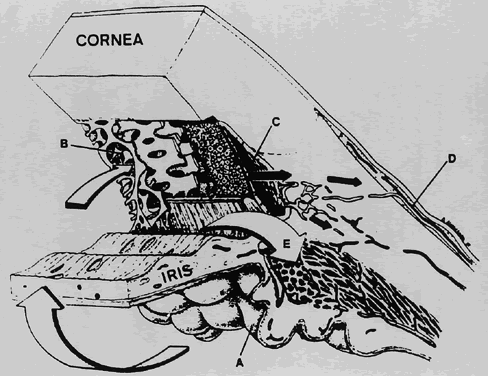
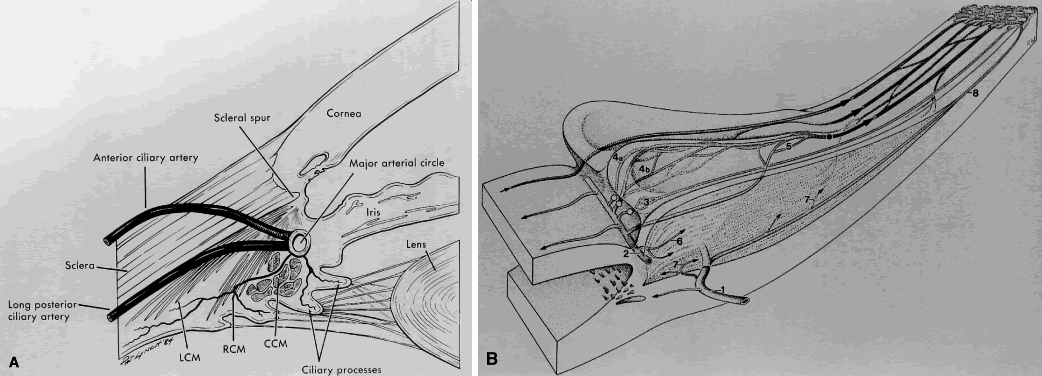
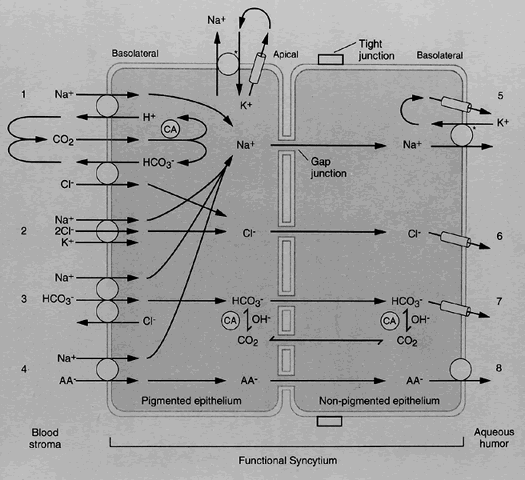
1. Millar C, Kaufman PL: Aqueous humor: Secretion and dynamics. In Tasman
W, Jaeger EA (eds): Duane's Foundations of Clinical Ophthalmology. Philadelphia: Lippincott-Raven, 1995 2. Bennett AG, Rabbetts RB: The schematic eye. In Clinical Visual Optics, p 249. 2nd
ed. London: Butterworths, 1989 3. Kaufman PL: Aqueous humor outflow. In Zadunaisky JA, Davson H (eds): Current
Topics in Eye Research. New York: Academic Press, 1984 4. Bill A: The aqueous humor drainage mechanism in the cynomolgus monkey (Macaca irius) with
evidence for unconventional routes. Invest Ophthalmol 4:911, 1965 5. Kaufman PL, Crawford K: Aqueous humor dynamics: How PGF2a lowers intraocular pressure. Prog Clin Biol Res 312:387, 1989 6. Nilsson SFE, Samuelsson M, Bill A, Stjernschantz J. Increased uveoscleral
outflow as a possible mechanism of ocular hypotension caused by prostaglandin
F2a-1-isopropylester in the cynomolgus monkey. Exp Eye Res 48:707, 1989 7. Sperber GO, Bill A: A method for near-continuous determination of aqueous humor flow: Effects
of anaesthetics, temperature and indomethacin. Exp Eye Res 39:435, 1984 8. Townsend DJ, Brubaker RF: Immediate effect of epinephrine on aqueous formation in the normal human
eye as measured by fluorophotometry. Invest Ophthalmol Vis Sci 19:256, 1980 9. Kaufman PL: Physiology and pharmacology of aqueous humor outflow: Implications
for treatment. Ophthalmol Clin North Am 1991:781 10. Bill A, Phillips I: Uveoscleral drainage of aqueous humor in human eyes. Exp Eye Res 21:275, 1971 11. Bill A: Blood circulation and fluid dynamics in the eye. Physiol Rev 55:383, 1975 12. Bill A: Basic physiology of the drainage of aqueous humor. Exp Eye Res 25(Suppl), 1997 13. Anderson L, Wilson WS: Inhibition by indomethacin of the increased facility of outflow induced
by adrenaline. Exp Eye Res 50:119, 1990 14. Araie M, Takase M: Effects of S-596 and carteolol, new β-adrenergic blockers, and flurbiprofen
on the human eye: A fluorometric study. Graefe's Arch Clin Exp Ophthalmol 222:259, 1985 15. Bárány EH: Topical epinephrine effects on true outflow resistance and pseudofacility
in vervet monkeys studied by a new anterior chamber perfusion technique. Invest Ophthalmol 7:88, 1968 16. Bill A: Early effects of epinephrine on aqueous humor dynamics in vervet
monkeys (Cercopithecus ethiops) Exp Eye Res 8:35, 1969 17. Bill A: Effects of norepinephrine, isoproterenol and sympathetic stimulation on
aqueous humour dynamics in vervet monkeys. Exp Eye Res 10:31, 1970 18. Bonomi L, Steindler P: Effect of pindolol on intraocular pressure. Br J Ophthalmol 59:301, 1975 19. Bouzoubaa M, Leclerc G, Decker N et al: Synthesis and β-adrenergic blocking activity of new aliphatic and
alicyclic oxime ethers. J Med Chem 27:1291, 1984 20. Coakes RL, Brubaker RF: The mechanism of timolol in lowering intraocular pressure. Arch Ophthalmol 96:2045, 1978 21. Dailey RA, Brubaker RF, Bourne WM: The effects of timolol maleate and acetazolamide on the rate of aqueous
formation in normal human subjects. Am J Ophthalmol 93:232, 1982 22. Kaufman PL, Bárány EH: Recent observations concerning the
effects of cholinergic drugs on outflow facility in monkeys. Exp Eye
Res 25(Suppl), 1997 23. Main BG, Tucker H: Recent advances in beta-adrenergic blocking agents. In
Ellis GP, West GB (eds): Progress in Medicinal Chemistry, p 121. Amsterdam: Elsevier, 1985 24. Mills KB, Wright G: A blind randomized cross-over trial comparing metipranolol 0.3% with timolol 0.25% in
open-angle glaucoma: A pilot study. Br J Ophthalmol 70:39, 1986 25. Murray DL, Podos SM, Wei C et al: Ocular effects in normal rabbits of topically applied labetalol, a combined α- and β-adrenergic antagonist. Arch Ophthalmol 97:723, 1979 26. Nathanson JA: Biochemical and physiological effects of S-32-468, a new β-adrenoceptor
antagonist with possible oculoselectivity. Curr Eye Res 4:191, 1985 27. Novack GD: Ophthalmic β-blockers since timolol. Surv Ophthalmol 31:307, 1987 28. Phillips CI, Howitt G, Rowlands DJ: Propranolol as ocular hypotensive agent. Br J Ophthalmol 51:222, 1967 29. Schenker JI, Yablonski ME, Podos SM, Linder L. Fluorophotometric study
of epinephrine and timolol in human subjects. Arch Ophthalmol 99:1212, 1981 30. Sears ML, Neufeld AH: Adrenergic modulation of the outflow of aqueous humor. Invest Ophthalmol 14:83, 1975 31. Stewart RH, Kimbrough RL, Ward RL: Betaxolol vs. timolol. A six-month double-blind comparison. Arch Ophthalmol 104:46, 1986 32. Zimmerman TJ: Topical ophthalmic beta blockers. A comparative review. J Ocul Pharmacol 9:373, 1993 33. Barnett NL, Osborne NN: The presence of serotonin (5-HT1) receptors negatively coupled to adenylate
cyclase in rabbit and human ciliary processes. Exp Eye Res 57:209, 1993 34. Chang FW, Burke JA, Potter DE: Mechanism of the ocular hypotensive action of ketanserin. J Ocul Pharmacol 1:137, 1985 35. Krootila K, Palkama A, Uusitalo H: Effect of serotonin and its antagonist (ketanserin) on intraocular pressure
in the rabbit. J Ocul Pharmacol 3:279, 1987 36. Meyer-Bothling U, Bron AJ, Osborne NN: Topical application of serotonin or the 5-HT1a-agonist 5-CT increases intraocular
pressure in rabbits. Invest Ophthalmol Vis Sci 34:3035, 1993 37. Chiou GCY: Ocular hypotensive actions of haloperidol, a dopaminergic antagonist. Arch Ophthalmol 102:143, 1984 38. Chiou GCY: Treatment of ocular hypertension and glaucoma with dopamine antagonists. Ophthalmic Res 16:129, 1984 39. De Vries GW, Mobasser A, Wheeler LA: Stimulation of endogenous cyclic AMP levels in ciliary body by SK&F28526, a
novel dopamine receptor agonist. Curr Eye Res 5:449, 1986 40. Geyer O, Robinson D, Lazar M: Hypotensive effect of bromocriptine in normal eyes. J Ocul Pharmacol 3:291, 1987 41. Langer SZ: Presynaptic regulation of the release of catecholamines. Pharmacol Rev 32:337, 1980 42. Leopold IH, Duzman E: Observations on the pharmacology of glaucoma. Ann Rev Pharmacol Toxicol 26:401, 1986 43. Mekki QA, Hassan SM, Turner P: Bromocriptine lowers intraocular pressure without affecting blood pressure. Lancet 1:1250, 1983 44. Mekki QA, Warrington SJ, Turner P: Bromocriptine eye-drops lower intraocular pressure without affecting prolactin
levels. Lancet 1:287, 1984 45. Potter DE, Burke JA, Chang FW: Ocular hypotensive action of ergoline derivatives in rabbits: Effects of
sympathectomy and domperidone pretreatment. Curr Eye Res 3:307, 1984 46. Siegel MJ, Lee PY, Podos SM et al: Effect of topical pergolide on aqueous dynamics in normal and glaucomatous
monkeys. Exp Eye Res 44:227, 1987 47. Crosson CE, Gray T: Adenosine agonists alter aqueous flow and outflow facility in rabbits. Invest Ophthalmol Vis Sci 38(ARVO Abstracts):S821, 1997. 48. Crosson CE: Adenosine receptor activation modulates intraocular pressure in rabbits. J Pharmacol Exp Ther 273:320, 1995 49. Crosson CE, Gray T: Modulation of intraocular pressure by adenosine agonists. J Ocul Pharmacol 10:379, 1994 50. Crosson CE: Ocular hypotensive activity of the adenosine agonist (R)-phenylisopropyladenosine
in rabbits. Curr Eye Res 11:453, 1992 51. Crosson CE, Heath AR, DeVries GW, Potter DE. Pharmacological evidence for
heterogeneity of ocular a2 adrenoceptors. Curr Eye Res 11:963, 1992 52. Tian B, Gabelt BT, Crosson CE, Kaufman PL. Effects of adenosine agonists
on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp
Eye Res 64:979, 1997 53. Alm A, Widengard I, Kjellgren D et al. Latanoprost administered once daily
caused a maintained reduction of intraocular pressure in glaucoma
patients treated concomitantly with timolol. Br J Ophthalmol 79:12, 1995 54. Bito LZ, Stjernschantz J, Resul B et al: The ocular effects of prostaglandins and the therapeutic potential of a
new PGF2α analog, PhXA41 (latanoprost), for glaucoma management. J Lipid Mediat 6:535, 1993 55. Camras CB, Alm A, Watson P, Stjernschantz J: Latanoprost, a prostaglandin analog, for glaucoma therapy. Ophthalmology 103:1916, 1996 56. Crawford K, Kaufman PL, True-Gabelt B: Prostaglandins and aqueous humor
dynamics. In Shields MB, Pollack, IP, Kolker AE (eds): Perspectives in
Glaucoma, p 259. Transactions of the First Scientific Meeting of the
American Glaucoma Society. Thorofare, NJ: Slack, 1988 57. Gabelt BT, Kaufman PL: Prostaglandin F2a increases uveoscleral outflow in the cynomolgus monkey. Exp Eye Res 49:389, 1989 58. Giuffré G: The effects of prostaglandin F2a in the human eye. Graefe's Arch Clin Exp Ophthalmol 222:139, 1985 59. Hayashi M, Yablonski ME, Bito LZ: Eicosanoids as a new class of ocular hypotensive agents. 2. Comparison
of the apparent mechanism of the ocular hypotensive effects of A and F
type prostaglandins. Invest Ophthalmol Vis Sci 28:1639, 1987 60. Kashiwagi K, Lindsey JD, Kashiwagi F, Weinreb RN: Extracellular matrix changes induced by prostaglandins in cultured human
ciliary muscle cells. Invest Ophthalmol Vis Sci 36(ARVO Abstract):S729, 1995 61. Kerstetter JR, Brubaker RF, Wilson SE, Kullerstrand LJ: Prostaglandin F2a-1-isopropylester lowers intraocular pressure without decreasing aqueous
flow. Am J Ophthalmol 105:30, 1988 62. Lee P-Y, Shoo H, Xu L, Qu C-K: The effect of prostaglandin F2a on intraocular pressure in normotensive human subjects. Invest Ophthalmol Vis Sci 29:1474, 1988 63. Lindsey JD, Kashiwagi K, Kashiwagi F, Weinreb RN: Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: Implications
for uveoscleral outflow. Surv Ophthalmol 41(Suppl 2):S53–S59, 1997 64. Lütjen-Drecoll E, Tamm E: Morphological study of the anterior segments of cynomolgus monkey eyes
following treatment with prostaglandin F2a. Exp Eye Res 47:761, 1988 65. Poyer JF, Millar C, Kaufman PL: Prostaglandin F2a effects on isolated rhesus monkey ciliary muscle. Invest Ophthalmol Vis Sci 36:2461, 1995 66. Toris CB, Camras CB, Yablonski ME: Effects of PhXA41, a new prostaglandin F2 alpha humor dynamics in human
eyes [see comments]. Ophthalmology 100:1297, 1993 67. Ziai N, Dolan JW, Kacere RD, Brubaker RF: The effects on aqueous dynamics of PhXA41, a new prostaglandin F2a analogue, after
topical application in normal and ocular hypertensive human
eyes. Arch Ophthalmol 111:1351, 1993 68. Seidël E: Weitre experimentelle Untersuchungen über die Quelle und den Verlauf
der introkulären Saftsrömung. XII. Metteilung. Uber den manometrischen
Nachweis des physiologischen Druckgefalles zwishen Vorderkammer
und Schlemmshen Kanal. Graefe's Arch Clin Exp Ophthalmol 107:101, 1921 69. Seidël E: Weitre experimentelle Untersuchungen über die Quelle und den Verlauf
der introkulären Saftströmung. IX. Uber der Abfluss des Kammerwassers
aus der vorderen Augenkammer. Graefe's Arch Clin Exp Ophthalmol 104:357, 1921 70. Ascher KW: Aqueous veins: Preliminary note. Am J Ophthalmol 25:31, 1942 71. Ashton N: Anatomical study of Schlemm's canal and aqueous veins by means of
neoprene casts: Part I. Aqueous veins. Br J Ophthalmol 35:291, 1952 72. Goldmann H: Enhalten die Kammervasservener Kammervasser? Ophthalmologica. 117:240, 1949 73. Knutson SL, Sears ML: Herman Boerhaave and the history of vessels carrying aqueous humor from
the eye. Am J Opthalmol 76:648, 1973 74. Bill A: The role of ciliary blood flow and ultrafiltration in aqueous humor formation. Exp Eye Res 16:287, 1973 75. Brodwell J, Fischbarg J: The hydraulic conductivity of rabbit ciliary epithelium in vitro. Exp Eye Res 34:121, 1982 76. Caprioli J: The ciliary epithelia and aqueous humor. In Hart WM (ed): Adler's
Physiology of the Eye: Clinical Application, p 228. St. Louis: CV
Mosby—Year Book, 1992 77. Cole DF: Effects of some metabolic inhibitors upon the formation of the aqueous
humor in rabbits. Br J Ophthalmol 44:739, 1960 78. Cole DF: Secretion of the aqueous humor. Exp Eye Res 25(Suppl):161, 1977 79. Green K, Pederson JE: Contribution of secretion and filtration to aqueous humor formation. Am J Physiol 222:1218, 1972 80. Maren TH: HCO3-formation in aqueous humor: Mechanism and relation to the treatment
of glaucoma. Invest Ophthalmol Vis Sci 13:479, 1974 81. Pederson JE: Fluid permeability of monkey ciliary epithelium in vivo. Invest Ophthalmol Vis Sci 23:176, 1982 82. Wilson WS, Shahidullay M, Millar C: The bovine arterially-perfused eye: An in vitro method for the study of
