ARN was originally described in Japan in 1971 by Urayama and coworkers, who studied six healthy patients with acute onset of a unilateral panuveitis and retinal arteritis that resulted in widespread peripheral, confluent retinal necrosis and eventual retinal detachment.7 This entity was first reported in English medical literature in 1977 by Willerson and associates, who described two patients with a bilateral necrotizing vaso-occlusive retinitis.8 The term acute retinal necrosis (ARN; or BARN, when bilateral), was coined by Young and Bird in 1978.9 Although this syndrome was initially described in healthy patients, it subsequently has been reported in individuals with compromised systemic immunity—most notably in those with human immunodeficiency virus (HIV) infection.
ARN can be a visually devastating disease with a poor visual prognosis, although advances in antiviral therapy and vitreoretinal techniques for retinal detachment have improved visual outcomes. Prompt diagnosis and treatment are necessary to limit retinal damage and preserve vision.
CLINICAL PRESENTATION
Acute retinal necrosis does not appear to have any racial predilection; however, it may be more common in males. Although ARN typically affects individuals between 20 and 50 years of age, cases have been reported in patients as young as 4 years and as old as 89 years of age.10–13
Initially, patients may complain of mild to moderate ocular or periorbital pain, often accompanied by a red eye. Early visual symptoms may or may not be present and can be insidious. When symptoms are present, these usually consist of floaters, blurred vision, or rarely, decreased peripheral vision. Acute central visual loss is an atypical presentation because the posterior pole usually is spared from the retinopathy until late in the course of the disease. Optic nerve involvement, retinal artery occlusion, and retinal detachment can affect central vision but usually are not manifest at presentation.7–10,14,15
