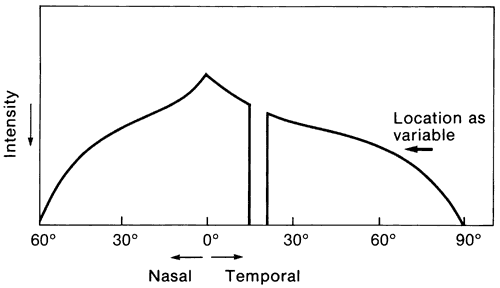
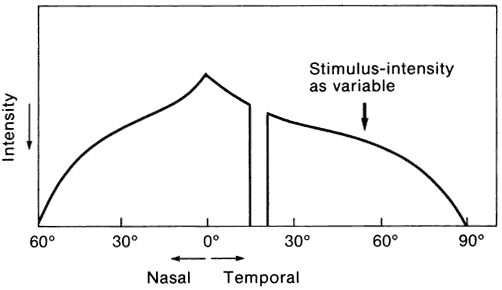
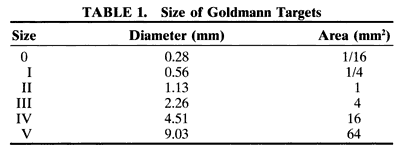
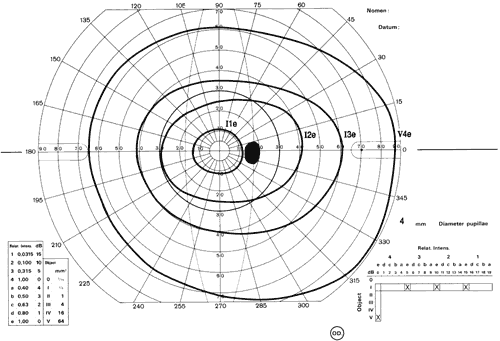
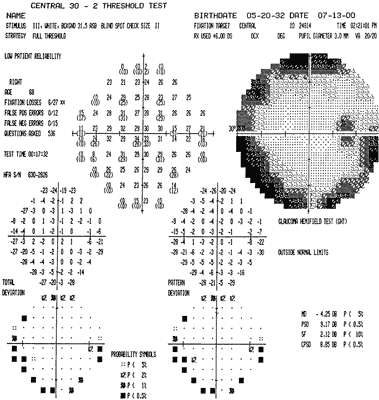
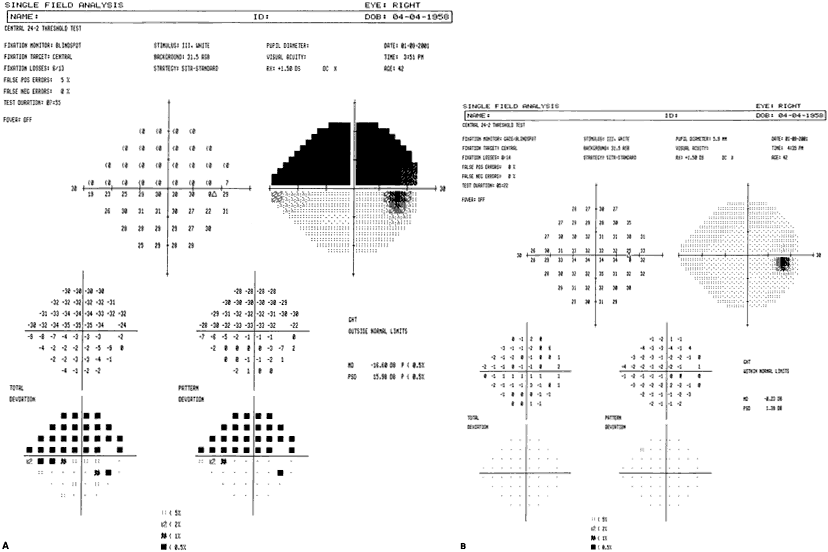
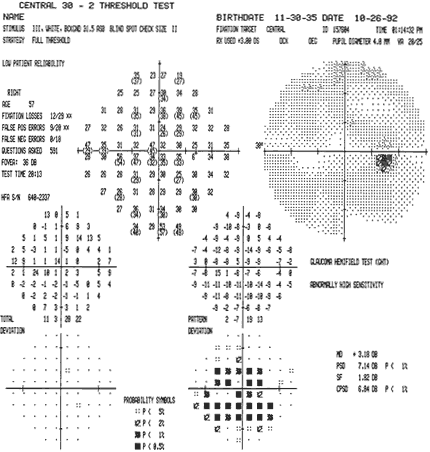
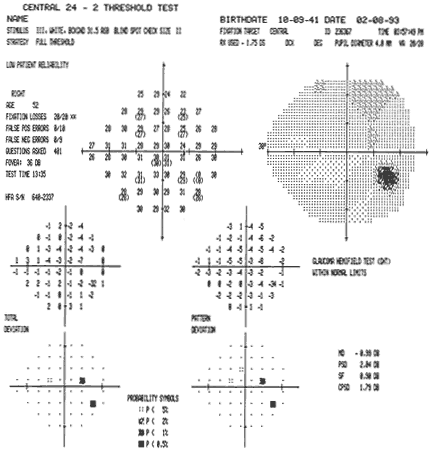
1. Traquair HM: Perimetry in the study of glaucoma. Trans Ophthalmol Soc UK 51:585, 1931 2. Aulhorn E, Harms H: Visual perimetry. In Jameson D, Horvich CM (eds): Handbook
of Sensory Physiology. Vol 7. Berlin: Springer-Verlag, 1972 3. Hattington DO, Drake MV: The Visual Fields: Textbook and Atlas of Clinical
Perimetry. St Louis: CV Mosby, 1990 4. Anderson DR: Perimetry With and Without Automation. 2nd ed. St Louis: CV
Mosby, 1987 5. Drance SM, Anderson DR: Automatic Perimetry in Glaucoma: A Practical Guide. New
York: Grune & Stratton, 1985 6. Minkler DS: The organization of nerve fiber bundles in the primate optic nerve head. Arch Ophthalmol 98:1630, 1989 7. Hayreh S: Blood supply of the anterior optic nerve. In Ritch R, Shields
MD, Krupin T (eds): The Glaucomas. Vol 1. St Louis: CV Mosby, 1989 8. Quigley HA, Addicks EM: Regional differences in the structure of the lamina cribrosa and their
relation to glaucomatous optic nerve damage. Arch Ophthalmol 99:137, 1981 9. Flammer J, Drance SM, Zulauf M: Differential light threshold: Short and long term fluctuation in patients
with glaucoma, normal controls and patients with suspected glaucoma. Arch Ophthalmol 102:704, 1984 10. Werner EB, Drance SM: Early visual field defects in glaucoma. Arch Ophthalmol 95:1173, 1977 11. Hart WM, Becker B: The onset and evolution of glaucomatous visual field defects. Ophthalmology 89:268, 1982 12. Phelps CD, Hayreh SS, Montague PR: Visual fields in low tension glaucoma, primary open angle glaucoma and
anterior ischemic optic neuropathy. Doc Ophthalmol 35:113, 1983 13. Piltz JR, Drance SM, Douglas GR et al: The relationship of peripheral vasospasm, diffuse