drug mechanisms on IOP, aqueous humour formation and uveal vasculature. Curr Eye Res 12:609, 1993 83. Goldmann H: On pseudofacility. Bibl Ophthalmol 76:1, 1968 84. Bárány EH: A mathematical formulation of intraocular pressure as dependent on secretion, ultrafiltration, bulk outflow, and osmotic reabsorption of fluid. Invest Ophthalmol 2:584, 1963 85. Bárány EH: Pseudofacility and uveoscleral outflow routes: Some
nontechnical difficulties in the determination of outflow facility
rate and rate of formation of aqueous humor. In Leydhecker W, (ed): Glaucoma
Symposium, Tutzing Castle, p 27. Basel: Karger, 1966 86. Bill A: Further studies on the influence of the intraocular presssure on aqueous
humor dynamics in cynomolgus monkeys. Invest Ophthamol Vis Sci 6:364, 1967 87. Bill A: Effects of longstanding stepwise increments in eye pressure on the rate
of aqueous humor formation in a primate (Cercopithecus ethiops). Exp Eye Res 12:184, 1971 88. Bill A, Bárány EH: Gross facility, facility of conventional routes, and pseudofacility of
aqueous humor outflow in the cynomolgus monkey. Arch Ophthalmol 75:665, 1966 89. Kaufman PL, Wiedman T, Robinson JR: Cholinergics. In Sears ML (ed): Handbook
of Experimental Pharmacology, p 149. Berlin: Springer-Verlag, 1984 90. Kaufman PL, Bill A, Bárány EH: Formation and drainage of aqueous humor following total iris removal and
ciliary muscle disinsertion in the cynomolgus monkey. Invest Ophthalmol Vis Sci 16:226, 1977 91. Gaasterland D, Kupfer C, Ross K: Studies of aqueous humor dynamics in man. IV. Effects of pilocarpine upon
measurements in young normal volunteers. Invest Ophthalmol 14:848, 1975 92. Pederson JE, Gaasterland DE, MacLellan HM: Anterior chamber volume determination in the rhesus monkey. Invest Ophthalmol Vis Sci 17:784, 1978 93. Johnson F, Maurice D: A simple method of measuring aqueous humor flow with intravitreal fluoresceinated
dextrans. Exp Eye Res 39:791, 1984 94. Erickson KA, Gonnering RS, Kaufman PL, Dortzbach RK. The cynomolgus monkey
as a model for orbital research. III. Effects on ocular physiology
of lateral orbitotomy and isolation of the ciliary ganglion. Curr Eye
Res 3:557, 1984 95. Colasanti BK: Aqueous humor dynamics in the enucleated deer eye. Comp Biochem 78:755, 1985 96. Bill A: Aqueous humor dynamics in monkeys (Macaca irus and Cercopithecus ethiops). Exp Eye Res 11:195, 1971 97. Yablonski ME, Zimmerman TJ, Waltman SR: A fluorophotometric study of the effect of topical timolol on aqueous humor
dynamics. Exp Eye Res 27:135, 1978 98. Topper JE, Brubaker RF: Effects of timolol, epinephrine, and acetazolamide on aqueous flow during
sleep. Invest Ophthalmol Vis Sci 26:1315, 1985 99. Krupin T, Civan MM: Physiologic basis of aqueous humor formation. In Ritch
R, Shields MB, Krupin T (eds): The Glaucomas, p 251. 2nd ed. St. Louis: Mosby, 1996 100. Gharagozloo NZ, Larson RS, Kullerstrand W: Terbutaline stimulates aqueous humor flow in humans during sleep. Arch Ophthalmol 106:1218, 1988 101. Davson H: The aqueous humor and the intraocular pressure. In Davson H (ed): Physiology
of the Eye, p 3. New York: Pergamon Press, 1990 102. Hart WM: Intraocular pressure. In Hart WM (ed): Adler's Physiology
of the Eye, p 248. 9th ed. St. Louis: Mosby, 1992 103. Millar JC, Carr RD, Humphries RG, Wilson WS: Drug effects on intraocular pressure and vascular flow in the bovine perfused
eye using radiolabelled microspheres. J Ocul Pharmacol Ther 11:11, 1995 104. Nilsson SFE, Bill A: Physiology and neurophysiology of aqueous humor inflow
and outflow. In Kaufman PL, Mittag TW (eds): Glaucoma, p 1.17. London: Mosby—Year
Book, 1994 105. Pederson JE, Green K: Aqueous humor dynamics: A mathematical approach to measurement of facility, pseudofacility, capillary pressure, active secretion and Xc. Exp Eye Res 15:265, 1973 106. Ballintine EJ, Maren TH: Carbonic anhydrase activity and the distribution of Diamox in the rabbit
eye. Am J Ophthalmol 40:148, 1955 107. Bhattacherjee P: Distribution of carbonic anhydrase in the rabbit eye as demonstrated histochemically. Exp Eye Res 12:356, 1971 108. Bonting SL, Becker B: Studies on sodium-potassium activated adenosinetriphosphatase: XIV. Inhibition
of enzyme activity and aqueous humor flow in the rabbit eye after
intravitreal injection of ouabain. Invest Ophthalmol 3:523, 1964 109. Bonting SL, Simon KA, Hawkins NM: Studies on sodium-potassium-activated adenosine triphosphatase. I. Quantitative
distribution in several tissues of the cat. Arch Biochem 95:416, 1961 110. Coca-Prados M, Lopez-Briones LG: Evidence that the a and the a(+ ) isoforms of the catalytic subunit
of (Na+ , K+ )-ATPase reside in distinct ciliary epithelial
cells of the mammalian eye. Biochem Biophys Res Commun 145:460, 1987 111. Flügel C, Lütjen-Drecoll E: Presence and distribution of Na+ /K+ -ATPase in the ciliary epithelium
of the rabbit. Histochemistry 88:613, 1988 112. Mishima H, Searx M, Bausher L, Gregory D: Ultracytochemistry of cholera toxin binding sites in ciliary processes. Cell Tissue Res 223:241, 1982 113. Riley MV: The sodium-potassium-stimulated adenosine triphosphatase of rabbit ciliary
epithelium. Exp Eye Res 3:76, 1964 114. Riley MV, Kishida K: ATPases of ciliary epithelium: Cellular and subcellular distribution and
probable role in secretion of aqueous humor. Exp Eye Res 42:559, 1986 115. Tsukahara S, Maezara N: Cytochemical localization of adenyl cyclase in the rabbit ciliary body. Exp Eye Res 26:99, 1978 116. Usukura J, Fain GL, Bok D: [3H]Ouabain localization of Na-K ATPase in the epithelium of
the rabbit ciliary body pars plicata. Invest Ophthalmol Vis Sci 29:606, 1988 117. Wistrand PJ: Carbonic anhydrase in the anterior uvea of the rabbit. Acta Physiol Scand 24:144, 1951 118. White A, Handler P, Smith EL: Introduction to metabolism. In White A, Handler
P, Smith EL (eds): Principles of Biochemistry, p 279. New York: McGraw-Hill, 1973 119. Becker B: Vanadate and aqueous humor dynamics. Proctor Lecture. Invest Ophthalmol Vis Sci 19:1156, 1980 120. Maren TH: Biochemistry of aqueous humor inflow. In Kaufman PL, Mittag TW (eds): Glaucoma, p 1.35. London: Mosby—Year Book, 1994 121. Becker B: Ouabain and aqueous humor dynamics in the rabbit eye. Invest Ophthalmol 2:325, 1963 122. Krupin T, Becker B, Podos SM: Topical vanadate lowers intraocular pressure in rabbits. Invest Ophthalmol Vis Sci 19:1360, 1980 123. Podos SM, Lee PY, Severin C, Mittag T: The effect of vanadate on aqueous humor dynamics in cynomolgus monkeys. Invest Ophthalmol Vis Sci 25:359, 1984 124. Simon KA, Bonting SL: Possible usefulness of cardiac glycosides in treatment of glaucoma. Arch Ophthalmol 68:227, 1962 125. Korenbrot JI: Ion transport in membranes: Incorporation of biological ion-translocating
proteins in model membrane systems. Ann Rev Physiol 39:19, 1977 126. Simon KA, Bonting SL, Hawkins NM: Studies on sodium-potassium-activated adenosine triphosphatase. II. Formation
of aqueous humor. Exp Eye Res 1:253, 1962 127. Lütjen-Drecoll E, Lonnerholm G: Carbonic anhydrase distribution in the rabbit eye by light and electron
microscopy. Invest Ophthalmol Vis Sci 21:782, 1981 128. Lütjen-Drecoll E, Lonnerholm G, Eichhorn M: Carbonic anhydrase distribution in the human and monkey eye by light and
electron microscopy. Graefe's Arch Clin Exp Ophthalmol 220:285, 1983 129. Maren TH: Carbonic anhydrase: Chemistry, physiology, and inhibition. Physiol Rev 47:595, 1967 130. Maren TH: Bicarbonate formation in cerebrospinal fluid: Role in sodium transport
and pH regulation. Am J Physiol 222:885, 1972 131. Mudge GH, Weiner IM: Agents affecting volume and composition of body fluids. In
Gilman AG, Rall TW, Nies AS, Taylowr P (eds): The Pharmacological
Basis of Therapeutics, p 682. 8th ed. New York: McGraw-Hill, 1990 132. Muther TF, Friedland BR: Autoradiographic localization of carbonic anhydrase in the rabbit ciliary
body. J Histochem Cytochem 28:1119, 1980 133. Friedenwald JS: The formation of the intraocular fluid. Proctor Award Lecture of the Association
for Research in Ophthalmology. Am J Ophthalmol 32:9, 1949 134. Becker B: The mechanism of the fall in intraocular pressure induced by the carbonic
anhydrase inhibitor Diamox. Am J Ophthalmol 39:177, 1955 135. Friedenwald JS: Carbonic anhydrase inhibition and aqueous flow. Am J Ophthalmol 39:59, 1955 136. Friedenwald JS: Current studies on acetazolamide (Diamox) and aqueous humor flow. Am J Ophthalmol 40:139, 1955 137. Kaufman PL, Mittag TW: Medical therapy of glaucoma. In Kaufman PL, Mittag
TW (eds): Glaucoma, p 9.7. London: Mosby—Year Book, 1994 138. Maren TH, Mayer E, Wadsworth BC: Carbonic anhydrase inhibition. I. The pharmacology of Diamox (1-acetylamino 1,3,4-thiadiazole-5-sulfonamide). Bull Johns Hopk Hosp 95:199, 1954 139. Maren TH: The development of ideas concerning the role of carbonic anhydrase
in the secretion of aqueous humor: Relations to the treatment of
glaucoma. In Drance SM, Neufeld AH (eds): Glaucoma, p 325. Applied Pharmacology
in Medical Treatment. Orlando: Grune & Stratton, 1984 140. Eller MG, Schoenwald RD, Dixson JA: Topical carbonic anhydrase inhibitors. III. Optimization model for corneal
penetration of ethoxzolamide analogues. J Pharm Sci 74:155, 1985 141. Maren TH, Jankowska L, Sanyal G, Edelhauser HF: The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors
and their effect on aqueous humor secretion. Exp Eye Res 36:457, 1983 142. Becker B: Decrease in intraocular pressure in man by a carbonic anhydrase inhibitor, Diamox. Am J Ophthalmol 37:13, 1954 143. Hoskins HD Jr, Kass MA: Aqueous humor formation. In Klein EA (ed): Becker-Shaffers's
Diagnosis and Therapy of the Glaucomas, p 18. 6th ed. St. Louis: CV
Mosby, 1989 144. Pierce WMJ, Sharir M, Waite KJ: Topically active ocular carbonic anhydrase inhibitors-novel biscarbonylamidothiadiazole
sulfonamides as ocular hypotensive agents. Proc Soc Exp Biol Med 203:360, 1993 145. Schoenwald RD, Eller MG, Dixson JA, Barfknecht CF: Topical carbonic anhydrase inhibitors. J Med Chem 27:810, 1984 146. Sugrue MF, Gautheron P, Grove J: MK-927: A topically active ocular hypotensive carbonic anhydrase inhibitor. J Ocul Pharmacol 6:9, 1990 147. Sugrue MF, Mallorga P, Schwamm H: A comparison of L-671,152 and MK927, two topically effective ocular hypotensive
carbonic anhydrase inhibitors, in experimental animals. Curr Eye Res 9:607, 1990 148. Woltersdorf OWJ, Schwam H, Bicking JB: Topically active carbonic anhydrase inhibitors. I. O-acyl derivatives of 6-hydroxybenzothiazole-2-sulfonamide. J Med Chem 32:2486, 1989 149. Hoskins HD Jr, Kass MA: Medical treatment. In Hoskins HD Jr, Kass MA (eds): Becker-Shaffer's
Diagnosis and Therapy of the Glaucomas, p 405. 6th
ed. St. Louis: CV Mosby, 1989 150. Kaufman PL: Aqueous humor dynamics. In Duane TD (ed): Clinical Ophthalmology. Philadelphia: Harper & Row, 1985 151. Maren TH, Haywood JR, Chapman SK: The pharmacology of methazolamide in relation to the treatment of glaucoma. Invest Ophthalmol Vis Sci 16:730, 1977 152. Gunning FP, Greve EL, Bron AM et al: Two topical carbonic anhydrase inhibitors sezolamide and dorzolamide in
Gelrite vehicle: A multiple-dose efficacy study. Graefes Arch Clin Exp Ophthalmol 231:384, 1993 153. Lippa EA, Carlson LE, Ehinger B et al: Dose response and duration of action of dorzolamide, a topical carbonic
anhydrase inhibitor. Arch Ophthalmol 110:495, 1992 154. Wang RF, Serle JB, Podos SM, Sugrue MF: The ocular hypotensive effect of the topical carbonic anhydrase inhibitor
L-671,152 in glaucomatous monkeys. Arch Ophthalmol 108:511, 1990 155. Wilkerson M, Cyrlin M, Lippa EA et al: Four-week safety and efficacy study of dorzolamide, a novel, active topical
carbonic anhydrase inhibitor. Arch Ophthalmol 111:1343, 1993 156. DiMatteo J: Active transport of ascorbic acid into lens epithelium of the rat. Exp Eye Res 49:873, 1989 157. Fielder AR, Rahi AHS: Immunoglobulins of normal aqueous humor. Trans Ophthalmol Soc UK 99:120, 1979 158. Ghose T, Quigley JH, Landrigan PL, Asif A: Immunoglobulins in aqueous humor and iris from patients with endogenous