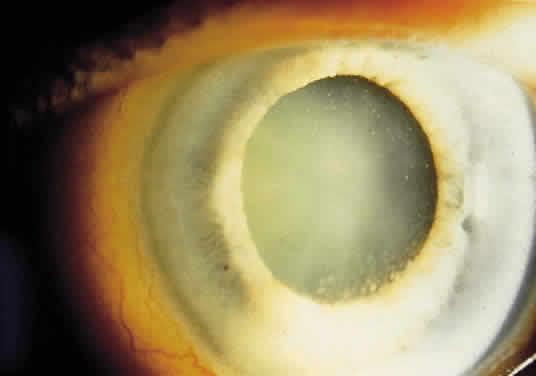
External examination may reveal diffuse episcleritis, scleritis, or orbital inflammatory disease. Mild to moderate anterior segment cellular reaction is common and either granulomatous or fine keratic precipitates typically are present 10,16,17(Fig. 1). A hypopyon typically does not develop.8 The intraocular pressure may be low, even in the absence of retinal detachment; however, elevated intraocular pressure secondary to anterior segment inflammation also may occur. Concurrent herpesvirus infection of the cornea with dendritic or stromal keratitis is not a typical feature of ARN syndrome in healthy individuals, although it has been described in patients with HIV infection.18,19 One case of simultaneous HSV type 1 keratitis and ARN was reported in a patient with Ramsay Hunt syndrome.20,21
|
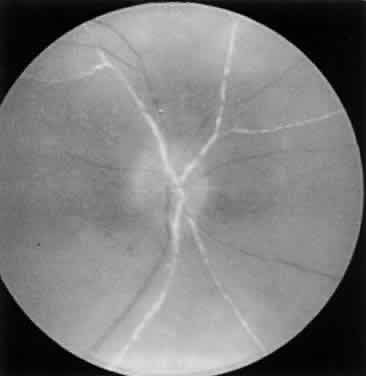
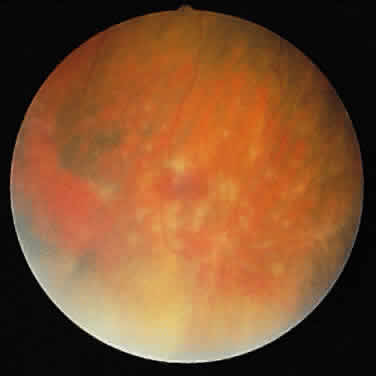
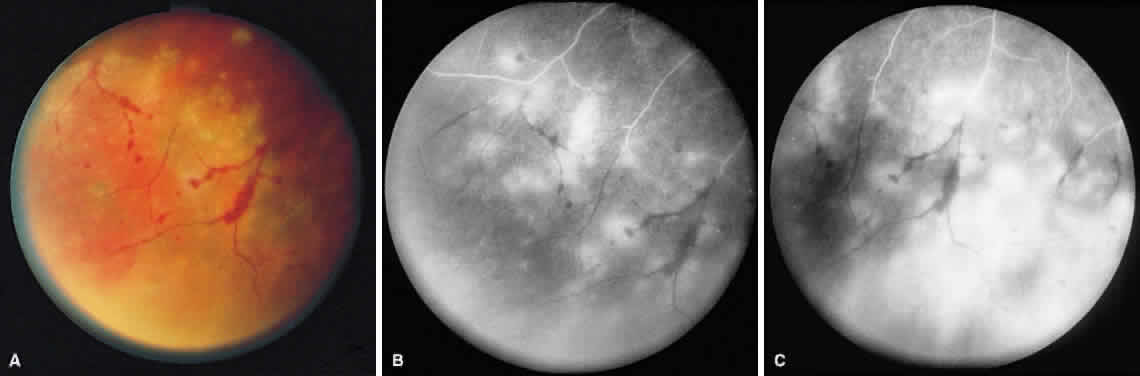
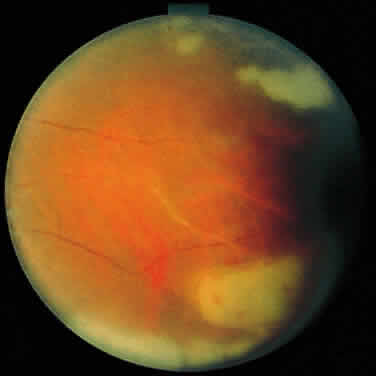
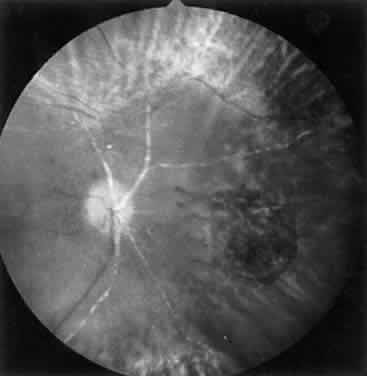
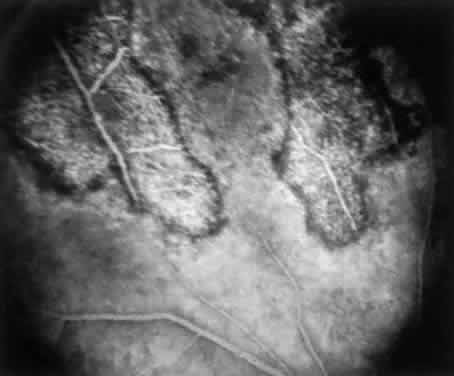
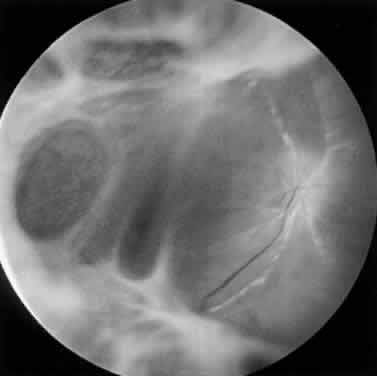
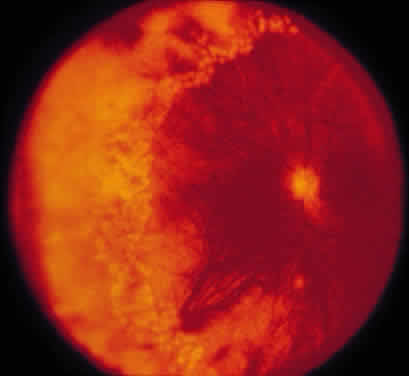
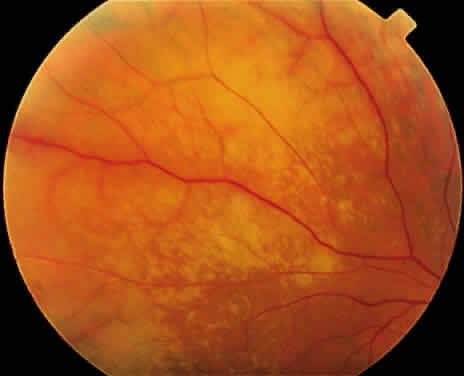
ARN is characterized by a retinal vasculitis affecting both the arteries and veins in the fundus, which is manifested by sheathing of the larger vessels (Fig. 2).7–10,16 Initially, patchy areas of peripheral retinal whitening (“thumbprint lesions”) representing full-thickness retinal necrosis are present or develop shortly after the vasculitis (Fig. 3). During a course that may span days or weeks, these patches coalesce into geographic areas (Fig. 4A). The entire peripheral retina (360 degrees) may be involved, or, more commonly, there are several noncontiguous patches of necrosis, each covering from a half to three clock hours (Figs. 5 and 6). The posterior segment lesions may not be detected without examination of the peripheral retina.
