IRIDOCORNEAL ENDOTHELIAL SYNDROME
The iridocorneal endothelial (ICE) syndrome consists of three rare clinical entities and their variations that, although separately described, are now considered as a spectrum of disease with a common pathogenetic mechanism. Patients with the ICE syndrome usually present in the third to fifth decades of life having noticed unilateral iris abnormalities or complaining of visual disturbances or ocular discomfort due to corneal edema or elevated intraocular pressure. The syndrome is more common in women, appears to occur sporadically, and has been recognized almost exclusively in whites. There are no known systemic associations.1,2
Clinical Features
The iris, cornea, and anterior chamber angle are affected in patients with the ICE syndrome. The major clinical variations within this syndrome are described below and are distinguished by particular patterns of involvement of these structures.
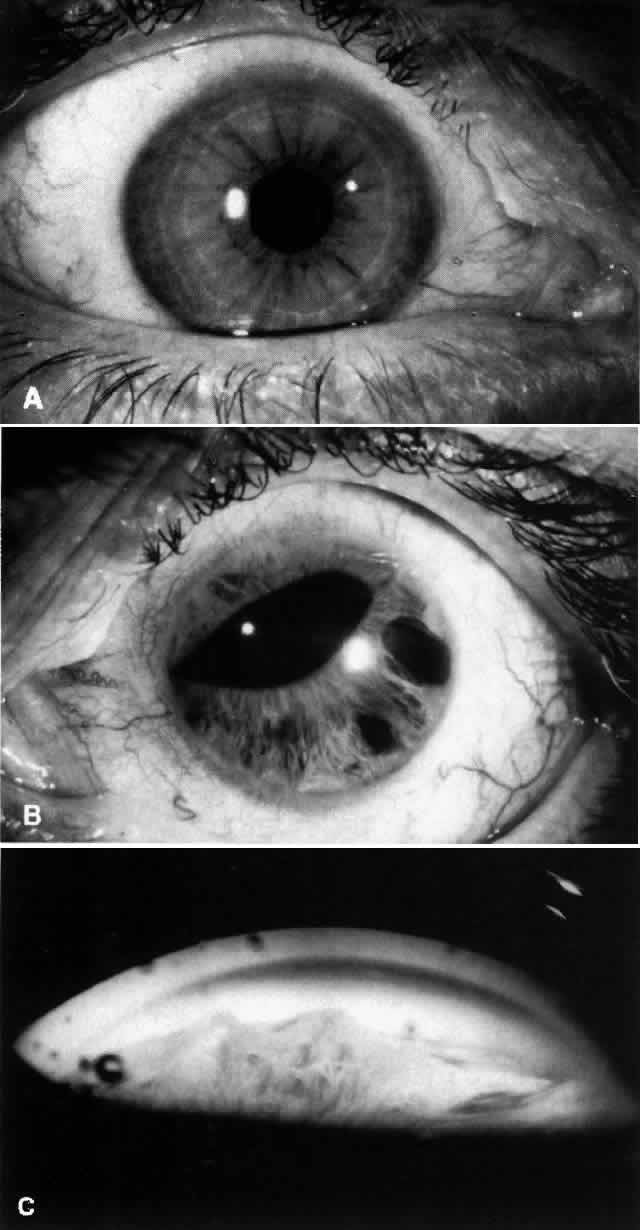
PROGRESSIVE (ESSENTIAL) IRIS ATROPHY. Progressive iris atrophy is characterized by prominent atrophy of the iris, with iris stromal thinning progressing to full-thickness iris holes.1,2 Peripheral anterior synechiae develop early, and progress both circumferentially and onto the cornea.1,2 Pupillary distortion and ectropion uveae occur, typically toward the most prominent peripheral anterior synechiae.1,3 Large and sometimes bizarrely shaped iris “stretch” holes occur, apparently from traction of the iris between peripheral anterior synechiae on opposite sides of the globe (Fig. 1).1,3,4 Less common, smaller oval holes may develop adjacent to peripheral anterior synechiae. These “melting” holes are presumed to be caused by iris ischemia resulting from obstruction of iris vessels within the synechiae.1,3 Multiple, fine, pedunculated iris nodules may occur, generally appearing late in the disease process.1,5