uveitis and patients with cataract. Br J Ophthalmol 57:897, 1973 159. Krause U, Raunio V: Proteins of the normal human aqueous humor. Ophthalmologica 159:178, 1969 160. Mondino BJ, Rao H: Complement levels in normal and inflamed aqueous humor. Invest Ophthalmol Vis Sci 24:380, 1983 161. Sen DK, Saren GS, Saha K: Immunoglobulins in human aqueous humor. Br J Ophthalmol 61:216, 1977 162. Stjernschantz J, Uusitalo R, Palkama A: The aqueous proteins of the rat in the normal eye and after aqueous withdrawal. Exp Eye Res 16:215, 1973 163. Bill A: The drainage of albumin from the uvea. Exp Eye Res 3:179, 1964 164. Bill A: Capillary permeability to and extravascular dynamics of myoglobin, albumin, and
gammaglobulin in the uvea. Acta Physiol Scand 73:204, 1968 165. Alm A: Ocular circulation. In Hart WMJ (ed): Adler's Physiology of
the Eye, p 198. 9th ed. St. Louis: Mosby—Year Book, 1992 166. Hogan MJ, Alvarado JA, Weddell JE: Ciliary body and posterior chamber. In
Hogan MJ, Alvarado JA, Weddell JE (eds): Histology of the Human Eye, p 260. Philadelphia: W
B Saunders, 1971 167. Rodriguez-Peralta L. The blood-aqueous barrier in five species. Am J Ophthalmol 80:713, 1975 168. Caprioli J: The ciliary epithelia and aqueous humor. In Hart WM (ed): Adler's
Physiology of the Eye: Clinical Application, p 228. St. Louis: Mosby—Year
Book, 1992 169. Shiose Y: Electron microscopic studies on blood-retinal and blood-aqueous barriers. Nippon Ganka Gakkai Zasshi- Acta Societatis Ophthalmologicae Japonicae 73:1606, 1969 170. Smith RS: Ultrastructural studies of the blood-aqueous barrier. I. Transport of an
electron dense tracer in the iris and ciliary body of the mouse. Am J Ophthalmol 71:1066, 1971 171. Uusitalo R, Palkama A, Stjernschantz J: An electron microscopic study of the blood-aqueous barrier in the ciliary
body and iris of the rabbit. Exp Eye Res 17:49, 1973 172. Uusitalo R, Stjernschantz J, Palkama A: Studies on the ultrastructure of the blood-aqueous barrier in the rabbit. Acta Ophthalmol 123:61, 1974 173. Vegge T: An epithelial blood-aqueous barrier to horseradish peroxidase in the ciliary
processes of the vervet monkey (Cercopithecus aethiops). Zeitschr Zellforsch Mikrosk Anat 114:309, 1971 174. Raviola G: Effects of paracentesis on the blood-aqueous barrier: An electron microscopic
study on Macaca mullata using horseradish peroxidase as a tracer. Invest Ophthalmol Vis Sci 13:828, 1974 175. Masuda M, Mishima: Effects of prostaglandins on inflow and outflow of the aqueous in rabbits. Jpn J of Ophthamol 17:300, 1973 176. Bill A: The effect of ocular hypertension caused by red cells on the rate of formation
of aqueous humor. Invest Ophthalmol Vis Sci 7:162, 1968 177. Toris CB, Pederson JE: Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci 28:477, 1987 178. Kaufman PL, Crawford K, Gabelt BT: The effects of prostaglandins on aqueous
humor dynamics. Ophthalmol Clin North Am 1989:141 179. Kaufman PL, Gabelt BT: Presbyopia, prostaglandins and primary open angle
glaucoma. In Krieglstein GK (ed): Glaucoma Update. V. Proceedings of
the Symposium of the Glaucoma Society of the International Congress of
Ophthalmology in Quebec City, June 1994, p 224. New York-Berlin: Springer-Verlag, 1995 180. Kaufman PL: Pressure-dependent outflow. In Ritch R, Shields MB, Krupin
T (eds): The Glaucomas, p 307. St. Louis: Mosby—Year Book, 1996 181. Bárány EH: Inhibition by hippurate and probenecid of in vitro uptake of iodipamide
and o-iodohippurate-composite uptake system for iodipamide in choroid
plexus, kidney cortex, and anterior uvea of several species. Acta Physiol Scand 86:12, 1972 182. Bárány EH: The liver-like anion transport system in rabbit kidney, uvea, and choroid
plexus. II. Efficiency of acidic drugs and other anions as inhibitors. Acta Physiol Scand 88:491, 1973 183. Bárány EH: The liver-like anion transport system in rabbit kidney, uvea, and choroid
plexus. I. Selectivity of some inhibitors, direction of transport, possible
physiological substrates. Acta Physiol Scand 88:412, 1973 184. Bárány EH: Bile acids as inhibitors of the liver-like anion transport system in the
rabbit kidney, uvea, and choroid plexus. Acta Physiol Scand 92:195, 1974 185. Bárány EH: In vitro uptake of bile acids by choroid plexus, kidney cortex, and anterior
uvea. I. The iodipamide sensitive transport systems in the rabbit. Acta Physiol Scand 93:250, 1975 186. Becker B: The transport of organic anions by the rabbit eye. I. In vitro iodopyracet (Diodrast) accumulation by ciliary body-iris preparations. Am J Ophthalmol 50:862, 1960 187. Becker B: Iodide transport by the rabbit eye. Am J Physiol 200:804, 1961 188. Bito LZ, Salvador EV: Intraocular fluid dynamics. III. The site and mechanism of prostaglandin
transfer across the blood intraocular fluid barriers. Exp Eye Res 14:233, 1972 189. Duke-Elder S: The aqueous humor. In Duke-Elder S (ed): The Physiology of
the Eye and of Vision, p 104. System of Ophthalmology. Vol 4. St. Louis: CV
Mosby, 1968 190. Forbes M, Becker B: The transport of organic anions by the rabbit eye. II. In vivo transport
of iodopyracet (Diodrast). Am J Ophthalmol 50:867, 1960 191. Stone RA: The transport of para-aminohippuric acid by the ciliary body and by the
iris of the primate eye. Invest Ophthalmol Vis Sci 18:807, 1979 192. Stone RA: Cholic acid accumulation by the ciliary body and by the iris of the primate
eye. Invest Ophthalmol Vis Sci 18:819, 1979 193. Bito LZ: Species differences in the response of the eye to irritation and trauma: A
hypothesis of divergence in ocular defense mechanisms, and the choice
of experimental animals for eye research. Exp Eye Res 39:807, 1984 194. Bito LZ: Prostaglandins: Old concepts and new perspectives. Arch Ophthalmol 105:1036, 1987 195. Bito L, Davson H, Salvador EV: Inhibition of in vitro concentrative prostaglandin accumulation by prostaglandins, prostaglandin
analogues, and by some inhibitors of organic
anion transport. J Physiol 256:257, 1976 196. Bito LZ: Accumulation and apparent active transport of prostaglandins by some rabbit
tissues in vitro. J Physiol 221:371, 1972 197. Bito LZ: Comparative study of concentrative prostaglandin accumulation by various
tissues of mammals and marine vertebrates and invertebrates. Comp Biochem Physiol 43:65, 1972 198. Bárány EH: Organic cation uptake in vitro by the rabbit iris-ciliary body, renal cortex, and
choroid plexus. Invest Ophthalmol 15:341, 1976 199. Bárány EH: Elimination of organic cations from the eye. Klinische Monatsblatter fur Augenheilkunde 184:290, 1984 200. Ehinger B: Adrenergic nerves to the eye and to related structures in man and in the
cynomolgus monkey (Macaca irus). Invest Ophthalmol Vis Sci 5:42, 1966 201. Ehinger B: A comparative study of the adrenergic nerves to the anterior segment of
some primates. Z Zellforschung 116:157, 1971 202. Laties AMD, Jacobowitz D: A comparative study of the autonomic innervation of the eye in monkey, cat
and rabbit. Anat Rec 156:383, 1966 203. Neufeld AH, Bartels SP: Receptor mechanisms for epinephrine and timolol. In
Lütjen-Drecoll E (ed): Basic Aspects of Glaucoma Research, p 113. Stuttgart: Schattauer-Verlag, 1982 204. Ruskell GL: Innervation of the anterior segment of the eye. In Lütjen-Drecoll
E (ed): Basic Aspects of Glaucoma Research, p 49. Stuttgart: Schattauer, 1982 205. Bryson JM, Wolter JR, O'Keefe NT: Ganglion cells in the human ciliary body. Arch Ophthalmol 75:57, 1966 206. Macri FJ, Cevario SJ: A possible vascular mechanism for the inhibition of aqueous humor formation
by ouabain and acetazolamide. Exp Eye Res 20:563, 1975 207. Barbelivian A, MacKenzie ET, Dauphin F: Regional cerebral blood flow responses to neurochemical stimulation of
the substantia innominata in the anesthetized rat. Neurosci Lett 190:81, 1995 208. Burlington RF, Milsom WK: Differential effects of acetylcholine on coronary flow in isolated hypothermic
hearts from rats and ground squirrels. J Exp Biol 185:17, 1993 209. Sato A, Sato Y: Cholinergic neural regulation of regional cerebral blood flow. Alzheimer Dis Assoc Disord 9:28, 1995 210. Alm A, Bill A, Young FA: The effects of pilocarpine and neostigmine on the blood flow through the
anterior uvea in monkeys. A study with radioactively labelled microspheres. Exp Eye Res 15:31, 1973 211. Bill A: Autonomic control of uveal blood flow. Acta Physiol Scand 56:70, 1962 212. Gartner S: Blood vessels of the conjunctiva. Arch Ophthalmol 32:464, 1944 213. Wilke K: Early effects of epinephrine and pilocarpine on the intraocular pressure
and the episcleral venous pressure in the normal human eye. Acta Ophthalmol 52:231, 1974 214. Janes RG, Calkins JP: Effect of certain drugs on iris vessels. Arch Ophthalmol 57:414, 1957 215. Yang S, Silldorf EP, Pallone TL: Effect of norepinephrine and acetylcholine on outer medullary descending
vasa recta. Am J Physiol 269:H710–H716, 1995 216. Bill A, Stjernschantz J: Cholinergic vasoconstrictor effects in the rabbit eye: Vasomotor effects
of pentobarbital anesthesia. Acta Physiol Scand 108:419, 1980 217. Nilsson S, Sperber GO, Bill A: Effects of vasoactive intestinal polypeptide (VIP) on intraocular pressure, facility
of outflow and formation of aqueous humor in the monkey. Exp Eye Res 43:849, 1986 218. Ruskell GL: Facial parasympathetic innervation of the choroidal blood vessels in monkeys. Exp Eye Res 12:166, 1971 219. Hoskins HD, Kass MA: Cholinergic drugs. In Hoskins HD, Kass MA (eds): Becker-Shaffer's
Diagnosis and Therapy of the Glaucomas, p 420. St. Louis: CV
Mosby, 1989 220. Becker B: The measurement of rate of aqueous flow with iodide. Invest Ophthalmol Vis Sci 1:52, 1962 221. Swan K, Hart W: A comparative study of the effects of mecholyl, doryl, pilocarpine, atropine, and
epinephrine on the blood-aqueous barrier. Am J Ophthalmol 23:1311, 1940 222. Shabo AL, Maxwell DS, Kreiger AE: Structural alterations in the ciliary process and the blood-aqueous barrier
of the monkey after systemic urea injections. Am J Ophthalmol 81:162, 1976 223. Bito LZ, Davson H, Snider N: The effects of autonomic drugs on mitosis and DNA synthesis in the lens
epithelium and on the composition of the aqueous humor. Exp Eye Res 4:54, 1965 224. Wålinder P-E: Influence of pilocarpine on iodopyracet and iodide accumulation by rabbit
ciliary body-iris preparations. Invest Ophthalmol 5:378, 1966 225. Wålinder P-E, Bill A: Influence of intraocular pressure and some drugs on aqueous flow and entry
of cycloleucine into the aqueous humor of vervet monkeys (Cercopithecus
ethiops). Invest Ophthalmol 8:446, 1969 226. Berggren L: Effect of parasympathomimetic and sympathomimetic drugs on secretion in
vitro by the ciliary processes of the rabbit eye. Invest Ophthalmol 4:91, 1965 227. Berggren L: Further studies on the effect of autonomic drugs on in vivo secretory activity
of the rabbit eye ciliary processes. A. Inhibition of the pilocarpine
effect by isopilocarpine, arecoline, and atropine. B. Influence
of isoproterenol and norepinephrine. Acta Ophthalmol 48:293, 1970 228. Bill A, Walinder P-E: The effects of pilocarpine on the dynamics of aqueous humor in a primate (macaca
irus). Invest Ophthalmol 5:170, 1966 229. Bill A: Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus
monkeys (macaca irus). Exp Eye Res 6:120, 1967 230. Chiou GC, Liu HK, Trzeciakowski J: Studies of action mechanism of antiglaucoma drugs with a newly developed
cat model. Life Sci 27:2445, 1980 231. Green K, Padgett D: Effects of various drugs on pseudofacility and aqueous humor formation
in rabbit eye. Exp Eye Res 28:239, 1979 232. Kupfer C: Clinical significance of pseudofacility. Sanford R. Gifford memorial lecture. Am J Ophthalmol 75:193, 1973 233. Liu HK, Chiou GCY: Continuous, simultaneous, and instant display of aqueous humor dynamics
with a microspectrophotometer and a sensitive drop counter. Exp Eye Res 32:583, 1981 234. Macri FJ, Cevario SJ: The induction of aqueous humor formation by the use of Ach + eserine. Invest Opthalmol 12:910, 1973 235. Macri FJ, Cevario SJ: The dual nature of pilocarpine to stimulate or inhibit the formation of
aqueous humor. Invest Ophthalmol Vis Sci 13:617, 1974 236. Nagataki S, Brubaker RF: The effect of pilocarpine on aqueous humor formation in human beings. Arch Ophthalmol 100:818, 1982 237. Stjernschantz J: Effect of parasympathetic stimulation on intraocular pressure, formation
of aqueous humor and outflow facility in rabbits. Exp Eye Res 22:639, 1976 238. Uusitalo R: The action of physostigmine, morphine, cyclopentolate and homatropine on