|
|
|
|
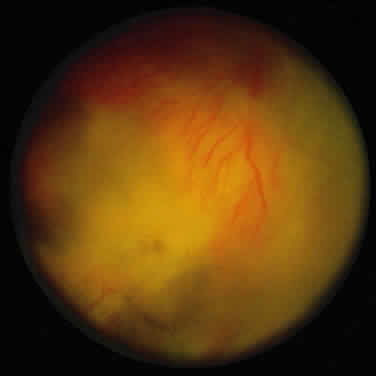
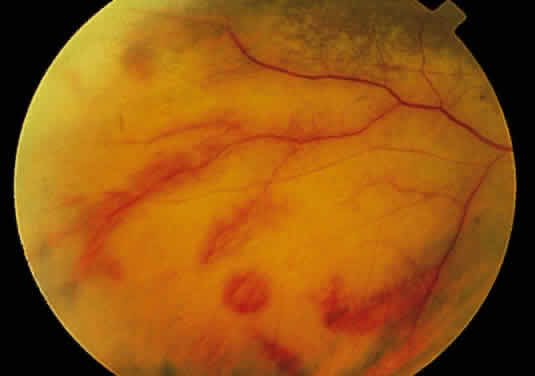
As the infection progresses, the leading edge of confluent retinal whitening advances toward the posterior pole (Fig. 7A). The retinitis may not progress posteriorly to the vascular arcades, sparing the macula and central vision. It is hypothesized that the retinal necrosis in ARN results from the combined effect of intracellular viral replication with subsequent cell death and vascular occlusion secondary to acute vasculitis. In some patients, the retinal vasculitic component may be much more prominent than the retinal necrosis.7 Optic disc swelling, either hyperemic or pallid, is a common feature of the ARN syndrome.1 Perivascular hemorrhages may be present (Fig. 8); however, widespread areas of retinal hemorrhage are atypical. Retinal vascular occlusion, often involving the arteries, can occur at any point during the clinical course. Without treatment, the inflammatory component of ARN typically burns out in 6 to 12 weeks, leaving behind a thin atrophic retina with associated pigmentary changes.10
|
Along with anterior uveitis, a mild to moderate vitritis is present early in the course of ARN (Fig. 9). As the retina becomes necrotic, increasing amounts of vitreous cells and debris are seen. The vitritis may impair visualization of the posterior segment. Eventual opacification and organization of the vitreous can occur. Severe vitreous fibrosis with traction resembling proliferative vitreoretinopathy is not an uncommon late complication.1,8–12,14,15,17 Other features described in ARN include exudative retinal detachment, macular edema, glaucoma, and cataract.4
|
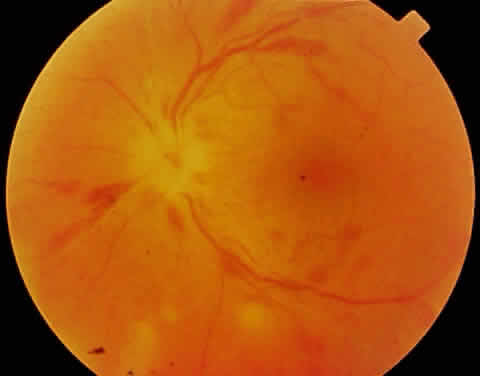
As the active retinal inflammatory process recedes, either spontaneously or with treatment, the retinal areas previously infected appear thinned and atrophic, with underlying retinal pigment epithelial alterations (Fig. 10). These pigmentary changes usually begin to develop around 4 weeks after the onset of the infection.22 Often a distinct scalloped pattern can be seen that clearly demarcates previously involved and spared retina (Fig. 11). The pigmentary alterations result from both retinal pigment epithelial cell death by direct infection and secondary inflammatory changes and hyperplasia induced by the adjacent retinal necrosis. Full-thickness retinal breaks are a prominent feature of the ARN syndrome, and often develop during the recovery stage of ARN. Retinal breaks may be large, irregular, and multiple and classically occur at the junction of normal and affected retina (Fig. 12)23; they can, however, occur elsewhere in the retina, with or without accompanying vitreous fibrosis and traction. Clarkson and coworkers noted traction-associated retinal tears in uninvolved retina.14 Rhegmatogenous retinal detachment is a major cause of visual loss and occurs in 50% to 75% of cases not treated with prophylactic laser photocoagulation.1,10,14 Retinal detachment caused by ARN usually develops several weeks after the onset of inflammation; however, retinal detachment has been reported as early as 6 days or as late as several months after the onset of symptoms.24 These detachments are often complicated because of the combined effect of vitreous organization and multifocal retinal breaks. Unusual late sequelae of ARN syndrome include neovascularization of the retina and optic disc with vitreous hemorrhage (Fig. 13).25,26 In contrast to other uveitic syndromes, chronic or relapsing episodes of intraocular inflammation are not common in ARN.27
|
|
|
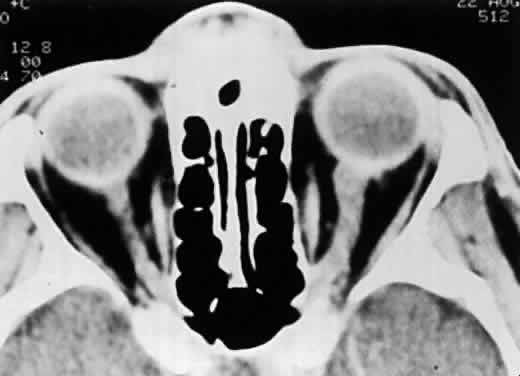
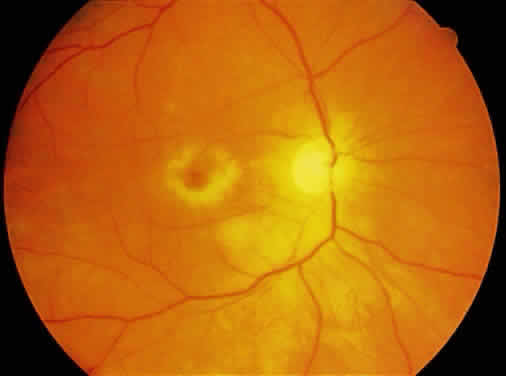