and localized visual field defects and intraocular pressure
in glaucomatous eyes. In Perimetry Update 1990/91: Proceedings
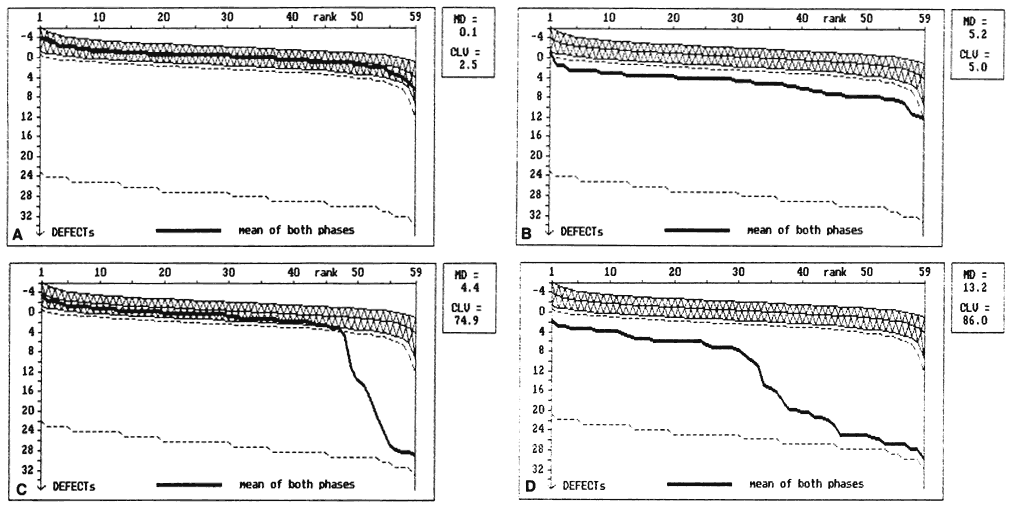
of the IX International Perimetric Society Meeting. Amsterdam: Kugler & Ghedini, 1991:465 14. Bebie H, Flammer J, Bebie H: The cumulative defect curve: Separation of local and diffuse components
of visual field damage. Graefes Arch Clin Exp Ophthalmol 227:9, 1989 15. Mikelberg F, Drance SM: The mode of progression of visual field defects in glaucoma. Am J Ophthalmol 98:443, 1984 16. Martinez-Bello C, Chauhan BC, Nicolela MT et al: Intraocular pressure and progression of glaucomatous visual field loss. Am J Ophthalmol 129:302, 2000 17. Hoskins HD, Kass M: Becker-Shaffer's Diagnosis and Therapy of the
Glaucomas. St Louis: CV Mosby, 1989 18. The Field Analyzer Primer. San Leandro, CA: Humphrey Instruments, 1986 19. Spry PGD, Henson DB, Sparrow JM et al: Quantitative comparison of static perimetric strategies in early glaucoma: Test-retest
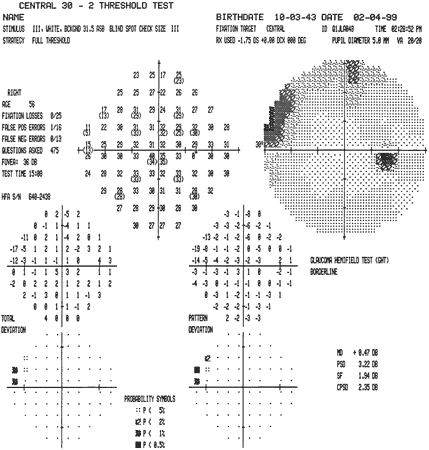
variability. J Glaucoma 9:247, 2000 20. Humphrey Field Analyzer II User's Guide. Dublin, CA: Humphrey Systems, 1998 21. Sekhar GC, Naduvilath TJ, Lakkai M et al: Sensitivity of Swedish Interactive Threshold Algorithm in Humphrey visual
field testing. Ophthalmology 107:1303, 2000 22. Johnson CA, Adams AJ, Casson EJ et al: Blue-on-yellow perimetry can predict the development of glaucomatous visual
field loss. Arch Ophthalmol 111:645, 1993 23. Blumenthal EZ, Sample PA, Zangwill L et al: Comparison of long-term variability for standard and short-wavelength automated
perimetry in stable glaucoma patients. Am J Ophthalmol 129:309, 2000 24. Kelly DH: Nonlinear visual responses to flickering sinusoidal gratings. J Opt Soc Am A 71:1051, 1981 25. Cello KE, Nelson-Quigg JM, Johnson CA: Frequency doubling technology perimetry for detection of glaucomatous visual
field loss. Am J Ophthalmol 129:314, 2000 26. Fellman RC, Lynn JR, Starita RJ et al: Clinical importance of spatial summation