the secretion and outflow of aqueous humor in the rabbit eye. Acta Physiol Scand 86:239, 1972 239. Uusitalo R: Effect of sympathetic and parasympathetic stimulation on the secretion
and outflow of aqueous humor in the rabbit eye. Acta Physiol Scand 86:315, 1972 240. Hoskins HD, Kass MA: Adrenergic agonists. In Hoskins HD, Kass MA (eds): Becker-Shaffer's
Diagnosis and Therapy of the Glaucomas, p 435. St. Louis: CV
Mosby, 1989 241. Gregory D, Sears M, Bausher L: Intraocular pressure and aqueous flow are decreased by cholera toxin. Invest Ophthalmol Vis Sci 20:371, 1981 242. Neufeld AH, Sears ML: Cyclic-AMP in ocular tissues of the rabbit, monkey, and human. Invest Ophthalmol 13:475, 1974 243. Waitzman MB, Woods WB: Some characteristics of an adenyl cyclase preparation from rabbit ciliary
process tissue. Exp Eye Res 12:99, 1971 244. Bartels SP, Lee SR, Neufeld AH: The effects of forskolin on cyclic AMP, intraocular pressure and aqueous
humor formation in rabbits. Curr Eye Res 6:307, 1987 245. Caprioli J, Sears M, Bausher L: Forskolin lowers intraocular pressure by reducing aqueous inflow. Invest Ophthalmol Vis Sci 25:268, 1984 246. Caprioli J, Sears ML: Forskolin lowers intraocular pressure in rabbits, monkeys, and man. Lancet 1:958, 1983 247. Bartels SP, Lee SR, Neufeld AH: Forskolin stimulates cyclic AMP synthesis, lowers intraocular pressure
and increases outflow facility in rabbits. Curr Eye Res 2:673, 1982 248. Shibata T, Mishima H, Kurokawa T: Ocular pigmentation and intraocular pressure response to forskolin. Curr Eye Res 7:667, 1988 249. Smith BR, Gaster RN, Leopold IH, Zeleznick LD: Forskolin, a potent adenylate cyclase activator, lowers rabbit intraocular
pressure. Arch Ophthalmol 102:146, 1984 250. Shahidullah M, Wilson WS, Millar C: Effects of timolol, terbutaline and forskolin on IOP, aqueous humour formation
and ciliary cyclic AMP levels in the bovine eye. Curr Eye Res 14:519, 1995 251. Watanabe K, Chiou GCY: Action mechanism of timolol to lower the intraocular pressure in rabbits. Ophthalmic Res 15:160, 1983 252. Baxter GM, Williamson TH, McKillop G, Dutton GN: Color doppler ultrasound of orbital and optic nerve blood flow: Effects
of posture and timolol 0.5%. Invest Ophthalmol Vis Sci 33:604, 1992 253. Chiou GCY, Yan HY: Effects of antiglaucoma drugs on the blood flow in rabbit eyes. Ophthalmic Res 18:265, 1986 254. Henkind P: Ocular circulation. In Records RE (ed): Physiology of the Human
Eye and Visual System, p 98. New York: Harper and Row, 1979 255. Morgan TR, Green K, Bowman K: Effects of adrenergic agonists upon regional ocular blood flow in normal
and ganglionectomized rabbits. Exp Eye Res 32:691, 1981 256. Pillunat L, Stodtmeister R: Effect of different antiglaucomatous drugs on ocular perfusion pressures. J Ocul Pharmacol 4:231, 1988 257. Coakes RL, Siah PB: Effects of adrenergic drugs on aqueous humor dynamics in the normal human
eye. I. Salbutamol. Br J Ophthalmol 68:393, 1984 258. Larson RS, Brubaker RF: Isoproterenol stimulates aqueous flow in humans with Horner's syndrome. Invest Ophthalmol Vis Sci 29:621, 1988 259. Yablonski ME, Novack GD, Burke PJ: The effect of levobunolol on aqueous humor dynamics. Exp Eye Res 44:49, 1987 260. Lotti VJ, LeDouarec JC, Stone CA: Autonomic nervous system: Adrenergic
antagonists. In Sears ML (ed): Pharmacology of the Eye: Handbook of Experimental
Pharmacology, p 249. Berlin: Springer-Verlag, 1984 261. Nathanson JA: Adrenergic regulation of intraocular pressure: Identification of beta 2-adrenergic-stimulated
adenylate cyclase in the ciliary process epithelium. Proc Natl Acad Sci USA 77:7420, 1980 262. Nathanson JA: Human ciliary process adrenergic receptor: Pharmacological characterization. Invest Ophthalmol Vis Sci 21:798, 1981 263. Sears ML: Autonomic nervous system: Adrenergic agonists. In Sears ML (ed): Pharmacology
of the Eye: Handbook of Experimental Pharmacology, p 193. Berlin: Springer-Verlag, 1984 264. Allen RC, Epstein DL: Additive effects of betaxolol and epinephrine in primary open angle glaucoma. Arch Ophthalmol 104:1178, 1986 265. Allen RC, Hertzmark, E, Walker AM, Epstein DL: A double-masked comparison of betaxolol vs timolol in the treatment of
open-angle glaucoma. Am J Ophthalmol 101:535, 1986 266. Berrospi AR, Leibowitz HM: Betaxolol. A new β-adrenergic blocking agent for the treatment of
glaucoma. Arch Ophthalmol 100:943, 1982 267. Berry DPJ, van Buskirk EM, Shields MB: Betaxolol and timolol: A comparison of efficacy and side-effects. Arch Ophthalmol 102:42, 1984 268. Caldwell DR, Salisbury CR, Guzek JP: Effects of topical betaxolol in ocular hypertensive patients. Arch Ophthalmol 102:539, 1984 269. Levy NS, Boone L, Ellis E: A controlled comparison of betaxolol and timolol with long-term evaluation
of safety and efficacy. Glaucoma 7:54, 1985 270. Chiou GCY, Watanabe K, McLaughlin MA, Liu HK: Are β-adrenergic mechanisms involved in ocular hypotensive actions
of adrenergic drugs? Ophthalmic Res 17:49, 1985 271. Keates EU, Stone R: The effect of d-timolol on intraocular pressure in patients with ocular
hypertension. Am J Ophthalmol 98:73, 1984 272. Liu JHK, Bartels SP, Neufeld AH: Effects of l- and d-timolol on cyclic AMP synthesis and intraocular pressure
in water-loaded, albino and pigmented rabbits. Invest Ophthalmol Vis Sci 24:1276, 1983 273. Merte HJ, Stryz J: Present day possibilities of glaucoma therapy. In Merte
HJ (ed): Metipranolol. Pharmacology of Beta-Blocking Agents and Use
of Metipranolol in Ophthalmology, p 22. Contributions to the First Metipranolol
Symposium. Vienna: Springer-Verlag, 1983 274. Mills KB, Jacobs NJ, Vogel R: A study of the effects of four concentrations of d-timolol, 0.25% l-timolol, and
placebo on intraocular pressure in patients with raised intraocular
pressure. Br J Ophthalmol 72:469, 1988 275. Richards R, Tattersfield AE: Bronchial β-adrenoceptor blockade following eyedrops of timolol and
its isomer L-714,465 in asthmatic subjects. Br J Clin Pharmacol 24:459, 1985 276. Richards R, Tattersfield AE: Comparison of the airway response to eye drops of timolol and its isomer
L-714,465 in asthmatic subjects. Br J Clin Pharmacol 24:485, 1987 277. Schmitt CJ, Lotti VJ, Varailles P, Le Douarec JC: β-adrenergic blockers: Lack
of relationship between antagonism of isoproterenol and lowering
of intraocular pressure in rabbits. In Sears M (ed): New Directions
in Ophthalmic Research, p 147. New Haven: Yale University Press, 1981 278. Share NN, Lotti VJ, Gautheron P et al: R-Enantiomer of timolol: A potential selective ocular antihypertensive
agent. Graefe's Arch Clin Exp Ophthalmol 221:234, 1984 279. Vareilles P, Silverstone D, Plazonnet B et al: Comparison of the effects of timolol and other adrenergic agents on intraocular
pressure in the rabbit. Invest Ophthalmol Vis Sci 16:987, 1977 280. Wentworth WO, Brubaker RF: Aqueous humor dynamics in a series of patients with third neuron Horner's
syndrome. Am J Ophthalmol 92:407, 1981 281. Crook RB, Riese K: Beta-adrenergic stimulation of Na+ , K+ , Cl- cotransport in
fetal nonpigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci 37:1047, 1996 282. Crook RB, Kim F, Polansky JR, Dinkelspiel KJ: Isoproterenol stimulates Na+ ,K+ ,Cl- cotransport in fetal human
nonpigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci 35(ARVO Abstracts):1660, 1994. 283. Robinson JC, Kaufman PL: Dose-dependent suppression of aqueous humor formation by timolol in the
cynomolgus monkey. J Glaucoma 2:251, 1993 284. Exley KA: Depression of autonomic ganglia by barbiturates. Br J Pharmacol Chemother 9:170, 1954 285. Lee DA, Brubaker RF: Effect of phenylephrine on aqueous humor flow. Curr Eye Res 83:89, 1982 286. Lee DA, Brubaker RF, Nagataki S: Effect of thymoxamine on aqueous humor formation in the normal human eye
as measured by fluorophotometry. Invest Ophthalmol Vis Sci 21:805, 1981 287. Hedler L, Stamm G, Weitzell R, Starke K: Functional characterization of central a-adrenoceptors by yohimbine diastereoisomers. Eur J Pharmacol 70:43, 1981 288. Krupin T, Feitl M, Becker B: Effect of prazosin on aqueous humor dynamics in rabbits. Arch Ophthalmol 98:1639, 1980 289. Mittag TW: Ocular effects of selective alpha-adrenergic agents: A new drug paradox? Ann Ophthalmol 15:201, 1983 290. Mittag TW, Tormay A, Severin C, Podos SM: Alpha-adrenergic antagonists: Correlation of the effect on intraocular
pressure and on α2-adrenergic receptor binding specificity in the
rabbit eye. Exp Eye Res 40:591, 1985 291. Serle JB, Stein AJ, Podos SM, Severin CH: Coryanthine and aqueous humor dynamics in rabbits and monkeys. Arch Ophthalmol 102:1385, 1984 292. Higgins RG, Brubaker RF: Acute effect of epinephrine on aqueous humor formation in the timolol-treated
normal eye as measured by fluorophotometry. Invest Ophthalmol Vis Sci 19:420, 1980 293. Thomas JV, Epstein DL: Transient additive effect of timolol and epinephrine in primary open-angle
glaucoma. Arch Ophthalmol 99:91, 1981 294. Bill A, Heilmann K: Ocular effects of clonidine in cats and monkeys (Macaca irus). Exp Eye Res 21:481, 1975 295. Gharagozloo NZ, Relf SJ, Brubaker RF: Aqueous flow is reduced by the alpha-adrenergic agonist, apraclonidine
hydrochloride (ALO 2145). Ophthalmology 95:1217, 1988 296. Krieglstein GK, Langham ME, Leydhecker W: The peripheral and central neural actions of clonidine in normal and glaucomatous
eyes. Invest Ophthalmol Vis Sci 17:149, 1978 297. Lee DA, Topper JE, Brubaker RF: Effect of clonidine on aqueous humor flow in normal human eyes. Exp Eye Res 38:239, 1984 298. Liu JH, Gallar J: In vivo cAMP level in rabbit iris-ciliary body after topical epinephrine
treatment. Curr Eye Res 15:1025, 1996 299. Nakamura T, Ariyoshi H, Kambayashi J et al: Signal transduction system in epinephrine stimulated plaelets: Comparison
between epinephrine sensitive and insensitive platelets. Thrombosis Res 85:83, 1997 300. Schutte M, Diadori A, Wang C, Wolosin JM: Comparative adrenocholinergic control of intracellular Ca2+ in the layers of the ciliary body epithelium. Invest Ophthalmol Vis Sci 37:212, 1996 301. Jin Y, Verstappen A, Yorio T: Characterization of alpha 2-adrenoceptor binding sites in rabbit ciliary
body membranes. Invest Ophthalmol Vis Sci 35:2500, 1994 302. Kaufman PL, Gabelt B: α2-Adrenergic agonist effects on aqueous humor dynamics. J Glaucoma 4(Suppl 1):S8–S14, 1995. 303. Borrás T, Tamm E, Zigler JS Jr: Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol Vis Sci 37:1282, 1996 304. Lindsey JD, Kashiwagi K, Boyle D et al: Prostaglandins increase proMMP-1 and proMMP-3 secretion by human ciliary
smooth muscle cells. Curr Eye Res 15:869, 1996 305. Clark AF, Lane D, Wilson K et al: Inhibition of dexamethasone-induced cytoskeletal changes in cultured human
trabecular meshwork cells by tetrahydrocortisol. Invest Ophthalmol Vis Sci 37:805, 1996 306. Hubbard WC, Gabelt BT, Kaufman PL: Effect of 3α,5β-tetrahydrocortisol on total outflow facility in
ocular normotensive cyonomolgus monkeys. Invest Ophthalmol Vis Sci 38(ARVO Abstracts):S812, 1997 307. Samuelsson-Almén M, Nilsson SFE, Mäepea O, Bill A: Effects of atrial natriuretic factor (ANF) on intraocular pressure and
aqueous humor flow in the cynomolgus monkey. Exp Eye Res 53:253, 1991 308. Korenfeld MS, Becker B: Atrial natriuretic peptides: Effects on intraocular pressure, cGMP, and
aqueous flow. Invest Ophthalmol Vis Sci 30:2385, 1989 309. Lütjen-Drecoll E, Kaufman PL: Acute and chronic structural effects
of pilocarpine on monkey outflow tissues. Trans Am Ophthalmol Soc 1998 (in
press) 310. Stone EM, Fingert JH, Alward WLM et al: Identification of a gene that causes primary open angle glaucoma. Science 275:668, 1997 311. Lütjen-Drecoll E, Kaufman PL: Morphological changes in primate aqueous humor formation and drainage tissues
after long-term treatment with antiglaucomatous drugs. J Glaucoma 2:316, 1993 312. Kaufman PL, Lütjen-Drecoll E, Hubbard WC, Erickson KA: Obstruction of aqueous humor outflow by cross-linked polyacrylamide microgels
in bovine, monkey and human eyes. Ophthalmology 101:1672, 1994 313. Bill A, Svedbergh B: Scanning electron microscopic studies of the trabecular meshwork and the
canal of Schlemm: An attempt to localize the main resistance to outflow