In the absence of direct macular involvement, arterial obstruction, or retinal detachment, decreased central vision that is inconsistent with the associated retinal findings is most likely secondary to optic nerve involvement.28 The presence of optic disc swelling with ARN was noted by early investigators.10 Histopathologic studies have confirmed the presence of intraneural inflammation and necrosis.1,29–31 Both direct inflammation of the optic nerve substance and a secondary ischemic component are believed to produce ARN optic neuropathy, paralleling the pattern of visual dysfunction in the retina. When optic nerve involvement occurs in ARN, an acquired dyschromatopsia, a relative afferent pupillary defect in disproportion to the amount of retinal necrosis, central or arcuate visual field defects, and an enlarged optic nerve sheath can be detected with neuroimaging28,31 (Fig. 14). It often is difficult to distinguish whether visual loss is caused by optic neuropathy or retinal disease in these severely affected patients. An accurate assessment of the incidence of ARN optic neuropathy has not been made. Clinically, optic nerve swelling occurs in a significant number of patients with ARN and may resolve rapidly with therapy.
Bilateral involvement in ARN eventually occurs in up to 65% of patients.10,23 There is no correlation between the severity of ARN in the first eye and severity of the disease in the fellow eye. Involvement of the second eye may not be apparent initially; there may be a delay of several weeks. Thirty-four years is the longest reported interval for involvement of the contralateral eye in ARN.32 Although this is an atypical scenario, it emphasizes the need for long-term ophthalmologic examinations in patients with ARN.
Initial reports indicated that the long-term visual prognosis in ARN syndrome was poor; 60% of eyes had a final visual acuity of 20/200 or worse.10,31Potential causes of visual loss in ARN syndrome include the following:
- Media opacity
- Retinal cell necrosis, infection, ischemia, retinal detachment, macular
pucker
- Optic neuropathy
A modified classification of the stages of ARN syndrome is presented in Table 1. However, not all cases of ARN have a fulminant course and result in a poor outcome. Mild cases of ARN with limited peripheral retinitis and good visual outcomes have been reported, and some cases resolve spontaneously without treatment.33 In a recent study of eyes with limited retinal involvement at the time of diagnosis, final visual acuity was 20/40 or better in 50% and 20/400 or better in 92% of eyes after treatment with acyclovir and prophylactic laser photocoagulation.34 The growing number of ARN cases with a good visual outcome is likely owing to increased physician awareness of this disorder, which leads to earlier diagnosis and aggressive therapy. The features of ARN may vary in severity depending on the specific etiologic agent and the immune status of the individual. For example, ARN associated with primary VZV infection (chickenpox) may have less severe features and a better visual prognosis.35
TABLE 28-1. Stages of Acute Retinal Necrosis
| Stage | Description |
| I | Necrotizing retinitis |
| Ia | Discrete areas of peripheral retinitis |
| Ib | Confluent areas of peripheral retinitis, papillitis, macular edema |
| II | Vitreous opacification or organization |
| III | Regression of retinal necrosis; secondary pigmentation of the lesions with contraction and condensation of the vitreous base |
| IV | Retinal detachment |
| IVa | Acute retinal tears or detachment with traction, or proliferative vitreoretinopathy |
| IVb | Chronic retinal detachment |
Central nervous system abnormalities occasionally have been associated with ARN syndrome. Cerebrospinal fluid pleocytosis is well documented.10,28,36 One case of labyrinthine deafness in association with diffuse cerebral atrophy has occurred.37 True viral encephalitis usually is not concomitant with ARN syndrome; however, posterior segment inflammatory conditions that resemble ARN have been reported in association with florid viral encephalitis.38,39 A rapidly progressive, bilateral retinal necrosis caused by HSV has been described in two patients with concomitant HSV encephalitis and acquired immunodeficiency syndrome (AIDS).5 Because of the presence of central nervous system abnormalities as well as an acute ARN optic neuropathy, it has been suggested that ARN be reclassified as a “uveo-meningeal syndrome” instead of an isolated retinitis.28