in glaucoma. In: Perimetry Update 1988/89: Proceedings of the
VIII International Perimetric Society Meeting. Amsterdam: Kugler & Ghedini, 1989 27. Wilensky JT, Metmelstein JR, Sietel HG: The use of different sized stimuli in automated perimetry. Am J Ophthalmol 101:710, 1986 28. Stewart WC, Shields MB: The peripheral visual field in glaucoma: Reevaluation in the age of automated
perimetry. Surv Ophthalmol 36:59, 1991 29. Weinreb RN, Perlman JP: The effect of refractive correction on automated
perimetric thresholds. Am J Ophthalmol 101:iO6, 1986 30. Goldstick BJ, Weinreb RN: The effect of refractive error on automated global analysis program Gel. Am J Ophthalmol 104:229, 1987 31. Drance SM, Betty V, Hughes A: Studies on the effects of age on the central and peripheral isopters of
the visual field in normal subjects. Am J Ophthalmol 63:1667, 1967 32. Jaffe GJ, Alvarado JA, Juster RP: Age-related changes of the normal visual field. Arch Ophthalmol 104:1021, 1986 33. Heijl A, Lindgren G, Olsson J: The effect of petimettic experience on normal subjects. Arch Ophthalmol 107:81, 1989 34. Heijl A, Drance SM: Changes in differential threshold in patients with glaucoma during prolonged
perimetry. Br J Ophthalmol 67:512, 1983 35. Katz J, Sommer A, Witt K: Reliability of visual field results over repeated testing. Ophthalmology 98:70, 1991 36. Katz J. Sommer A: Reliability indexes of automated petimetric tests. Arch Ophthalmol 106:1252, 1988 37. Bengtsson B, Heijl A: False-negative responses in glaucoma perimetry: Indicators of patient performance
or test reliability? Invest Ophthalmol Vis Sci 41:2201, 2000. 38. Flammer J, Drance SM, Fankhauser F et al: Differential light threshold in automated static perimetry: Factors influencing