of aqueous humor in man. Acta Ophthalmol 50:295, 1972 314. Sears ML: The mechanism of action of adrenergic drugs in glaucoma. Invest Ophthalmol 5:115, 1966 315. Hoffman BF, Bigger JTJ: Digitalis and allied cardiac glyosides. In Gilman
AG, Rall TW, Nies AS, Taylor P (eds): Goodman and Gilman's The
Pharmacological Basis of Therapeutics, p 814. New York: McGraw-Hill, 1990 316. Brubaker RF: Flow of aqueous humor in humans. Invest Ophthalmol Vis Sci 32:3145, 1991 317. Armaly MF: On the distribution of applanation pressure. Arch Ophthalmol 73:11, 1965 318. Hoskins HD, Kass MA: Aqueous humor outflow. In Hoskins HD, Kass MA (eds): Becker-Shaffer's
Diagnosis and Therapy of the Glaucomas, p 41. St. Louis: CV
Mosby, 1989 319. Kupfer C, Ross K: Studies of aqueous humour dynamics in man. I. Measurements in young normal
subjects. Invest Ophthalmol Vis Sci 10:518, 1971 320. Beneyto MP, Fernandez-Vila PC, Perez TM: Determination of the pseudofacility by fluorophotometry in the human eye. Internat Ophthalmol 19:219, 1995-1996 321. Bill A: The protein exchange in the eye with aspects on conventional and
uveoscleral bulk drainage of aqueous humor in primates. In Rohen JW (ed): The
Structure of the Eye, Second Symposium, p 313. Stuttgart: Schattauer-Verlag, 1965 322. Bill A: Conventional and uveoscleral drainage of aqueous humor in the cynomolgus
monkey (Macaca irus) at normal and high intraocular pressures. Exp Eye Res 5:45, 1966 323. Bill A, Hellsing K: Production and drainage of aqueous humor in the cynomolgus monkey (Macaca
irus). Invest Ophthalmol Vis Sci 4:920, 1965 324. van Alphen GW: On emmetropia and ametropia. Ophthamologica 142(suppl):1, 1961 325. Bill A: The routes for bulk drainage of aqueous humor in rabbits with and without
cyclodialysis. Documenta Ophthalmol 20:157, 1966 326. Hoskins HD, Kass MA: Secondary open-angle glaucoma. In Hoskins HD, Kass
MA (eds): Becker-Shaffer's Diagnosis and Therapy of the Glaucomas, p 308. St. Louis: CV
Mosby, 1989 327. Bill A: Effects of atropine on aqueous humor dynamics in the vervet monkey (Cercopithecus
ethiops). Exp Eye Res 8:284, 1969 328. Gaasterland D, Kupfer C, Ross K: Studies of aqueous humor dynamics in man. III. Measurements in young normal
subjects using norepinephrine and isoproterenol. Invest Ophthalmol Vis Sci 12:267, 1973 329. Kupfer C, Gaasterland D, Ross K: Studies of aqueous humor dynamics in man. II. Measurements in young normal
subjects using acetazolamide and l-epinephrine. Invest Ophthalmol Vis Sci 10:523, 1971 330. Rohen JW, Lütjen E , Bárány E: The relation between the ciliary muscle and the trabecular meshwork and
its importance for the effect of miotics on aqueous outflow resistance. Graefe's Arch Clin Exp Ophthalmol 172:23, 1967 331. Armaly MF, Burian HM: Changes in the tonogram during accommodation. Arch Ophthalmol 60:60, 1958 332. Armaly MF: Studies on intraocular effects of the orbital parasympathetic pathway. I. Technique
and effects on morphology. Arch Ophthalmol 61:14, 1959 333. Armaly MG: Studies on intraocular effects of the orbital parasympathetic pathway. III. Effect
on steady state dynamics. Arch Ophthalmol 62:817, 1959 334. Armaly MF: Studies on intraocular effects of the orbital parasympathetics. II. Effects
on intraocular pressure. Arch Ophthalmol 62:117, 1959 335. Bárány EH, Rohen JW: Localized contraction and relaxation
within the ciliary muscle of the vervet monkey (Cercopithecus ethiops). In
J. W. Rohen (ed): The Structure of the Eye, Second Symposium, p 287. Stuttgart: FK
Schattauer Verlag, 1965 336. Kaufman PL: Accommodation and presbyopia: Neuromuscular and biophysical
aspects. In Hart WM Jr (ed): Adler's Physiology of the Eye, p 391. St. Louis: Mosby, 1992 337. van Buskirk EM, Grant WM: Lens depression and aqueous outflow in enucleated primate eyes. Am J Ophthalmol 76:632, 1973 338. Bárány EH, Christensen RE: Cycloplegia and outflow resistance. Arch Ophthalmol 77:757, 1967 339. Galin MA: Mydriasis provocative test. Arch Ophthalmol 66:353, 1961 340. Harris LS: Cycloplegic-induced intraocular pressure elevations. Arch Ophthalmol 79:242, 1968 341. Schimek RA, Lieberman WJ: The influence of Cyclogyl and Neosynephrine on tonographic studies of miotic
control in open angle glaucoma. Am J Ophthalmol 51:781, 1961 342. Bárány EH: The immediate effect on outflow resistance of intravenous pilocarpine in
the vervet monkey. Invest Ophthalmol 6:373, 1967 343. Bárány EH: The mode of action of pilocarpine on outflow resistance in the eye of a
primate (Cercopithecus ethiops). Invest Ophthalmol 1:712, 1962 344. Bárány EH: The mode of action of miotics on outflow resistance. A study of pilocarpine
in the vervet monkey (Cercopithecus ethiops). Trans Ophthalmol Soc UK 86:539, 1966 345. Shaffer RN: Glaucoma: Transactions of the fifth conference. In Newell FW (ed):, p 234. New
York: Josiah Macy Jr Foundation, 1960 346. Kaufman PL, Bárány EH: Residual pilocarpine effects on outflow facility after ciliary muscle disinsertion
in the cynomolgus monkey. Invest Ophthalmol 15:558, 1976 347. Kaufman PL, Lütjen-Drecoll E: Total iridectomy in the primate in vivo: Surgical technique and postoperative
anatomy. Invest Ophthalmol 14:766, 1975 348. Kaufman PL, Bárány EH: Loss of acute pilocarpine effect on outflow facility following surgical
disinsertion and retrodisplacement of the ciliary muscle from the scleral
spur in the cynomolgus monkey. Invest Ophthalmol Vis Sci 15:793, 1976 349. Kaufman PL: Aqueous humor dynamics following total iridectomy in the cynomolgus monkey. Invest Ophthalmol Vis Sci 18:870, 1979 350. Nomura T, Smelser GK: The identification of adrenergic and cholinergic nerve endings in the trabecular
meshwork. Invest Ophthalmol 13:525, 1974 351. Ruskell GL: The source of nerve fibres of the trabeculae and adjacent structures in
monkey eyes. Exp Eye Res 23:449, 1976 352. Gupta N, Drance SM, McAllister R et al: Localization of M3 muscarinic receptor subtype and mRNA in the human eye. Ophthalmic Res 26:207, 1994 353. Shade DL, Clark A, Pang IH: Effects of muscarinic agents on cultured human trabecular meshwork cells. Exp Eye Res 62:201, 1996 354. Wiederholt M, Schafer R, Wagner U, Lepple-Wienhues A: Contractile response of the isolated trabecular meshwork and ciliary muscle
to cholinergic and adrenergic agents. German J Ophthalmol 5:146, 1996 355. de Kater AW, Spurr-Michaud SJ, Gipson IK: Localization of smooth muscle myosin-containing cells in the aqueous outflow
pathway. Invest Ophthalmol Vis Sci 31:347, 1990 356. de Kater AW, Shahsafaei A, Epstein DL: Localization of smooth muscle and nonmuscle actin isoforms in the human
aqueous outflow pathway. Invest Ophthalmol Vis Sci 33:424, 1992 357. Coroneo MT, Korbmacher C, Fügel C et al: Electrical and morphological evidence for heterogeneous populations of
cultured bovine trabecular meshwork cells. Exp Eye Res 52:375, 1991 358. Flügel C, Tamm E, Lütjen-Drecoll E, Stefani FH: Age-related loss of α-smooth muscle actin in normal and glaucomatous
human trabecular meshwork of different age groups. J Glaucoma 1:165, 1992 359. Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L: Preliminary characterization of a transformed cell strain derived from
human trabecular meshwork. Curr Eye Res 13:51, 1994 360. Lepple-Wienhues A, Rauch R, Clark AF et al: Electrophysiological properties of cultured human trabecular meshwork cells. Exp Eye Res 59:305, 1994 361. Lepple-Wienhues A, Stahl F, Willner U et al: Endothelin-evoked contractions in bovine ciliary muscle and trabecular
meshwork: Interaction with calcium, nifedipine and nickel. Curr Eye Res 10:983, 1991 362. Lepple-Wienhues A, Stahl F, Wiederholt M: Differential smooth muscle-like contractile properties of trabecular meshwork
and ciliary muscle. Exp Eye Res 53:33, 1991 363. Wiederholt M, Bielka S, Schweig F, Lütjen-Drecoll E: Regulation of outflow rate and resistance in the perfused anterior segment
of the bovine eye. Exp Eye Res 61:223, 1995 364. Schroeder A, Erickson K: Low dose cholinergic agonists increase trabecular outflow facility in the
human eye in vitro. Invest Ophthalmol Vis Sci 36(Suppl):S722, 1995 365. Schroeder A, Erickson K: Cholinergic agonists do not increase trabecular outflow facility in the
human eye. Invest Ophthalmol Vis Sci 34(Suppl):2054, 1994 366. Fortin EP: Canal de Schlemm y Ligamento Pectineo. Arch Ophthalmol 4:454, 1925 367. Asayama J: Zur Anatomie des Ligamentum pectinatum. Albecht Von Graefe's Arch Ophthalmol 53:113, 1902 368. Fortin EP: Action du muscle ciliare sur la circulation de l'oeil; Insertion du muscle
ciliare sur la paroi du canal de Schlemm. Signification physiologique
et pathologique. CR Soc Biol 102:432, 1929 369. Holmberg Å, Bárány EH: The effect of pilocarpine on the endothelium forming the inner wall of
Schlemm's canal: An electron microcsopic study in the monkey Cercopithecus
ethiops. Invest Ophthalmol Vis Sci 5:53, 1966 370. Moses RAJ, Grodzki WJJ, Etheridge EL: Schlemm's canal: The effect of intraocular pressure. Invest Ophthalmol Vis Sci 20:61, 1981 371. Lütjen-Drecoll E: Structural factors influencing outflow facility and its changeability under
drugs: A study of Macaca arctoides. Invest Ophthalmol 12:280, 1973 372. Grierson I, Lee WR, Abraham S: Effects of pilocarpine on the morphology of the human outflow apparatus. Br J Ophthalmol 62:302, 1978 373. Gupta N, McAllister R, Drance SM et al: Muscarinic receptor M1 and M2 subtypes in the human eye: QNB, pirenzipine, oxotremorine, and
AFDX-116 in vitro autoradiography. Br J Ophthalmol 78:555, 1994 374. Zhang X, Hernandez MR, Yang H, Erickson K: Expression of muscarinic receptor subtype mRNA in the human ciliary muscle. Invest Ophthalmol Vis Sci 36:1645, 1995 375. Gabelt BT, Kaufman PL: Inhibition of outflow facility, accommodative, and miotic responses to
pilocarpine in rhesus monkeys by muscarinic receptor subtype antagonists. J Pharmacol Exp Ther 263:1133, 1992 376. Gabelt BT, Kaufman PL: Inhibition of aceclidine-stimulated outflow facility, accommodation and
miosis by muscarinic receptor subtype antagonists in rhesus monkeys. Exp Eye Res 58:623, 1994 377. Flügel C, Bárány EH, Lütjen-Drecoll E: Histochemical differences within the ciliary muscle and its function in
accommodation. Exp Eye Res 50:219, 1990 378. Erickson-Lamy K, Schroeder A: Dissociation between the effect of aceclidine on outflow facility and accommodation. Exp Eye Res 50:143, 1990 379. Demailly PH: Place de l'acéclidine dans le traitement du glaucome chronique simple à angle
ouvert. Arch Ophthalmol 28:735, 1968 380. Étienne R, Barut C, Gonzalès-Bouchon J: Un nouvel hypotenseur oculaire: L'acéclidine. Ann Oculist 200:287, 1967 381. Kaufman PL, Gabelt BT: Cholinergic mechanisms and aqueous humor dynamics. In
Drance SM, Van Buskirk EM, Neufeld AH (eds): Pharmacology of Glaucoma, p 64. Baltimore: Williams & Wilkins, 1992 382. Lieberman TW, Leopold IH: The use of aceclidine in the treatment of glaucoma. Its effect on intraocular
pressure and facility of aqueous humor outflow as compared to that
of pilocarpine. Am J Ophthalmol 64:405, 1967 383. Romano JH: Double-blind crossover comparison of aceclidine and pilocarpine in open-angle
glaucoma. Br J Ophthalmol 54:510, 1970 384. Poyer JF, Gabelt BT , Kaufman PL: The effect of muscarinic agonists and selective receptor subtype antagonists
on the contractile response of the isolated rhesus monkeys ciliary
muscle. Exp Eye Res 59:729, 1994 385. Hubbard WC, Kee C, Kaufman PL: Aceclidine effects on outflow facility after ciliary muscle disinsertion. Ophthalmologica 210:303, 1996 386. Hart WM: Intraocular pressure. In Hart WM (ed): Adler's Physiology
of the Eye, p 248. 9th ed. St. Louis: Mosby, 1992 387. Croft MA, Kaufman PL, Erickson-Lamy KA et al: Accommodation and ciliary muscle muscarinic receptors after echothiophate. Invest Ophthalmol Vis Sci 32:3288, 1991 388. Bárány E: Pilocarpine-induced subsensitivity to carbachol and pilocarpine of ciliary
muscle in vervet and cynomolgus monkeys. Acta Ophthalmol 55:141, 1977 389. Bárány E, Berrie CP, Birdsall NJM et al: The binding properties of the muscarinic receptors of the cynomolgus monkey
ciliary body and the response to the induction of agonist subsensitivity. Br J Pharmacol 77:731, 1982 390. Bárány EH: Muscarinic subsensitivity without receptor change in monkey ciliary muscle. Br J Pharmacol 84:193, 1985 391. Kaufman PL, Bárány EH: Subsensitivity to pilocarpine in primate ciliary muscle following topical
