 1994 120. Levin PS, Stormo-Gipson J: Endocanalicular laser-assisted dacryocystorhinostomy: An anatomic study. Arch Ophthalmol 110:1488, 1992 121. Silkiss RZ, Axelrod RN, Iwach AG et al: Transcanalicular THC:YAG dacryocystorhinostomy. Ophthalmic Surg 23:351, 1992 122. Patel BCK, Phillips B, McLeish WM et al: Transcanalicular Neodymium:YAG laser for revision of dacryocystorhinostomy. Ophthalmology 104:1191, 1997 123. Welham RAN, Henderson PH: Results of dacryocystorhinostomy analysis of causes for failure. Trans Ophthalmol Soc UK 93:601, 1973 124. Welham RAN, Wulc AE: Management of unsuccessful lacrimal surgery. Br J Ophthalmol 71:152, 1987 125. Singh G, Wilson MR, Foster CS: Mitomycin eye drops as treatment for pterygium. Ophthalmology 95:813, 1988 126. Yamamoto T, Varani J, Soong HK et al: Effect of 5-fluorouracil and mitomycin C on cultured rabbit subconjunctival
fibroblasts. Ophthalmol 97:1204, 1990 127. Krupin TH, Juzych MS, Shin DH et al: Adjunctive mitomycin C in primary trabeculectomy in phakic eyes. Am J Ophthalmol 119:30, 1995 128. Kao S, Liao CL, Tseng JH et al: Dacryocystorhinostomy with intraoperative mitomycin C. Ophthalmology 104:86, 1997 129. Camara JG, Bengzon AU, Henson RD: The safety and efficacy of Mitomycin C in endonasal endoscopic laser-assisted
dacryocystorhinostomy. Ophthalmic Plast Reconstr Surg 16:114, 2000 130. Hu D, Sires BS, Tong DC et al: Effect of brief exposure to mitomycin C on cultured human nasal mucosa
fibroblasts. Ophthalmic Plast Reconstr Surg 16:119, 2000 131. Allen KM, Berlin AJ, Levine HL: Intranasal endoscopic analysis of dacryocystorhinostomy failure. Ophthalmic Plastic Reconstr Surg 4:143, 1988 132. Orcutt JC, Hillel A, Weymuller EA: Endoscopic repair of failed dacryocystorhinostomy. Ophthalmic Plastic Reconstr Surg 6:197, 1990 133. Reinicke RD, Carroll JM: Silicone lacrimal tube implantation. Trans Am Acad Ophthalmol Otolaryngol 73:85, 1969 134. Jones LT: The cure of epiphora due to canalicular disorders, trauma, and surgical
failures on the lacrimal passages. Trans Am Acad Ophthalmol Otolaryngol 66:506, 1962 135. Steinsapir KD, Glatt HJ, Putterman AM: A 16-year study of conjunctival dacryocystorhinostomy. Am J Ophthalmol 109:387, 1990 136. Rose G, Welham RAN: Jones' lacrimal canalicular bypass tubes: Twenty-five years' experience. Eye 5:13, 1991 137. Putterman AM: Fixation of Pyrex tubes and conjunctivo-dacryocystorhinostomy. Am J Ophthalmol 78:1024, 1974 138. Gladstone GJ, Putterman AM: A modified glass tube for conjunctivodacryocystorhinostomy. Arch Ophthalmol 103:1229, 1985 139. Sekhar GC, Dortzbach RK, Gonnering RS et al: Problems associated with conjunctivodacryocystorhinostomy. Am J Ophthalmol 112:502, 1991 140. Rosen N, Ashkenazi I, Rosner M: Patient dissatisfaction after functionally successful conjunctivodacryocystorhinostomy
with Jones' tube. Am J Ophthalmol 117:636, 1994 141. Doucet TW, Hurwitz JJ: The broken Lester Jones tube. Can J Ophthalmol 17:32, 1982 142. Wood JR, Anderson RL, Edwards JJ: Phospholine iodide toxicity and Jones' tubes. Ophthalmology 87:346, 1980 143. Bartley GB, Gustafson RO: Complications of malpositioned Jones' tubes. Am J Ophthalmol 109:66, 1990 |