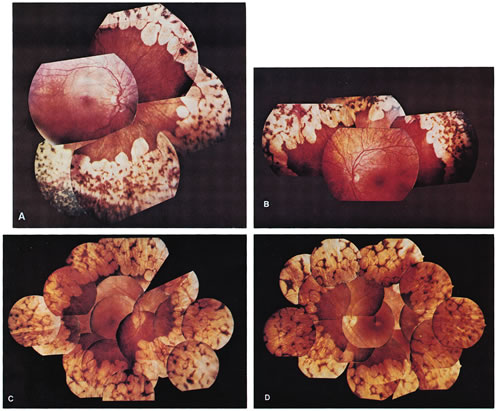
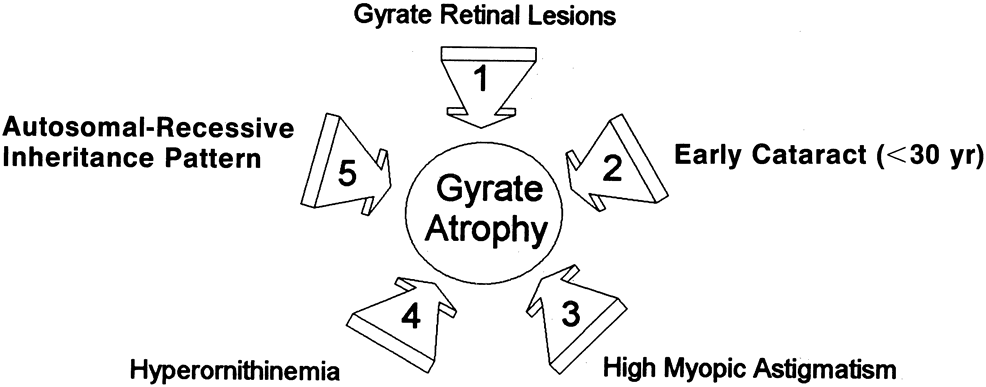
- Typical gyrate retinal and choroidal lesions (Fig. 1): The appearance of confluent arcuate equatorial full-thickness
lesions of the choroid and retina, sparing some of the larger choroidal
vessels and separated from one another by thin margins of pigment, is
characteristic of GA. The lesions extend without interruption
to the ora serrata, where a dense aggregation of pigment can be seen, but
they invade the circummacular vascular arcade only in advanced cases. Peripapillary
gyrate lesions are common, but foveal lesions are rare
until late in the course of the disease.
- Early cataract: Posterior subcapsular lens changes usually begin in the late teens, and
fully developed posterior subcapsular cataracts with diffuse cortical
opacities are almost invariably present by age 30.
- High myopia with marked astigmatism: Myopia of –6 to –10 diopters (D) or more, accompanied
by 2 D or more of astigmatism is present in about 90% of
cases so far reported. The occasional case may be only mildly myopic and
astigmatic.
- Hyperornithinemia: Extreme hyperornithinemia is universal in this disease. Its absence necessitates
a search for another diagnosis. All body fluids measured to
date (whole blood, plasma, cerebrospinal fluid, aqueous humor, and
urine) have been found to contain 10 to 20 times the normal levels
of ornithine.9,11
- Autosomal recessive inheritance pattern: To date, all patients with the oculometabolic disease have family trees
and laboratory genetic findings consistent with autosomal recessive
inheritance. It is possible to diagnose heterozygotes and to confirm the
inheritance pattern in a family with a single affected member (see
below).12
OPHTHALMIC FINDINGS
A detailed ophthalmic examination of a patient with GA usually reveals the following findings:
Visual acuity: Acuity in most cases is normal with correction, up until about age 10. There
appears to be a gradual decline in visual acuity thereafter, unrelated
to the development of cataracts. The slowly progressive irreversible
loss of vision from retinal degeneration often leads to blindness, usually
by the fourth or fifth decade of life.13 The appearance of a large peripapillary gyrate lesion is often accompanied
by a decline in central vision. Rarely, a patient may develop a foveal
gyrate lesion during the early stages of the disease, but these
lesions are often present in advanced cases. Occasionally, acuity is inexplicably
decreased in one eye, when compared with its fellow, in the
same patient. Variation in the severity of visual loss among patients
from different families is considerable and may be due to the tremendous
variety in the specific mutations in the GA gene found in different
families (see metabolic discussion below; e.g., pyridoxine-responsive
mutations tend to be less severe).
Pupils: Pupillary appearance and reactivity to light and near are generally normal. Patients
with marked asymmetry in acuity, visual fields, and other
parameters may have an afferent pupillary defect in the worse eye.
Motility: Extraocular muscle balance is usually normal, except for a moderate exophoria
not unlike that seen in other myopes. Patients with significantly
worse vision in one eye may have manifest exotropia.
Stereopsis: Stereopsis is normal as long as the visual acuity remains symmetric. A
decrease in stereopsis may be an early sign of developing exotropia and
decline in the visual acuity of one eye.