The corneal endothelium in involved eyes is described as showing a fine, beaten metal irregularity, sometimes in focal areas. Stromal and/or epithelial edema may be present. In some cases, no definite corneal abnormality is evident.1
CHANDLER'S SYNDROME. The dominant feature in this variant of the ICE syndrome is usually corneal edema, often at normal or only moderate levels of intraocular pressure elevation. Chandler described the corneal endothelium as having a “fine hammered silver appearance.” ,6 Iris atrophy is less prominent in Chandler's syndrome than in progressive iris atrophy and, when detectable, is often limited to the anterior iris stroma. The pupil is usually round or slightly oval.6
COGAN-REESE (IRIS NEVUS) SYNDROME. Patients with Cogan-Reese syndrome are characterized by iris pigmented lesions that range from multiple, pedunculated, nodular lesions to a more diffuse, smooth, velvety change of the iris. The iris surface tends to lose its normal pattern, and it usually appears darker than that of the fellow eye. Ectropion uveae, breaks in the iris stroma, and an ectopic pupil are often present. Peripheral anterior synechiae, corneal edema, and labile glaucoma are characteristic features.7
Pathogenesis
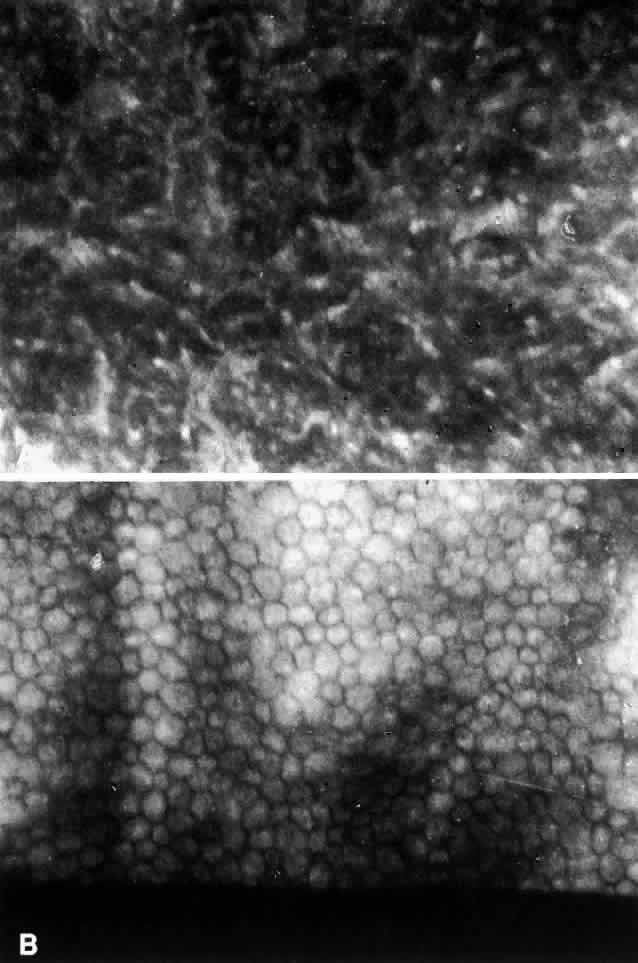
On the basis of careful clinical observations and histopathologic study, Campbell and co-workers3 and Eagle and co-workers8 have advanced the now generally accepted hypothesis that the primary abnormality in the ICE syndrome is proliferation of abnormal corneal endothelium. This abnormal endothelium correlates with the beaten metal appearance visible on slit lamp biomicroscopy. If the patient is examined in the early stages of the disease, demarcations between normal and abnormal corneal endothelium may be visible biomicroscopically.9,10 With time, the areas of abnormal endothelium enlarge, so that eventually the entire corneal endothelium is involved.9 The stroma and epithelium overlying the regions of abnormal endothelium may be clear or may become edematous.