anticholinesterase treatment. Invest Ophthalmol 14:302, 1975 392. Kaufman PL, Bárány EH: Subsensitivity to pilocarpine of the aqueous outflow system in monkey eyes
after topical anticholinesterase treatment. Am J Ophthalmol 82:883, 1976 393. Kaufman PL: Anticholinesterase-induced cholinergic subsensitivity in primate accommodative
mechanism. Am J Ophthalmol 85:622, 1978 394. Kaufman PL, Gabelt BT: Direct, indirect and dual action parasympathetic
drugs. In Zimmerman TJ (ed): Textbook of Ocular Pharmacology, p 221. Philadelphia: Lippincott-Raven, 1997 395. Erickson-Lamy KA, Polansky JR, Kaufman PL, Zlock DM. Cholinergic drugs
alter ciliary muscle response and receptor content. Invest Ophthalmol
Vis Sci 28:375, 1987 396. Quigley HA, Pollack IP, Harbin TS Jr: Pilocarpine ocuserts: Long-term clinical trials and selected pharmacodynamics. Arch Ophthalmol 93:771, 1975 397. Worthen DM, Zimmerman TJ, Wind CA: An evaluation of the pilocarpine ocusert. Invest Ophthalmol Vis Sci 13:296, 1974 398. Ballintine EJ, Garner LL: Improvement of the coefficient of outflow in glaucomatous eyes. Prolonged
local treatment with epinephrine. Arch Ophthalmol 66:314, 1961 399. Becker B, Pettit TH, Gay AJ: Topical epinephrine therapy of open-angle glaucoma. Arch Ophthalmol 66:219, 1961 400. Criswick VG, Drance SM: Comparative study of four different epinephrine salts on intraocular pressure. Arch Ophthalmol 75:768, 1966 401. Krill AE, Newell FW, Novak KM: Early and long-term effects of levo-epinephrine on ocular tension and outflow. Am J Ophthalmol 59:833, 1965 402. Kronfeld PC: Dose-effect relationships as an aid in the evaluation of ocular hypotensive
drugs. Invest Ophthalmol 3:258, 1964 403. Kronfeld PC: The efficacy of combinations of ocular hypotensive drugs: A tonographic
approach. Arch Ophthalmol 78:140, 1967 404. Kronfeld PC: Early effect of single and repeated doses of L-epinephrine in man. Am J Ophthalmol 72:1058, 1971 405. Neufeld AH, Sears ML: Adenosine 3',5'-monophosphate analogue increases the outflow
facility of the primate eye. Invest Ophthalmol Vis Sci 14:688, 1975 406. Prijot E: Contribution to the study of tonometry and tonography in ophthalmology. Doc Ophthalmol 15:1, 1961 407. Weekers R, Prijot E, Gustin J: Recent advances and future prospects in the medical treatment of ocular
hypertension. Br J Ophthalmol 38:742, 1954 408. Törnqvist G: Effect of cervical sympathetic stimulation on accommodation in monkeys: An
example of a beta-adrenergic inhibitory effect. Acta Physiol Scand 67:363, 1966 409. van Alphen GWHM, Kern R, Robinette SL: Adrenergic receptors of the intraocular muscles. Comparison to cat, rabbit, and
monkey. Arch Ophthalmol 74:253, 1965 410. van Alphen GWHM, Robinette SL, Macri FJ: Drug effects on ciliary muscle and choroid preparations in vitro. Arch Ophthalmol 68:111, 1962 411. Lograno MD, Reibaldi A: Receptor-responses in fresh human ciliary muscle. Br J Pharmacol 87:379, 1986 412. Kaufman PL, Rentzhog L: Effect of total iridectomy on outflow facility responses to adrenergic
drugs in cynomolgus monkeys. Exp Eye Res 33:65, 1981 413. Kaufman PL, Bárány EH: Adrenergic drug effects on aqueous outflow facility following ciliary muscle
retrodisplacement in the cynomolgus monkey. Invest Ophthalmol Vis Sci 20:644, 1981 414. Langham ME: The aqueous outflow system and its response to autonomic receptor
agonists. In Bito LZ, Davson H, Fenstermacher JD (eds): The Ocular
and Cerebrospinal Fluids. Fogarty International Center Symposium. London: Academic
Press, 1977 Exp Eye Res 25(Suppl): 311 415. Kaufman PL, Bárány EH, Erickson KA: Effect of serotonin, histamine, and bradykinin on outflow facility following
ciliary muscle retrodisplacement in the cynomolgus monkey. Exp Eye Res 35:191, 1982 416. Kaufman PL, Bill A, Bárány EH: Formation and drainage of aqueous humor following total iris removal and
ciliary muscle disinsertion in the cynomolgus monkey. Invest Ophthalmol Vis Sci 16:226, 1977 417. Bárány EH: Relative importance of autonomic nervous tone
and structure as determinants of outflow resistance in normal monkey eyes (Cercopithecus
ethiops and Macaca irus). In Rohen JW (ed): The Structure
of the Eye, 2nd symposium, p 223. Stuttgart: Schattauer, 1965 418. Wax MB, Molinoff PB, Alvarado JA et al: Characterization of beta-adrenergic receptors in cultured human trabecular
cells and in human trabecular meshwork. Invest Ophthalmol Vis Sci 30:51, 1989 419. Alvarado JA: Epinephrine effects on major cell types of the aqueous outflow pathway: In
vitro studies/clinical implications. Trans Am Ophthalmol Soc 88:267, 1990 420. Busch MJWM, van Oosterhout JGM, Hoyng PFJ: Effects of cyclic nucleotide analogs on intraocular pressure and trauma-induced
inflammation in the rabbit eye. Curr Eye Res 11:5, 1992 421. Kaufman PL: Adenosine 3',5' cyclic monophosphate and outflow facility in
monkey eyes with intact and retrodisplaced ciliary muscle. Exp Eye Res 44:415, 1987 422. Neufeld AH, Jampol LM, Sears ML: Cyclic-AMP in the aqueous humor: The effects of adrenergic agonists. Exp Eye Res 14:242, 1972 423. Neufeld AH: Influences of cyclic nucleotides on outflow facility in the vervet monkey. Exp Eye Res 27:387, 1978 424. Erickson-Lamy KA, Nathanson JA: Epinephrine increases facility of outflow and cyclic AMP content in the
human eye in vitro. Invest Ophthalmol Vis Sci 33:2672, 1992 425. Thomas JV, Epstein DL. Timolol and epinephrine in primary open angle glaucoma. Transient
additive effect. Arch Ophthalmol 99:91, 1981 426. Cyrlin MN, Thomas JV, Epstein DL: Additive effect of epinephrine to timolol therapy in primary open-angle
glaucoma. Arch Ophthalmol 100:414, 1982 427. Robinson JC, Kaufman PL: Effects and interactions of epinephrine, norepinephrine, timolol and betaxolol
on outflow facility in the cynomolgus monkey. Am J Ophthalmol 109:189, 1990 428. Allen RC, Epstein DL: Additive effects of betaxolol and epinephrine in primary open angle glaucoma. Arch Ophthalmol 104:1178, 1986 429. Aurbach GD, Spiegel AM, Gardner JD: Beta-adrenergic receptors, cyclic AMP, and ion transport in the avian erythrocyte. Adv Cyclic Nucl Res 5:117, 1975 430. Bilezikian JP: A beta-adrenergic receptor of the turkey erythrocyte. I. Binding of catecholamine
and relationship to adenylate cyclase activity. J Biol Chem 248:5577, 1973 431. Bilezikian JP, Aurbach GD: A beta-adrenergic receptor of the turkey erythrocyte. II. Characterization
and solubilization of the receptor. J Biol Chem 248:5584, 1973 432. Levitzki A: The binding characteristics and number of beta-adrenergic receptors in
the turkey erythrocyte. Proc Natl Acad Sci USA 71:2773, 1974 433. Robinson JC, Kaufman PL: Cytochalasin B potentiates epinephrine's outflow facility increasing
effect. Invest Ophthalmol Vis Sci 32:1614, 1991 434. Robinson JC, Kaufman PL: Phalloidin inhibits epinephrine's and cytochalasin B's facilitation
of aqueous outflow. Arch Ophthalmol 112:1610, 1994 435. Tripathi BJ, Tripathi RC: Effect of epinephrine in vitro on the morphology, phagocytosis, and mitotic
activity of human trabecular endothelium. Exp Eye Res 39:731, 1984 436. Shattil SJ, Haimovich B, Cunningham M et al: Tyrosine phosphorylation of pp125FAK in platelets requires coordinated
signalling through integrin and agonist receptors. J Biol Chem 269:14738–14745, 1994 437. Petty HR, Martin SM: Combinative ligand-receptor interactions: effects of cAMP, epinephrine, and
met-enkephalin on RAW264 macrophage morphology, spreading, adherence, and
microfilaments. J Cell Physiol 138:247, 1989 438. Benschop RJ, Oostveen FG, Heijnen CJ et al: β2-Adrenergic stimulation causes detachment of natural killer cells
from cultured endothelium. Eur J Immunol 23:3242, 1993 439. Hamachi T, Hirata M, Koga T: Effect of cAMP-elevating drugs on Ca2+ efflux and actin polymerization
in peritoneal macrophages stimulated with N-formyl chemotactic peptide. Biochim Biophys Acta 804:230, 1984 440. Casey WJ: Cervical sympathetic stimulation in monkeys and the effects on outflow
facility and intraocular volume. A study in the East African vervet (Cercopithecus
aethiops). Invest Ophthalmol Vis Sci 5:33, 1966 441. Engstrom P, Dunham EW: Alpha-adrenergic stimulation of prostaglandin release from rabbit iris-ciliary
body in vitro. Invest Ophthalmol Vis Sci 22:757, 1982 442. Yousufzai SY, Abdel-Latif AA: Effects of norepinephrine and other pharmacological agents on prostaglandin
E2 release by rabbit and bovine irides. Exp Eye Res 37:279, 1983 443. Bhattacherjee P, Hammond BR: Effect of indomethacin on the ocular hypotensive action of adrenaline in
the rabbit. Exp Eye Res 24:307, 1977 444. Camras CB, Feldman SG, Podos SM et al: Inhibition of the epinephrine-induced reduction of intraocular pressure
by systemic indomethacin in humans. Am J Ophthalmol 100:169, 1985 445. Gilmartin B, Hogan RE, Thompson SM: The effect of timolol maleate on tonic accommodation, tonic vergence, and
pupil diameter. Invest Ophthalmol Vis Sci 25:763, 1984 446. Lee P-Y, Serle JB, Podos SM et al: Time course of the effect of UK 14304-18 (brimonidine tartrate) on rabbit
uveoscleral outflow. Invest Ophthalmol Vis Sci 33(ARVO Abstracts):1118, 1992 447. Poyer JF, Gabelt BT, Kaufman PL: The effect of topical PGF2a on uveoscleral outflow and outflow facility in the rabbit eye. Exp Eye Res 54:277, 1992 448. Toris CB, Tafoya ME, Camras CB et al: Effects of apraclonidine on aqueous humor dynamics in human eyes. Ophthalmology 102:456, 1995 449. Toris CB, Gleason ML, Camras CB et al: Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol 113:1514, 1995 450. Tamm E, Rittig M, Lütjen-Drecoll E: Elektronenmikroskopische und immunhistochemische Untersuchungen zur augendrucksenkenden
Wirkung von Prostaglandin F2a. Fortschr Ophthalmol 87:623, 1990 451. Lindsey JD, Kashiwagi K, Kashiwagi F et al: Prostaglandin treatment reduces collagen types I and III in cultures of
human ciliary smooth muscle cells. Invest Ophthalmol Vis Sci 38:2214, 1997 452. Lindsey JD, Kashiwagi K, Kashiwagi F et al: Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: Implications
for uveoscleral outflow. Surv Ophthalmol 41(Suppl 2):S53–S59, 1997 453. Lindsey JD, To HD, Weinreb RN: Induction of c-fos by prostaglandin F2 alpha in human ciliary smooth muscle
cells. Invest Ophthalmol Vis Sci 35:242, 1994 454. Kaufman PL, Crawford K, Gabelt BT: The effects of prostaglandins on aqueous
humor dynamics. Ophthalmol Clin North Am 1989:141 455. Toris CB, Pederson JE: Aqueous humor dynamics in experimental iridocyclitis. Invest Ophthalmol Vis Sci 28:477, 1987 456. Villumsen J, Alm A: Prostaglandin F2a-isopropylester eye drops: Effects in normal human eyes. Br J Ophthalmol 73:419, 1989 457. Camras CB, Zhan GL, Wang YL et al: Effect of sympathectomy (SX) and prostaglandin (PG) analog (latanoprost) treatment
on iris color in pigmented rabbits. Invest Ophthalmol Vis Sci 36(ARVO Abstracts):S720, 1995 458. Alm A, Villumsen J: PhXA34, a new potent ocular hypotensive drug. A study on dose-response
relationship and on aqueous humor dynamics in healthy volunteers. Arch Ophthalmol 109:1564, 1991 459. Alm A, Villumsen J, Tornquist P et al: Intraocular-pressure-reducing effect of PhXA41 in patients with increased
eye pressure. A one-month study. Ophthalmology 100:1312, 1993 460. Hotehama Y, Mishima HK, Kitazawa Y et al: Ocular hypotensive effect of PhXA41 in patients with ocular hypotension
or primary open-angle glaucoma. Jpn J Ophthalmol 37:270, 1993 461. Villumsen J, Alm A: PhXA34—a prostaglandin F2a analogue: Effect on intraocular pressure in patients with ocular hypertension. Br J Ophthalmol 76:214, 1992 462. Azuma I, Masuda K, Kitazawa Y et al: Double-masked comparative study of UF-021 and timolol ophthalmic solutions
in patients with primary open-angle glaucoma or ocular hypertension. Jpn J Ophthalmol 37:514, 1993 463. Fujimori C, Yamabayashi S, Hosoda M et al: The clinical evaluation of UF-021, a new prostaglandin related compound, in
low tension glaucoma patients. Nippon Ganka Gakkai Zasshi-Acta Societatis Ophthalmologicae Japonicae 97:1231, 1993 464. Yamamoto T, Kitazawa Y, Azuma I, Masuda K: Clinical evaluation of UF-021 (Rescula; isopropyl unoprostone). Surv Ophthalmol 41(Suppl 2):S99, 1997 465. Kolker AE, Becker B: Topical corticosteroids and glaucoma: Current status. In