short-term fluctuations. Arch Ophthalmol 102:876, 1984 39. Flammer J. Drance SM, Schulzer M: Covariates of the long-term fluctuation of the differential light threshold. Arch Ophthalmol 102:880, 1984 40. Starita RJ, Piltz JR, Lynn JR et al: Total variance of serial Octopus visual fields in glaucomatous eyes. Doc Ophthalmol 49:85, 1987 41. Heijl A, Asman P: A clinical study of perimetric probability maps. Arch Ophthalmol 107:199, 1989 42. Statpac Users Guide. San Leandro, CA: Allergan Humphrey Instruments, 1987 43. Flammer J, Drance SM, Augustiny L et al: Quantification of glaucomatous visual field defects with automated perimetry. Invest Ophthalmol Vis Sci 26:176, 1985 44. Hirsch J: Statistical analysis in computerized petimetry. In Whalen WR, Spaeth
GL (eds): Computerized Visual Fields: What They Are and How to
Use Them. Thorofare, NJ: Slack, 1985:309 45. Duggan C, Sommer A, Auer C et al: Automated differential threshold perimetry for detecting glaucomatous visual
field loss. Am J Ophthalmol 100:420, 1985 46. Introducing Statpac 2. San Leandro, CA: Humphrey Instruments, 1989 47. Brenton RS, Phelps CD, Rojas P et al: Interocular differences of the visual field in normal subjects. Invest Ophthalmol Vis Sci 27:799, 1986 48. Werner EB, Petrig B, Krupin T et al: Variability of automated visual fields in clinically stable glaucoma patients. Invest Ophthalmol Vis Sci 30:1083, 1988 49. Heidl A, Lindgren A, Lindgren G: Test-retest variability in glaucomatous visual fields. Am J Ophthalmol 108:130, 1989 50. Piltz JR, Starita RJ: Test-retest variables in glaucomatous visual fields [letter]. Am J Ophthalmol 109:109, 1990 51. Holmin C, Krakau CET: Regression analysis of the central visual field in chronic glaucoma cases: A
follow-up study using automated perimetry. Acta Ophthalmol 60:267, 1982 52. Chauhan BC, Drance SM, Douglas GR: The use of visual field indices in detecting changes in the visual field. Invest Ophthalmol Vis Sci 31:512, 1990 53. Heijl A, Lindgren G. Olsson J et al: Visual field interpretation with empiric probability maps. Arch Ophthalmol 107:204, 1989 54. Anderson DR: Automated Static Perimetry. St Louis: Mosby-Year Book, 1992 |