Initial reports of ARN were in healthy patients with normal systemic immunity and no extraocular manifestations of herpetic infection. This continues to be true in most ARN cases. Most cases of ARN are believed to be secondary to reactivation of a latent herpetic infection, and often patients have a prior history of dermatomal VZV (herpes zoster) or primary VZV (chickenpox) infection, or perioral blisters, presumably caused by HSV infection. Reactivation of the viral genome from the trigeminal ganglion may result in the development of ARN after transneural spread. Trauma-induced reactivation of latent congenital HSV type 2 infection has been reported in three cases of ARN.13 It is uncommon for healthy individuals to develop ARN simultaneously with cutaneous VZV or HSV infection.40,41 Concurrent aphthous ulcers have been reported in two patients with ARN.42 Rare cases of ARN have been described in immunocompetent individuals (primarily adults) with primary VZV infection and in those who have had close contact with an individual with chickenpox but who did not experience skin lesions.35,43
ARN subsequently has been reported in immunocompromised patients with HIV infection, autoimmune disorders, cancer, and organ transplants.44–46 It has been recommended that ARN syndrome be defined by its ocular features rather than by the immune status of the affected individual.4 In patients with HIV infection and ARN, the CD4+ T lymphocyte count generally is greater than 60 cells/μl, although ARN is sufficiently infrequent in these patients to allow any conclusions to be drawn regarding the CD4+ count. HIV-infected patients are more likely to have simultaneous or recurrent VZV or HSV infections.45 Bilateral ARN after unilateral herpes zoster ophthalmicus has been reported as a presenting sign of AIDS.45 In a study of patients with VZV involving the ophthalmic division of the trigeminal nerve, 5 of 29 (17%) patients with HIV infection developed ARN within 9 months of follow-up, but there were no cases of ARN in immunocompetent individuals.47 ARN syndrome associated with HIV infection is typical in that it is rapidly progressive, peripheral in location, and responsive to acyclovir treatment. However, the retinitis often is more severe and bilateral, and often results in a worse visual outcome than in immunocompetent individuals.5,47
ETIOLOGY AND HISTOPATHOLOGY
Similarities between ARN syndrome and forms of viral retinitis such as neonatal HSV infections and CMV retinitis have been noted.8,9,16 Early studies either failed to detect rising serum antibody titers to common viruses or failed to culture virus from the vitreous of patients with ARN, and the viral theory remained speculative.17,48,49 In 1982, Culbertson and coworkers found viral particles consistent with a member of the herpes family of viruses in retinal tissue from an individual with ARN.1 Positive serum VZV titers and positive intraocular VZV antigen studies have been documented in some patients with ARN2,50–52; VZV also has been cultured from the vitreous during the active phase of ARN.2 Along with VZV, it is now clear that HSV types 1 and 2 also can produce ARN syndrome13,15,30,53; HSV has been cultured from the vitreous and HSV-DNA types 1 and 2 have been identified by polymerase chain reaction.3–5
Two members of the herpesvirus family, HSV and VZV, most commonly produce the clinical syndrome of ARN. Although CMV antigen was noted histopathologically in an enucleation specimen from a patient with bilateral ARN, subsequent studies have not supported an association between CMV and ARN syndrome.29 Under various clinical situations, HSV and VZV have been associated with a retinitis or choroiditis distinct from ARN and it is not clear what specific circumstances result in the ARN syndrome as opposed to other clinical ocular inflammatory syndromes. Most cases of cutaneous VZV and HSV are not associated with ARN syndrome; other factors must play a role in viral reactivation. An association between ARN and certain HLA haplotypes, such as the HLA-DQw7 antigen and the phenotype HLA-Bw62, DR4, has been postulated to account for the tendency of some individuals to develop ARN syndrome.54 It has been hypothesized that ARN may be a specific syndrome caused by a relatively recent mutation in the herpesvirus.42 There were few reports of similar cases before 1971 and most clinicians believe that ARN syndrome is a new disease.