The specular microscopic changes of the corneal endothelium are sufficiently specific that a masked observer can usually differentiate the ICE syndrome from other endothelial conditions.11 The earliest visible changes are a loss of the uniform hexagonal shape of the endothelial cells. Dark areas begin to appear in individual cells. In more affected corneas there is increased cellular pleomorphism and the dark areas within individual cells become larger, eventually reaching the point at which the endothelial mosaic can no longer be recognized (Fig. 2).9,11 The darker areas within the abnormal endothelial cells may be accompanied by a brighter than normal reflection from the cell borders.12 In clinically normal-appearing areas in some affected eyes, specular microscopy has shown a normal endothelial mosaic but cells that are much smaller than usual9,10; the significance of this is unknown. In some cases, the uninvolved fellow eyes of patients with the ICE syndrome have shown abnormal endothelial cell pleomorphism with lower than expected cell counts.11,13
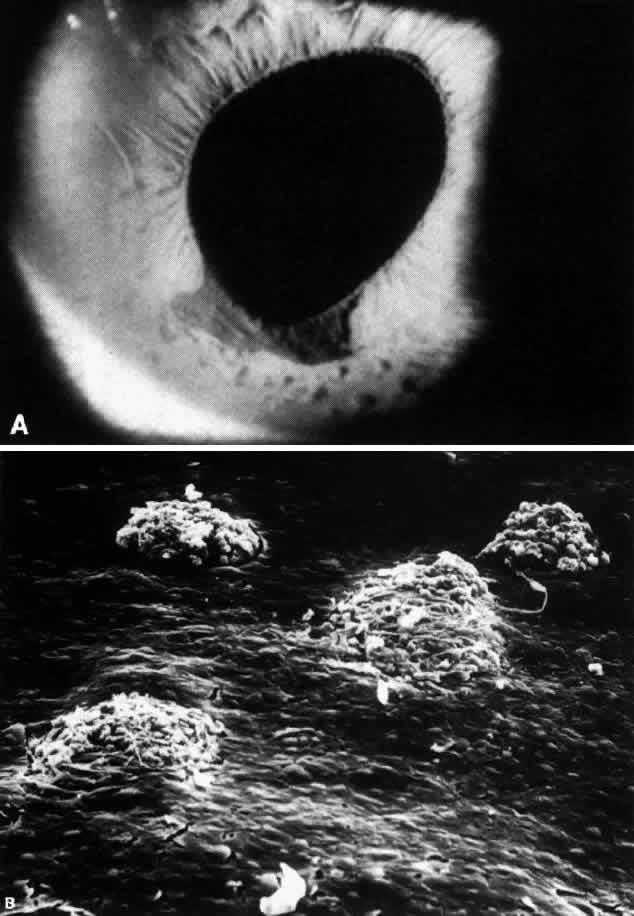
The abnormal endothelium and the basement membrane that it elaborates eventually spread from the cornea onto the trabecular meshwork and the surface of the iris.3,8 Contraction of this membrane results in the development of peripheral anterior synechiae in areas of previously open angle and may also result in ectropion uveae.3,8 Iris atrophy and full-thickness holes develop as a result of stretching of the iris between synechiae. Since the iris atrophy is actually a secondary phenomenon,8 the term progressive iris atrophy is preferred to the historically used term essential iris atrophy.2Iris nodules may develop in areas involved with the endothelium-basement membrane complex (Fig. 3); it has been suggested that the nodules form as a result of encircling and “pinching off” portions of the iris by the cellular membrane.3,8,14 The nodules are thus a marker for areas of iris endothelialization.14
The various clinical manifestations of progressive iris atrophy, Chandler's syndrome, and the Cogan-Reese syndrome can thus all be explained by the proliferation of this abnormal endothelium.2,3,8 The location, extent and specific pattern of proliferation account for the different clinical variations seen from patient to patient. Continued growth of the abnormal endothelium and basement membrane and its contraction results in the progressive changes that occur in a patient over years.
The cause of the endothelial proliferation is unknown. Ultrastructural study of Descemet's membrane in cases of the ICE syndrome shows normal anterior banded and posterior nonbanded zones, posterior to which is an abnormal collagenous layer.15 The abrupt transition from normal Descemets membrane to an abnormal posterior collagenous layer suggests that endothelial function was altered suddenly after having functioned normally for some time after birth. This damaged endothelium responds by proliferation.15 The nature of the event precipitating this presumed change in the endothelium is unknown. An alternate hypothesis has been proposed by Bahn and co-workers, who suggest that a primary neural crest abnormality could explain endothelial proliferation in these patients.16
Glaucoma in the ICE syndrome results from progressive loss of the chamber angle. Formation of peripheral anterior synechiae increases with time. Intraocular pressure may, however, be higher than expected based on the areas affected by peripheral anterior synechiae. Histologic study has confirmed that abnormal endothelium and basement membrane often overlie the trabecular meshwork even when the angle appears gonioscopically open.3,8,17 This membrane probably interferes with aqueous outflow even prior to the development of synechiae, contributing to elevated intraocular pressure.