Bellows JG (ed): Contemporary Ophthalmology, p 161. Baltimore: Williams & Wilkins, 1972 466. Schwartz B: The response of ocular pressure to corticosteroids. In Schwartz
B (ed): Corticosteroids and the Eye. Int Ophthalmol Clin, p 929. Vol 6, no 4. Boston: Little, Brown, 1966 467. Armaly MF: Steroids and glaucoma. In Samson CLM, Bahn GC, Schoel RE, Allen
JH (eds): Symposium on Glaucoma: Transactions of the New Orleans Academy
of Ophthalmology, p 74. St. Louis: CV Mosby, 1967 468. Becker B , Hahn K: Topical corticosteroids and heredity in primary open-angle glaucoma. Am J Ophthalmol 57:543, 1964 469. Hernandez MR, Wenk EJ, Weinstein BI et al: Glucocorticoid target cells in human outflow pathway: Autopsy and surgical
specimens. Invest Ophthalmol Vis Sci 24:1612, 1983 470. Tchernitchiv A, Wenk EJ, Hernandez MR et al: Glucocorticoid localization by radioautography in the rabbit eye following
systemic administration of 3H-dexamethasone. Invest Ophthalmol Vis Sci 19:1231, 1980 471. Weinreb RN, Bloom E, Baxter JD: Detection of glucocorticoid receptors in cultured human trabecular cells. Invest Ophthalmol Vis Sci 21:403, 1981 472. Southren AL, L'Hommedieu D, Gordon GG, Weinstein BI. Intraocular hypotensive effect of a topically applied cortisol metabolite: 3α, 5β-tetrahydrocortisol. Invest Ophthalmol Vis Sci 28:901, 1987 473. Levi L, Schwartz B: Decrease of ocular pressure with oral metyrapone: A double masked crossover
trial. Arch Ophthalmol 105:777, 1987 474. Polansky JR, Palmberg P, Matulich D: Cellular sensitivity to glucocorticoids in patients with POAG: Steroid
receptors and responses in cultured skin fibroblasts. Invest Ophthalmol Vis Sci 26:805, 1985 475. Schwartz B, Levene RZ: Plasma cortisol differences between normal and glaucomatous patients before
and after dexamethasone suppression. Arch Ophthalmol 87:369, 1972 476. Schwartz B, McCarty G, Rosner B: Increased plasma free cortisol in ocular hypertension and open-angle glaucoma. Arch Ophthalmol 105:1060, 1987 477. Weinreb RN, Polansky JR, Kramer SG, Baxter JD: Acute effects of dexamethasone on intraocular pressure in glaucoma. Invest Ophthalmol Vis Sci 26:170, 1985 478. Hernandez MR, Weinstein BI, Dunn MW et al: The effect of dexamethasone on the synthesis of collagen in normal human
trabecular meshwork explants. Invest Ophthalmo Vis Sci 26:1784, 1985 479. Hernandez MR, Weinstein BI, Wenk EJ et al: The effect of dexamethasone on the in vitro incorporation of precursors
of extracellular matrix components in the outflow pathway region of the
rabbit eye. Invest Ophthalmol Vis Sci 24:704, 1983 480. Knepper PA, Collins JA, Frederick R: Effects of dexamethasone, progesterone, and testosterone on IOP and GAGS
in the rabbit eye. Invest Ophthalmol Vis Sci 26:1093, 1985 481. Polansky JR, Wood IS, Alvarado JA: Trabecular meshwork cell culture in glaucoma research: Evaluation of biological
activity and structural properties of human trabecular cells
in vitro. Ophthalmology 91:580, 1984 482. Southren AL, Hernandez AL, L'Hommedieu D: Potentiation of collagen synthesis in explants of the rabbit eye by 5 beta-dihydrocortisol. Invest Ophthalmol Vis Sci 27:1757, 1986 483. Steely HT, Browder S, Julian MB: The effects of dexamethasone on fibronectin expression in cultured trabecular
meshwork cells. Invest Ophthalmol Vis Sci 33:2242, 1992 484. Tripathi BJ, Millard CB, Tripathi RC: Corticosteroids induce a sialated glycoprotein (Cort-GP) in trabecular
cells in vitro. Exp Eye Res 51:735, 1990 485. Tripathi RC, Tripathi BJ: Human trabecular endothelium, corneal endothelium, keratocytes, and scleral
fibroblasts in primary cell culture. A comparative study of growth
characteristics, morphology, and phagocytic activity by light and scanning
electron microscopy. Exp Eye Res 35:611, 1982 486. Polansky JR, Kurtz RM, Fauss DJ: In vitro correlates of glucocorticoid
effects on intraocular pressure. In Krieglestein GK (ed): Glaucoma Update
IV, p 20. Berlin Springer-Verlag, 1991 487. Shirato S, Murphy CG, Bloom E et al: Kinetics of phagocytosis in trabecular meshwork cells: Flow cytometry and
morphometry. Invest Ophthalmol Vis Sci 30:2499, 1989 488. Patridge CA, Weinstein BI, Southren AL, Gerritsen ME. Dexamethasone induces
specific proteins in human trabecular meshwork cells. Invest Ophthalmol
Vis Sci 30:1843, 1989 489. Polansky JR, Kurtz RM, Alvarado JA et al: Eicosanoid production and glucocorticoid
regulatory mechanisms in cultured human trabecular meshwork
cells. In Bito LZ, Stjernschantz J (eds): The Ocular Effects of Prostaglandins
and Other Eicosanoids. Progress in Clinical and Biological
Research, p 113. Vol 312. New York: Alan R. Liss, 1989 490. Yun AJ, Murphy CG, Polansky JR et al: Proteins secreted by human trabecular cells. Glucocorticoid and other effects. Invest Ophthalmol Vis Sci 30:2012, 1989 491. Southren AL, Gordon GG, Munnangi PR et al: Altered cortisol metabolism in cells cultured from trabecular meshwork
specimen obtained from patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci 26:1413, 1983 492. Weinstein BI, Munnangi P, Gordon GG, Southren AL. Defects in cortisol-metabolizing
enzymes in primary open-angle glaucoma. Invest Ophthalmol
Vis Sci 26:890, 1985 493. Southren AL, Wandel T, Gordon GG, Weinstein BI. Treatment of glaucoma with 3a,5B-tetrahydrocortisol: A new therapeutic
modality. J Ocul Pharmacol 10:385, 1994 494. Clark AF, Lane D, Wilson K et al: Inhibition of dexamethasone-induced cytoskeletal changes in cultured human
trabecular meshwork cells by tetrahydrocortisol. Invest Ophthalmol Vis Sci 37:805, 1996 495. Tripathi BJ, Tripathi RC, Swift HH: Hydrocortisone-induced DNA endoreplication in human trabecular cells in
vitro. Exp Eye Res 49:259, 1989 496. Polansky JR, Fauss DJ, Chen P et al: Cellular pharmacology and molecular biology of the trabecular meshwork
inducible glucocorticoid response gene product. Ophthalmologica 211:126, 1997 497. Nguyen TD, Huang WD, Bloom E et al: Glucocorticoid (GC) effects on HTM
cells: Molecular biology approaches. In Lütjen-Drecoll E (ed): Basic
Aspects of Glaucoma Research III, p 331. Stuttgart: Schattauer, 1993 498. Wilson K, McCartney MD, Miggans ST et al: Dexamethasone induced ultrastructural changes in cultured human trabecular
meshwork cells. Exp Eye Res 12:783, 1993 499. Clark AF, Wilson K, McCartney MD et al: Glucocorticoid-induced formation of cross-linked actin networks in cultured
human trabecular meshwork cells. Invest Ophthalmol Vis Sci 35:281, 1994 500. Underwood JL, Alvarado JA, Franse-Carman L: Steroids induce tight junction formation, narrowing of paracellular routes
and decreased hydraulic conductivity. Invest Ophthalmol Vis Sci 34(ARVO Abstracts):1140, 1993 501. Al-Aswad L, O'Donnell M, Brandt J et al: The effect of bumetanide on outflow facility in human and calf eyes in
vitro. Invest Opthalmol Vis Sci 38(ARVO Abstracts):S817, 1997 502. Petrovich M, Gray T, Crosson CE: Does adenosine receptor activation contribute to the ocular hypotensive
actions of epinephrine? Invest Ophthalmol Vis Sci 38(ARVO Abstracts):S820, 1997 503. Crosson CE, Gray T: Adenosine agonists alter aqueous flow and outflow facility in rabbits. Invest Ophthalmol Vis Sci 38(ARVO Abstracts):S821, 1997 504. Epstein DL, Roberts BC, Skinner LL: Nonsulfydryl-reactive phenoxyacetic acids increase aqueous humor outflow
facility. Invest Ophthalmol Vis Sci 38:1526, 1997 505. Bill A, Svedbergh B: Scanning electron microscopic studies of the trabecular meshwork and the
canal of Schlemm: An attempt to localize the main resistance to outflow
of aqueous humor in man. Acta Ophthalmol 50:295, 1972 506. Svedbergh B: Aspects of the aqueous humour drainage: Functional ultrastructure of Schlemm's
canal, the trabecular meshwork, and the corneal endothelium
at different intraocular pressures. Acta Univ Uppsl 256:1, 1976 507. Inomata H, Bill A, Smelser GK: Aqueous humor pathways through the trabecular meshwork and into Schlemm's
canal in the cynomolgus monkey (Macaca irus). Am J Ophthalmol 73:760, 1972 508. Grant WM: Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol 69:783, 1963 509. Ellingsen BA, Grant WM: Trabeculotomy and sinusotomy in enucleated human eyes. Invest Ophthalmol 11:21, 1972 510. Peterson WS, Jocson VL: Hyaluronidase effects on aqueous outflow resistance: Quantitative and localizing
studies in the rhesus monkey eye. Am J Ophthalmol 77:573, 1974 511. Ellingsen BA, Grant WM: Influence of intraocular pressure and trabeculotomy on aqueous outflow
in enucleated monkey eyes. Invest Ophthalmol 10:705, 1971 512. Hassel JR, Newsome DA, Martin GR: Isolation and characterization of the proteoglycans and collagens synthesized
by cells in culture. Vision Res 21:49, 1981 513. Richardson TM: Distribution of glycosaminoglycans in the aqueous outflow system of the
cat. Invest Ophthalmol Vis Sci 22:319, 1982 514. Rohen JW, Schachtschabel DO, Wehrmann R: Structural changes in human and monkey trabecular meshwork following in
vitro cultivation. Graefe's Arch Clin Exp Ophthalmol 218:225, 1982 515. Schachtschabel DO, Bigalke B, Rohen JW: Production of glycosaminoglycans by cell cultures of the trabecular meshwork
of the primate eye. Exp Eye Res 24:71, 1977 516. Schachtschabel DO, Rohen JW, Wever J et al: Synthesis and composition of glycosaminoglycans by cultured human trabecular
meshwork cells. Graefe's Arch Clin Exp Ophthalmol 218:113, 1982 517. Francois J: The importance of the mucopolysaccharides in intraocular pressure regulation. Invest Ophthalmol Vis Sci 14:173, 1975 518. Alvarado JA, Yun AJ, Murphy CG: Juxtacanalicular tissue in primary open-angle glaucoma and in nonglaucomatous
normals. Arch Ophthalmol 104:1517, 1986 519. Murphy CG, Johnson M, Alvarado JA: Juxtacanalicular tissue in pigmentary and primary open-angle glaucoma: The
hydrodynamic role of pigment and other constituents. Arch Ophthalmol 110:1779, 1992 520. Svedbergh B: Effects of intraocular pressure on the pores of the inner wall of Schlemm's
canal: A scanning electron microscopic study. Jpn J Ophthalmol, 3:127, 1976 521. Epstein DL, Rohen JW: Morphology of the trabecular meshwork and inner wall endothelium after
cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci 32:160, 1991 522. Lütjen-Drecoll E, Futa R, Rohen JW: Ultrahistochemical studies on tangential sections of the trabecular meshwork
in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci 21:563, 1981 523. Rohen JW, Futa R, Lütjen-Drecoll E: The fine structure of the cribriform meshwork in normal and glaucomatous
eyes as seen in tangential sections. Invest Ophthalmol Vis Sci 21:574, 1981 524. Grierson I, Lee WR: Erythrocyte phagocytosis in the human trabecular meshwork. Br J Ophthalmol 57:400, 1973 525. Rohen JW, Van der Zypen E: The phagocytic activity of the trabecular meshwork endothelium: An electron
microscopic study of the vervet (Cercopithecus ethiops). Graefe's Arch Clin Exp Ophthalmol 175:143, 1968 526. Alvarado JA, Murphy CG: Outflow obstruction in pigmentary and primary open angle glaucoma. Arch Ophthalmol 110:1769, 1992 527. Bill A: The drainage of aqueous humor [editorial]. Invest Ophthalmol Vis Sci 14:1, 1975 528. Gaasterland DE, Pederson JE, MacLellan HM: Perfusate effects upon resistance to aqueous humor outflow in the rhesus
monkey eye. Invest Ophthalmol Vis Sci 17:391, 1978 529. Gaasterland DE, Pederson JE, McLellan HM et al: Rhesus monkey aqueous humor composition and a primate ocular perfusate. Invest Ophthalmol Vis Sci 18:1139, 1979 530. Bárány EH: Simultaneous measurements of changing intraocular pressure and outflow
facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol Vis Sci 3:135, 1964 531. Erickson KA, Kaufman PL: Comparative effects of three ocular perfusates on outflow facility in the
cynomolgus monkey. Curr Eye Res 1:211, 1981 532. Kaufman PL, Bárány EH: Cytochalasin B reversibly increases outflow facility in the eye of the
cynomolgus monkey. Invest Ophthalmol Vis Sci 16:47, 1977 533. Kaufman PL, Bill A, Bárány EH: Effect of cytochalasin B on
conventional drainage of aqueous humor in the cynomolgus monkey. Exp