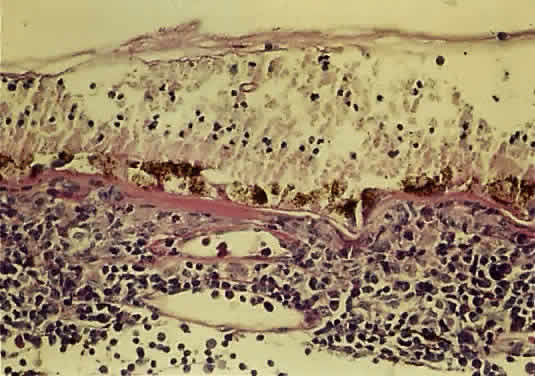
The histopathology of ARN shows profound necrosis of the retina, retinal arteritis, and viral inclusions in retinal cells.1 Examination of an eye with end-stage ARN secondary to VZV revealed diffuse full-thickness, necrotizing retinitis, replacement of sensory retinal structures by glial tissue, occlusive retinal arteritis, granulomatous choroiditis, optic neuritis with ischemic optic atrophy, and VZV in the choroid and the choriocapillaris55 (Fig. 15).
|
DIAGNOSTIC EVALUATION AND ANCILLARY TESTS
The diagnosis of ARN is based on a clinical examination and a characteristic funduscopic appearance. Although the signs of ARN may vary in severity, the American Uveitis Society proposed the following clinical criteria for diagnosis of ARN syndrome, regardless of an individual's systemic or immune status: a discrete peripheral necrotizing retinitis that progresses rapidly and circumferentially without antiviral therapy; prominent vitreous and anterior chamber inflammation; and an occlusive vasculitis with arteriolar involvement.6 Other features supporting the diagnosis of ARN include optic neuropathy or atrophy, scleritis, and ocular pain.
Diagnostic vitrectomy or retinal biopsy may be indicated in some atypical cases. However, evaluation of intraocular samples with various techniques, such as viral culture, serology, polymerase chain reaction, and histopathologic examination, do not always yield proof of a viral infection. Intraocular antibodies in the vitreous or aqueous have been measured to determine the cause of necrotizing retinitis.56 In one study, intraocular antibody production to VZV or HSV was detected in 57% of patients with typical ARN syndrome.57 The viral cause of ARN often is difficult to determine in an end-stage atrophic or detached retinal specimens, and may be more readily identified in ocular specimens obtained during acute stages of the disease.58
Systemic laboratory evaluations are suggested to help delineate the specific viral cause of ARN syndrome, to rule out possible associated immunodeficiency, and to guard against potential complications of therapy. Despite the fact that they may not be of benefit in the initial treatment of the patient, acute and convalescent serologic viral titers to HSV type 1, HSV type 2, VZV, and CMV may be obtained for diagnostic confirmation and epidemiologic data. Positive viral titers may prove particularly helpful in guiding therapy for an immunosuppressed patient who may harbor a subclinical, disseminated infection. A diagnostic increase or decrease in herpes viral antibody titers during serial sampling has been noted in only 40% of ARN cases because serum antibody levels may not reflect localized viral reactivation in the eye.59 Evaluation of the ratio of viral antibody levels in paired serum and intraocular fluid samples, which identified the etiologic agent of ARN syndrome in approximately 80% of cases, appears to be more efficacious.59 A complete blood cell count, as well as renal and liver function tests, should be monitored if acyclovir therapy will be used. Antibody testing for HIV should be obtained. If systemic corticosteroids will be used, exposure to tuberculosis should be ruled out with skin testing and chest roentgenography.