Management
In the early stages, glaucoma may be controlled medically. Since by the time intraocular pressure is elevated the angle is often largely closed by synechiae or covered by the abnormal membrane, drugs decreasing aqueous production are more useful than are miotics. Laser trabeculoplasty is generally not effective for the same reason. Thus, if intraocular pressure cannot be controlled medically, then filtering surgery is required. The success rate for trabeculectomy in the ICE syndrome is comparable to that for primary open-angle glaucoma.18 Some of the late failures have been attributed to proliferation of abnormal endothelium into the filtering bleb.8 In these cases, another filtering procedure performed in a different location is appropriate. The success rates for repeated trabeculectomies are comparable to those of the initial procedure and similar to repeat procedures in patients with primary open-angle glaucoma.18
Patients with corneal edema may benefit from lowering intraocular pressure even if it is already within normal limits.6,19 Filtering surgery cannot be recommended solely in an attempt to resolve corneal edema by reducing intraocular pressure, however, since the cornea may remain edematous even at the lowest achievable levels of pressure.19 Hypertonic saline drops may be helpful for mild epithelial edema. If visually significant corneal edema is present after intraocular pressure has been maximally lowered medically, then penetrating keratoplasty is usually required. Provided intraocular pressure remains controlled, the prognosis for the corneal graft is very good.20 Recurrences of the endothelial abnormalities that characterize the ICE syndrome have not been noted to develop on the donor cornea.11,20
POSTERIOR POLYMORPHOUS DYSTROPHY
Posterior polymorphous dystrophy (PPMD) is now believed to be a congenital condition, usually with an autosomal dominant inheritance pattern. The cornea, iris, and anterior chamber angle are involved, but there is a wide variation in expression of this dystrophy both within and between affected families. It can be stationary or slowly progressive. Although the condition is bilateral, involvement may be so asymmetric as to be clinically evident in only one eye.21,22
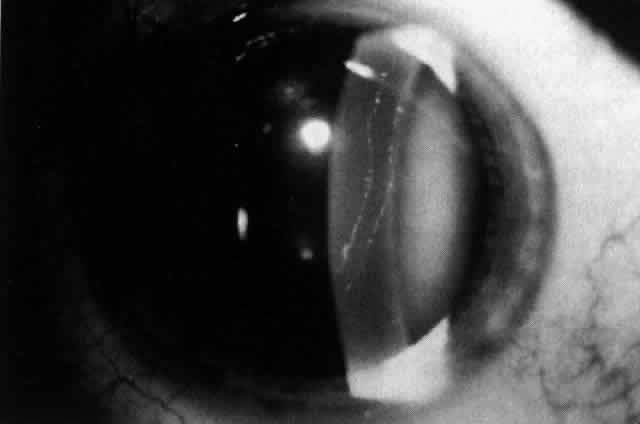
The corneal abnormalities are the most characteristic and the most varied. Lesions at Descemets membrane may take the form of individual or groups of vesicular-appearing lesions; bandlike lesions with scalloped, irregular edges (Fig. 4); or islands of abnormal-appearing endothelial cells. By specular microscopy, these areas consist of abnormal enlarged or irregularly shaped cells or black acellular zones.23 Areas of thickening of Descemet's membrane may occur, with involvement ranging from small areas to the entire posterior corneal surface.21,22
|
In the majority of patients, only limited regions of Descemet's and the endothelium are involved.21,22 These patients are usually asymptomatic and may be unaware of their condition. Even areas of cornea that appear normal on biomicroscopy may have specular microscopic abnormalities, including abnormally small endothelial cells or enlarged pleomorphic endothelial cells.23 In cases with large areas of endothelial involvement, there may be focal or diffuse overlying corneal edema that can be severe enough to require penetrating keratoplasty. The edema can be so advanced as to preclude slit lamp evaluation of the posterior cornea, in which case the diagnosis can sometimes be made by recognizing typical lesions in family members. Corneal edema has been noted more commonly in older patients, presumably due to progressive corneal changes, but can be present in childhood or even at birth.21,22
Histopathologically, the most unusual finding in PPMD is that some areas of damaged endothelial cells have epithelial-like characteristics. These cells contain extensive microvilli and keratofibrils and have desmosomal attachments.22,24 The epithelial character of these cells has been confirmed by the demonstration that they contain epithelial keratins.25 In other areas, endothelial cells are degenerated, attenuated, and vacuolated. Fibroblastic proliferation has also been noted.22,26 Abnormalities in Descemet's membrane include abnormal lamination, deposition of abnormal fibrillar collagenous material on the posterior surface with areas of irregular guttate excrescences,22,26 and pits.26,27 These changes are probably secondary to the endothelial abnormalities.22,26
The abnormal endothelial cells, both those with and without epithelial characteristics, have been found to extend across the trabecular meshwork and onto the surface of the iris.22,24 It is likely that this extension results in the iridocorneal adhesions that occur in some patients with PPMD. These adhesions vary from peripheral anterior synechiae visible only on gonioscopy to prominent adhesions easily visible by slit lamp examination. In some cases, translucent membranes apparently extending from the posterior cornea are visible on the surface of the iris; these cause ectropion uveae and corectopia in occasional patients.22,28