Eye Res 25(Suppl), 1997 534. Kaufman PL, Svedbergh B, Lütjen-Drecoll E: Medical trabeculocanalotomy in monkeys with cytochalasin B or EDTA. Ann Ophthalmol 11:795, 1979 535. Kaufman PL, Erickson KA: Cytochalasin B and D dose-outflow facility response relationships in the
cynomolgus monkey. Invest Ophthalmol Vis Sci 23:646, 1982 536. Bill A, Lutjen-Drecoll E, Svedbergh B: Effects of intracameral Na2EDTA and EGTA on aqueous outflow routes in the
monkey eye. Invest Ophthalmol Vis Sci 19:492, 1980 537. Croft MA, Hubbard WC, Kaufman PL: Effect of ethacrynic acid on aqueous outflow dynamics in monkeys. Invest Ophthalmol Vis Sci 35:1167, 1994 538. Croft MA, Kaufman PL: Effect of daily topical ethacrynic acid on aqueous humor dynamics in monkeys. Curr Eye Res 14:777, 1995 539. Liang L-L, Epstein DL, de Kater AW et al: Ethacrynic acid increases facility of outflow in the human eye in vitro. Arch Ophthalmol 110:106, 1992 540. Tian B, Kaufman PL, Geiger B et al: The protein kinase inhibitor H-7 increases outflow facility and inhibits
miotic but not accommodative responses to pilocarpine in monkeys. Invest Ophthalmol Vis Sci 37(ARVO Abstracts):S202, 1996 541. Peterson JA, Tian B, Kiland JA et al: Latrunculin (LAT-A) & staurosporin, but not swinholide (SWIN)-A, increase
outflow facility in the monkey. Invest Ophthalmol Vis Sci 37(ARVO Abstract):S825, 1996 542. Peterson JA, Tian B, Geiger B et al: In cynomolgus monkeys latrunculin (LAT)-A, LAT-B increase outflow facility, LAT-A
decreases intraocular pressure and initially increases aqueous
humor formation. Invest Ophthalmol Vis Sci 38(ARVO Abstracts):S243, 1997 543. Epstein DL: Open angle glaucoma. Why not a cure? [editorial]. Arch Ophthalmol 105:1187, 1987 544. Bárány EH, Scotchbrook S: Influence of testicular hyaluronidase on the resistance to flow through
the angle of the anterior chamber. Acta Physiol Scand 30:240, 1954 545. Francois J, Rabaey M, Neetens A: Perfusion studies on the outflow of aqueous humor in human eyes. Arch Ophthalmol 55:193, 1956 546. Pedler WS: The relationship of hyaluronidase to aqueous outflow resistance. Trans Ophthalmol Soc UK 76:51, 1956 547. Hubbard WC, Johnson M, Gong H et al: Intraocular pressure and outflow facility are unchanged following acute
and chronic intracameral chondroitinase ABC and hyaluronidase in monkeys. Exp Eye Res 65:177, 1997 548. Rees D, Lloyd CW, Thom D: Control of grip and stick in cell adhesion through lateral relationships
of membrane glycoproteins. Nature 267:124, 1977 549. Tokiwa T, Hoshika T, Shiraishi M et al: Mechanism of cell dissociation with trypsin and EDTA. Acta Med Okayama 33:1, 1979 550. Bill A: Effects of Na2EDTA and alpha-chymotrypsin on aqueous humor outflow conductance
in monkey eyes. Uppsala J Med Sci 85:311, 1980 551. Alexander JP, Conger DM, Acott TS: Effects growth factors on matrix metalloproteinase and TIMP production
by trabecular cells. Invest Ophthalmol Vis Sci 36(ARVO Abstract):S727, 1995 552. Bradley JMB, Conger DM, Fisk AS (eds): Matrix metalloproteinases increase outflow facility in perfused anterior
segment explants. Invest Ophthalmol Vis Sci 36(ARVO Abstract):S727, 1995 553. Geiger B, Yehuda-Levenberg S, Bershadsky AD: Molecular interactions in the submembrane plaque of cell-cell and cell-matrix
adhesions. Acta Anat 154:46, 1995 554. Grierson I, Lee WR: Pressure-induced changes in the ultrastructure of the endothelium lining
of Schlemm's canal. Am J Ophthalmol 80:863, 1975 555. Grierson I, Lee WR: The fine structure of the trabecular meshwork at graded levels of intraocular
pressure. II. Pressures outside the physiological range (0 and 50 mmHg). Exp Eye Res 20:523, 1975 556. Gipson IK, Anderson RA: Actin filaments in cells of human trabecular meshwork and Schlemm's
canal. Invest Ophthalmol Vis Sci 18:547, 1979 557. Grierson I, Rahi AH: Microfilaments in the cells of the human trabecular meshwork. Br J Ophthalmol 63:3, 1979 558. Ringvold A: Actin filaments in trabecular endothelial cells in eyes of the vervet monkey (Cercopithecus
aethiops). Acta Ophthalmologica 56:217, 1978 559. Tripathi RC, Tripathi BJ: Contractile protein alteration in trabecular endothelium in primary open-angle
glaucoma. Exp Eye Res 31:721, 1980 560. Brown SS, Spudich JA: Mechanism of action of cytochalasin: Evidence that it binds to actin filament
ends. J Cell Biol 88:487, 1981 561. Davies P, Allison AC: Effects of cytochalasin B on endocytosis and exocytosis. In
Tannenbaum SW (ed): Cytochalasins: Biochemical and Cell Biological
Aspects, p 143. Amsterdam: North Holland, 1978 562. Godman GC, Miranda AF: Cellular contractility and the visible effects of
cytochalasin. In Tannenbaum SW (ed): Cytochalasins: Biochemical and
Cell Biological Aspects, p 277. Amsterdam: North Holland, 1978 563. Plagemann PGW, Wohlheuter RM, Graff JC, Marz R: Inhibition of carrier mediated
and non-mediated permeation processes by cytochalasin B. In Tannenbaum
SW (ed): Cytochalasins: Biochemical and Cell Biological Aspects, p 445. Amsterdam: North Holland, 1978 564. Brenner SL, Korn ED: Substoichiometric concentrations of cytochalasin D inhibit actin polymerization: Additional
evidence for an F-actin treadmill. J Biol Chem 254:9982, 1979 565. Johnstone M, Tanner D, Chau B, Kopecky K: Concentration-dependent morphologic effects of cytochalasin B in the aqueous
outflow system. Invest Ophthalmol Vis Sci 19:835, 1980 566. Svedbergh B, Lütjen-Drecoll E, Ober M, Kaufman PL: Cytochalasin B-induced structural changes in the anterior ocular segment
of the cynomolgus monkey. Invest Ophthalmol Vis Sci 17:718, 1978 567. Erickson-Lamy KA, Schroeder A, Epstein DL: Ethacrynic acid induces reversible shape and cytoskeletal changes in cultured
cells. Invest Ophthalmol Vis Sci 33:2631, 1992 568. Johnson DH, Tschumper RC: Ethacrynic acid: Outflow effects and toxicity in human trabecular meshwork
in perfusion organ culture. Curr Eye Res 12:385, 1993 569. Croft MA, Wang RF, Podos SM et al: Effect of ticrynafen (TI) on aqueous dynamics in monkeys. Invest Ophthalmol Vis Sci 38(ARVO Abstracts):S243, 1997 570. Rodewald R, Newman SB, Karnovsky MJ: Contraction of isolated brush borders from the intestinal epithelium. J Cell Biol 70:541, 1976 571. Campbell DG, Simmons RJ, Grant WM: Ghost cells as a cause of glaucoma. Am J Ophthalmol 81:441, 1976 572. Campbell DG, Essingman EM: Hemolytic ghost cell glaucoma: Further studies. Arch Ophthalmol 97:2141, 1979 573. Goldberg MF: The diagnosis and treatment of sickled erythrocytes in human hyphemias. Trans Am Ophthalmol Soc 76:481, 1978 574. Flocks M, Littwin CS, Zimmerman LE: Phacolytic glaucoma: Clinicopathologic study of 138 cases of glaucoma associated
with hypermature cataract. Arch Ophthalmol 54:37, 1955 575. Fenton RH, Zimmerman LE: Hemolytic glaucoma. An unusual cause of acute open-angle secondary glaucoma. Arch Opthalmol 70:236, 1963 576. Yanoff M: Glaucoma mechanisms in ocular malignant melanomas. Am J Ophthalmol 70:898, 1970 577. Peterson HP: Can pigmentary deposits on the trabecular meshwork increase the resistance
of the aqueous outflow? Acta Ophthalmol 47:743, 1969 578. Quigley HA: Long-term follow-up of laser iridotomy. Ophthalmology 88:218, 1981 579. Anderson DR: Experimental alpha chymotrypsin glaucoma studied by scanning electron microscopy. Am J Ophthalmol 71:470, 1971 580. Worthen DM: Scanning electron microscopy after alpha chymotrypsin perfusion in man. Am J Ophthalmol 73:637, 1972 581. Channell MM et al: Intraocular pressure changes after Neodynium-YAG laser posterior capsulotomy. Arch Ophthalmol 102:1024, 1984 582. Kaufman PL, Lütjen-Drecoll E, Hubbard WC, Erickson KA: Obstruction of aqueous humor outflow by cross-linked polyacrylamide microgels
in bovine, monkey and human eyes. Ophthalmology 101:1672, 1994 583. Epstein DL, Jedziniak JA, Grant WM: Obstruction of aqueous outflow by lens particles and by heavy-molecular
weight soluble lens proteins. Invest Ophthalmol Vis Sci 17:272, 1978 584. Epstein DL, Jedziniak JA, Grant WM: Identification of heavy molecular weight soluble lens protein in aqueous
humor in phakolytic glaucoma. Invest Ophthalmol Vis Sci 17:398, 1978 585. Epstein DL, Hashimoto JM, Grant WM: Serum obstruction of aqueous outflow in enucleated eyes. Am J Ophthalmol 86:101, 1978 586. Davanger M: On the molecular composition and physiochemical properties of the pseudoexfoliation
material. Acta Ophthalmol 55:621, 1977 587. Barsotti M, Bartels SP, Barsotti MF, Kamm RD: The source of proteins in the aqueous humor of the normal monkey eye. Invest Ophthalmol Vis Sci 33:581, 1992 588. Freddo TF, Bartels SP, Barsotti MF, Kamm RD: The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci 31:125, 1990 589. Freddo TF: The Glenn A. Fry award lecture 1992: Aqueous humor proteins: A key for
unlocking glaucoma? Optom Vis Sci 70:263, 1993 590. Raviola G: The structural basis of the blood-ocular barriers. Exp Eye Res 25:27, 1977 591. Johnson M, Gong H, Freddo TF et al: Serum proteins and aqueous outflow resistance in bovine eyes. Invest Ophthalmol Vis Sci 34:3549, 1993 592. Sit AJ, Gong H, Ritter N et al: The role of soluble proteins in generating aqueous outflow resistance in
the bovine and human eye. Exp Eye Res 64:813, 1997 593. Kee C, Gabelt BT, Gange SJ, Kaufman PL: Serum effects on aqueous outflow during anterior chamber perfusion in monkeys. Invest Ophthalmol Vis Sci 37:1840, 1996 594. Chrzanowska-Wodnicka M, Burridge K: Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton
in response to serum or LPA stimulation. J Cell Sci 107:3643, 1994 595. Seufferlein T, Rozengurt E: Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion
kinase, paxillin, and p130. J Biol Chem 269:9345, 1994 596. Probst LE, Hakim OJ, Nichols BD: Phacoemulsification with aspirated or retained Viscoat. J Cataract Refract Surg 20:145, 1994 597. Duperre J, Grenier B, Lemire J et al: Effect of timolol vs acetazolamide on sodium hyaluronate-induced rise in
intraocular pressure after cataract surgery. Can J Ophthalmol 29:182, 1994 598. Dada VK, Sindhu N, Sachdev MS: Postoperative intraocular pressure changes with use of different viscoelastics. Ophthal Surg 25:540, 1994 599. Raitta C, Lehto I, Puska P et al: A randomized, prospective study on the use of sodium hyaluronate (Healon) in
trabeculectomy. Ophthal Surg 25:536, 1994 600. Shibasaki H, Kurome H, Hayasaka S et al: Viscoelastic substance in the anterior chamber elevates intraocular pressure. Ann Ophthalmol 26:10, 1994 601. Hager H: Besondere mickrochirurgische Eingraffe. II. Erste Erfahrugen mid dem Argon
Laser Gerät 800. Klin Monatsbl Augenheilkd 162:437, 1973 602. Krasnov MM: Laser puncture of anterior chamber angle in glaucoma. Am J Ophthalmol 75:674, 1973 603. Teichmann I, Teichmann KD, Fechner PU: Glaucoma operation with the argon laser. Eye Ear Nose Throat Monthly 55:58, 1976 604. Ticho U, Zauberman H: Argon laser application to the angle structures in the glaucomas. Arch Ophthalmol 94:61, 1976 605. Wickham MG, Worthen DM: Argon laser trabeculotomy: Long-term follow-up. Ophthalmology 86:495, 1979 606. Worthen DM, Wickham MG: Argon laser trabeculotomy. Trans Am Acad Ophthalmol
Otolaryngol 78:OP371, 1974 607. Gaasterland D, Kupfer C: Experimental glaucoma in the rhesus monkey. Invest Ophthalmol Vis Sci 13:455, 1974 608. Schwartz AL, Whitten ME, Bleiman B: Argon laser trabecular surgery in uncontrolled phakic open-angle glaucoma. Ophthalmology 88:203, 1981 609. Wilensky JT, Jampol LM: Laser therapy for open-angle glaucoma. Ophthalmology 88:213, 1981 610. Wise JB, Witter SL: Argon laser therapy for open-angle glaucoma: A pilot study. Arch Ophthalmol 97:319, 1979 611. Wise JB: Long-term control of adult open-angle glaucoma by argon laser treatment. Ophthalmology 88:197, 1981 612. Lyle WM, Cullen AP, Charman WN: Role of lasers in eye care. Optom Vis Sci 70:136, 1993 613. Moriarty AP: Diode lasers in ophthalmology. Int Ophthalmol 17:297, 1993-1994 614. McMillan TA, Stewart WC, Legler UF et al: Comparison of diode and argon laser trabeculoplasty in cadaver eyes. Invest Ophthalmol Vis Sci 35:706, 1994 615. Parshley DE, Bradley JM, Samples JR et al: Early changes in matrix metalloproteinases and inhibitors after in vitro
laser treatment to the trabecular meshwork. Curr Eye Res 14:537, 1995 616. Parshley DE, Bradley JM, Fisk A et al: Laser trabeculoplasty induces stromelysin expression by trabecular juxtacanalicular
cells. Invest Ophthalmol Vis Sci 37:795, 1996 |