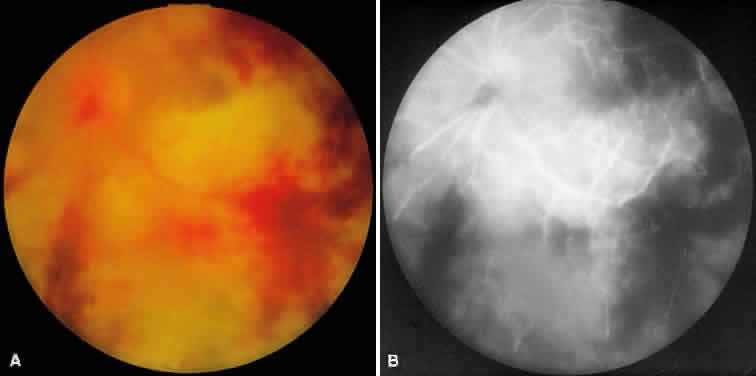
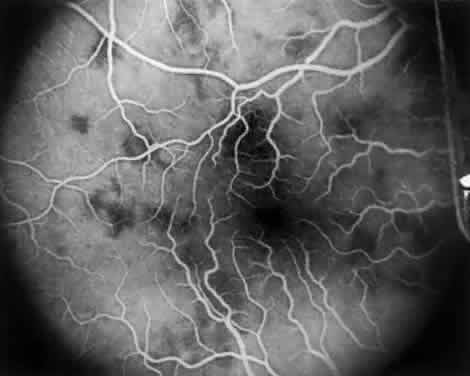
Intravenous fluorescein angiography may be helpful in delineating the extent of infection and elucidating the cause of central visual loss. In the early frames, choroidal perfusion defects may be seen; these defects are caused by areas of focal choroidal inflammatory cell accumulation and overlying retinal pigment epithelial damage (Fig. 16). Such choroidal perfusion defects may occur away from zones of active necrosis.42 Acute obstruction of the central retinal artery or any of its branches may be present. Peripheral views in the areas of active retinitis commonly show little or no intravascular fluorescein in the retinal arteries and veins. Often an abrupt “cut-off” of the intravascular fluorescein may be apparent at the edges of the retinal inflammation (see Fig. 4B and C). Areas of active retinitis show blockage of the underlying choroidal fluorescein pattern (see Fig. 7B). Recirculation phase views may reveal macular leakage, optic disc, and retinal vasculature staining.
|
Ultrasonography is invaluable for detecting retinal detachment when the degree of vitritis precludes examination of the posterior segment. In addition, ultrasound may be used to diagnose enlargement of the optic nerve sheath, which occurs with ARN optic neuropathy.28 Color Doppler imaging has identified hemodynamic compromise in the central retinal artery, suggesting that ischemia plays a role in the pathogenesis of ARN.60
The results of electroretinography have been reported for a limited number of patients. Bright-flash electroretinography tends either to be normal or to show reduced amplitudes, depending on the amount of retina affected.11,30 In a fulminant case of ARN caused by HSV type 1, the electroretinography was completely extinguished early in the disease course.4
Computed tomography scans often show optic nerve sheath enlargement on the side affected with ARN.28 Bilateral optic nerve sheath enlargement has been documented despite the presence of unilateral ARN.3 Magnetic resonance imaging (MRI) has shown concurrent lesions of the optic tracts, chiasm, and lateral geniculate body in a patient with ARN, suggesting that infection may spread through the axons of the ganglion cells.3
TREATMENT
Intravenous acyclovir is the current medical treatment of choice for active ARN. Acyclovir works by selectively inhibiting herpesvirus DNA polymerase. It has antiviral activity against HSV type 1, HSV type 2, and VZV. Most strains of CMV are resistant to acyclovir. The retinitis in ARN typically shows a rapid response to acyclovir therapy, in which progression of retinitis ceases in 3 to 5 days and eventual atrophy of the infected retina occurs. Untreated eyes tend to show regression of the necrotic lesions spontaneously over a period of 6 to 12 weeks. Acyclovir therapy speeds this regression and prevents new lesion formation. In unilateral cases of ARN, acyclovir reduces but does not completely eliminate the risk of fellow eye involvement.61 Initial treatment with a 10 day course of high dose intravenous acyclovir (10 mg/kg every 8 hours) is followed by oral acyclovir (800 mg taken 5 times a day) for up to 14 weeks.61 The time interval for therapy is based on the observation that ARN in the second eye most often occurs within 6 to 14 weeks of the initial symptoms in the first eye. ARN in healthy patients does not generally recur in the same eye after antiviral treatment.27 Long-term oral maintenance therapy may be required in immunosuppressed patients who develop recurrent lesions. Acyclovir has not conclusively been shown to decrease the incidence of subsequent retinal detachment.24 Ganciclovir and foscarnet are alternative intravenous agents that are effective against herpesviruses; however, their use is less favorable because of potential systemic toxicity. New antiviral agents such as famciclovir and valcyclovir are available, but there is no conclusive data regarding their efficacy for treatment of ARN. Intravitreal injections of ganciclovir or foscarnet may be considered to treat individuals limited by systemic drug toxicity.