The incidence of glaucoma in PPMD appears to be less than in the ICE syndrome. Krachmer reported that 14% of the referred patients he had examined with PPMD had elevated intraocular pressure.22 Since so many patients with PPMD are asymptomatic, the true incidence of elevated intraocular pressure is probably much lower. Most of the patients in Krachmer's group with elevated intraocular pressure had iridocorneal adhesions that closed portions of the filtration angle. A second group of PPMD patients with open angles and without iridocorneal adhesions have been recognized.21,22 The cause of the pressure increase in these patients is uncertain; in one such case examined histopathologically, an abnormally high insertion of the iris into the posterior trabecular meshwork with some collapse of the intertrabecular spaces was noted.29
Management
When present, glaucoma is best initially managed with medications that decrease aqueous secretion. Miotics may be helpful if areas of open angle are present. There are the same concerns with the use of laser trabeculoplasty in this condition as in the ICE syndrome: because of the growth of a cellular membrane across the chamber angle, trabeculoplasty might accelerate synechiae formation and thus aggravate rather than help the situation. In those patients whose intraocular pressure cannot be controlled medically, filtering surgery is probably the safest option.
Penetrating keratoplasty is required in only a minority of patients with PPMD. In those patients without iridocorneal adhesions or glaucoma, the prognosis for maintaining a clear graft is excellent. Patients with iridocorneal adhesions have a much lower success rate: only 31% with adhesions achieved 20/40 (6/12)* or better acuity compared with 78% without adhesions achieving this vision in Krachmer's series.22 Preoperative glaucoma, even though controlled at the time of surgery, was also found to be a poor prognostic factor for keratoplasty: 69% of eyes with glaucoma had final visual acuities of 20/400 (6/120) or less. In comparison, all eyes with normal intraocular pressure had better than 20/400 visual acuity after transplantation.22 All patients with preoperative glaucoma continued to have difficulties with glaucoma postoperatively.22 There was an overlap between these two groups since all eyes with glaucoma also had iridocorneal adhesions. Thus the presence of iridocorneal adhesions and preoperative glaucoma are poor prognostic factors for successful keratoplasty in PPMD and are more important to the outcome than is the severity of the corneal disease itself.22
* Metric equivalent given in parentheses following Snellen notation
FUCHS' ENDOTHELIAL DYSTROPHY
The terms Fuchs' endothelial dystrophy is applied to patients with extensive corneal guttata (often accompanied by flecks of endothelial pigment) who have developed corneal edema. Those patients with extensive guttata who have not yet developed corneal edema are said to have “endothelial dystrophy,”30 although in practice this strict distinction in terminology is not always followed. Although this dystrophy appears to be inherited in an autosomal dominant fashion, women are more frequently affected than men.30 The condition is bilateral but usually somewhat asymmetric. Once visually significant corneal edema develops, penetrating keratoplasty is generally required.
It has been taught that open-angle glaucoma is more common in patients with endothelial dystrophy and with Fuchs' dystrophy than in the general population.31 Data supporting this contention are difficult to locate, however. In a study of 64 families with endothelial dystrophy, only 1 of 71 patients with Fuchs' dystrophy (1.4%) was found to have open-angle glaucoma,32 which was considerably less than the 10% to 15% prevalence that had been previously estimated.31 There have been conflicting reports regarding the tonographic facility of aqueous outflow in patients with corneal guttata. Although a preliminary report in 1967 found that a high percentage of patients with corneal guttata had an abnormally low facility of aqueous outflow,33 Roberts and co-workers were unable to reproduce this finding in their 1984 study.34 They also graded the extent of endothelial guttata by slit lamp examination and by wide-field specular microscopy but could not demonstrate any relationship between outflow facility and the extent of guttata.34 Thus it is uncertain whether there is any increased likelihood of open-angle glaucoma in patients with Fuchs' dystrophy or its precursor states. When present, open-angle glaucoma in these patients should be treated with standard measures.
Occasionally, patients with Fuchs' dystrophy who have shallow anterior chambers may develop angle-closure glaucoma. In addition to thickening of the crystalline lens that occurs with aging, the angles in these patients may be further compromised by progressive edema of the peripheral cornea. Peripheral iridectomy is then required.