Anticoagulation to treat the vascular obstructive component of the ARN syndrome has been recommended by some investigators.11,42 Experimental evidence supporting this form of therapy was presented by Ando and associates, who found hyperaggregation of platelets in six of seven patients with bilateral ARN.62 Aspirin and corticosteroids in combination were found to ameliorate this hypercoagulation state. Aspirin may be included in the therapeutic regimen of ARN if there are no systemic contraindications. The efficacy of anticoagulants such as oral warfarin and intravenous heparin to prevent vaso-occlusive phenomena remains unknown; however, a beneficial effect has not been conclusively demonstrated and potentially fatal systemic side effects may occur.
Systemic and topical corticosteroids are advocated to suppress the inflammatory component of ARN and to speed clearing of the vitreous reaction.24 Systemic corticosteroids used alone have not demonstrated any beneficial effect in the early stages of ARN, and because of their immunoinhibitory effects, they should not be administered in active cases of ARN without concurrent acyclovir antiviral therapy.7–10,14,22 Other antiviral medications such as vidarabine have been given to patients with ARN without a clear-cut beneficial effect.10 Cytotoxic agents were used in some early cases based on the similarity between ARN and Behçet's disease; however, it currently is known that ARN is a viral retinitis and that cytotoxic therapy should not be used.8,48 If a patient with ARN syndrome is medically immunosuppressed, the immunosuppression should be reversed unless systemically contraindicated. Topical steroids and cycloplegics are useful in treating associated anterior segment inflammation.
Culbertson and coworkers recommended that confluent laser photocoagulation be applied posterior to the active retinitis in all cases of ARN.14 This treatment creates a “new” ora serrata within the healthy retina in order to localize to the periphery any subsequent retinal detachment. Laser treatment does not stop the progression of retinitis, and therefore repeat treatments may be necessary. Because clear media is required to perform laser treatment successfully, the cases manifesting the greatest vitreous reaction are least likely to be suitable for laser photocoagulation. Sternberg and associates noted retinal detachment in 17% of eyes treated prophylactically with laser compared with 67% of untreated eyes.12 Han and colleagues reported on five eyes with ARN that received laser treatment in which the retina did not subsequently detach.63 Most clinicians currently perform prophylactic laser photocoagulation circumferentially for 360 degrees at the junction of necrotic and healthy retina at the time of diagnosis of ARN if visualization is adequate (Fig. 17).
|
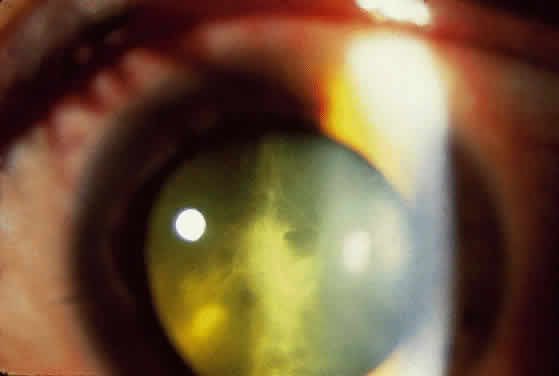
Of the first 52 cases of ARN reported, 72% progressed to retinal detachment and only 22% of those retinas were successfully reattached.10 In another study, a 50% incidence of ARN-associated retinal detachment was noted and 60% of these retinas were successfully reattached.14 Retinal detachment in eyes with a limited area of necrosis can be treated with scleral buckling, and a high posterior buckle is recommended to relieve vitreous traction on posterior breaks. In many cases, retinal detachments associated with ARN syndrome require pars plana vitrectomy for anatomic repair. This is owing to multiple factors, including media opacity, vitreous traction and fibrosis, and the nature, number, and posterior location of the retinal holes. Temporary or permanent retinal tamponade with long-acting gases or silicone oil often is necessary. Silicone oil tamponade has been shown to be efficacious and safe for repair of retinal detachments in necrotizing retinitis.64 Long-term complications of silicone oil include cataract, corneal opacification, hypotony, glaucoma, and silicone oil emulsification. Despite anatomical success, final visual acuity may be limited by optic atrophy secondary to optic nerve involvement, photoreceptor damage caused by detachment, or retinal cell death from infection. Prophylactic pars plana vitrectomy, scleral buckling, and intravitreal infusion of acyclovir has been reported to treat seven cases of ARN; although none of these patients subsequently developed retinal detachment, there was no control group available for comparison.11,15
Recently, increasing evidence has indicated that some of the acute loss of central vision in ARN is caused by an accompanying optic neuropathy.30 For this reason, Sergott and coworkers performed a modified optic nerve sheath decompression in selected patients with ARN who, after clinical examination and neuroimaging, were believed to have acute ARN optic neuropathy.31 Compared with a small group of concurrent patients who did not receive optic nerve surgery, those undergoing optic nerve sheath decompression were left with better central visual acuity. This work requires confirmation because it was based on an uncontrolled study.