Color vision: Decreased color discrimination occurs when there is a drop in visual acuity
on the basis of retinal lesions, or with the development of cataracts. Otherwise, there
are no remarkable changes in color vision, as
the central color sensitive cones appear to be preserved until late in
the disease.14
Visual fields: Visual fields coincide fairly well with the remaining area of healthy-appearing
retina (i.e., about 15 to 50 degrees), with
an enlarged blind spot corresponding to the peripapillary lesion, if
present. Using fundus photoperimetry, Enoch and associates15 found that the limit of the functional visual field typically conforms
to the observed gyrate lesions with a sharp drop-off in visual
function at the border of the lesions. However, the functional visual
field may occasionally be considerably smaller than the central area
of the retina that appears to be healthy on ophthalmoscopy, and this is
often associated with a large peripapillary lesion.11
Lens: Posterior subcapsular cataracts develop in the late teens or early twenties
and progress to include extensive cortical changes primarily involving
opacification of the lens along the suture lines, extending toward
the equator of the lens.16 Anterior subcapsular fibrous plaques have been reported.17
Ciliary body: Abnormally short and scant ciliary processes have been reported from cycloscopic
examination of these patients.18
Vitreous: The vitreous is typically syneretic and frequently contains clusters of
cloudy fibrils. Recurrent vitreous hemorrhages during adolescence have
been noted in a few patients.19–21
Fundus: Gyrate appearance is typical (see Diagnosis section). Takki
and associates have described the presence of crystals in the demarcation
lines of the peripheral gyrate lesions in some patients.9,12,22 Choroidal large vessels may be seen where the retina and pigment epithelium
have atrophied. At the macula, a granular appearance may be observed
before atrophic changes become evident. A zone of pigmentary change
may separate normal and atrophic areas. These areas may represent abnormally
functioning retina and pigment epithelium prior to the stage
of visible atrophy.22,23 Bakker and colleagues24 described subretinal neovascularization and foveal disciform hemorrhages
in a case of GA. Marano and colleagues25,26 described GA associated with low-activity subfoveal choroidal neovascularization (CNV) development, possibly a result of the
high myopia present.
Intraocular pressure: Alteration in intraocular pressure has not been described in the literature
as an associated finding in GA. Since 1977, however, the author (SAA) has
been following a patient who developed typical pigmentary
glaucoma 5 years after bilateral intracapsular cataract extraction, with
dense bands of presumed retinal pigment epithelial (RPE) pigment
in the trabecular meshwork bilaterally. This patient has
been successfully managed with filtration surgery and topical medications
bilaterally. Subsequent patients have had their cataracts managed
with extracapsular cataract extraction or phacoemulsification and have
not developed glaucoma, possibly because of preservation of the posterior
capsule as a barrier to pigment migration (Steve A. Arshinoff, unpublished data, 1977–2003).
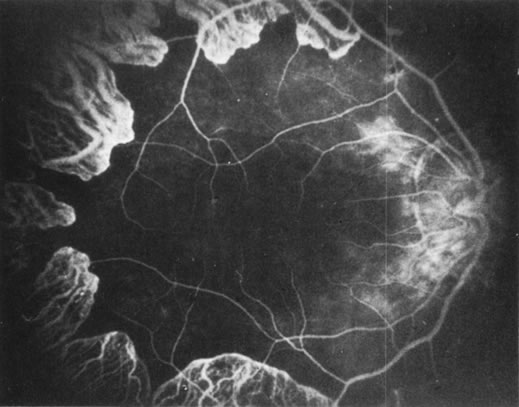
Fluorescein angiography: There is mild leakage of fluorescein at the margin of the healthy-appearing
retina, where it abuts the gyrate lesions (Fig. 2). The earliest detectable changes include pigment epithelial transmission
defects, which eventually progress into patches of atrophy with
drop-out of the choriocapillaris in the involved area. The edges
of such patches stain by leakage, indicating functioning choriocapillaris
in the surrounding tissue.27 The ophthalmoscopically normal areas of retina appear normal on fluorescein
angiography.
|
Electroretinography: The full-field electroretinogram (ERG) has been shown to be reduced in the early stages of GA, and no normal ERG recordings have been reported.28,29 Both rod- and cone-mediated a- and b-wave amplitudes are usually severely reduced (less than 10 μV) or even isoelectric, indicating that rod and cone systems are affected jointly, although occasionally a patient's ERG may be reduced by only 50% to 75% (i.e., recordable at 100 μV to 300 μV). Direct current ERG findings indicate that the c wave can be recorded only in the very early stages of the disease. The disappearance of the c wave prior to the a and b waves in GA suggests that the ocular disease is primarily RPE in origin.30 Some patients have a delayed implicit time on 30 Hz flicker testing. Computer averaging with narrow-band filtering has made it possible to detect ERG responses under 1 μV, allowing objective monitoring of the progress of even severely affected subjects.31
Electro-oculography: The ratio of the light peak to the dark trough is severely reduced, parallel to the reduction in the ERG.
Visual evoked response: The visual evoked response appears to vary as a direct function of visual acuity.
Conjunctival pathology: Three conjunctival biopsies of patients with GA have shown changes in the epithelial cells and in the stromal fibroblasts of the conjunctiva. The presence of osmiophilic particles (hypothesized to be lipidic or fatty acid drops), hypertrophy of the Golgi apparatus with rupture of intracellular membranes, and accumulation of lysosomes have been detected on electron microscopy.32
Whole-globe pathologic examinations: A postmortem histopathologic examination of whole globes from a 98-year-old woman with a well-documented (confirmed with fibroblast cultures and hyperornithinemia), atypically mild case of vitamin B6–responsive GA, who had retained 20/50 vision up until death, was reported by Wilson and associates.33 Distinctive, circumscribed patches of RPE atrophy were observed only at the periphery. The outer retina, RPE, choriocapillaris, and most of the choroidal vessels were absent from these patches. Where the RPE terminated, the photoreceptor cells directly abutted Bruch's membrane. On electron microscopy, the mitochondria in the corneal endothelium, nonpigmented ciliary epithelium, smooth muscle of the iris, and ciliary body were enlarged and had disrupted cristae and an electronlucent matrix. Similar but less severe mitochondrial abnormalities were found in the photoreceptors. No mitochondrial abnormalities were found in the RPE.
SYSTEMIC CLINICAL AND PATHOLOGIC FINDINGS
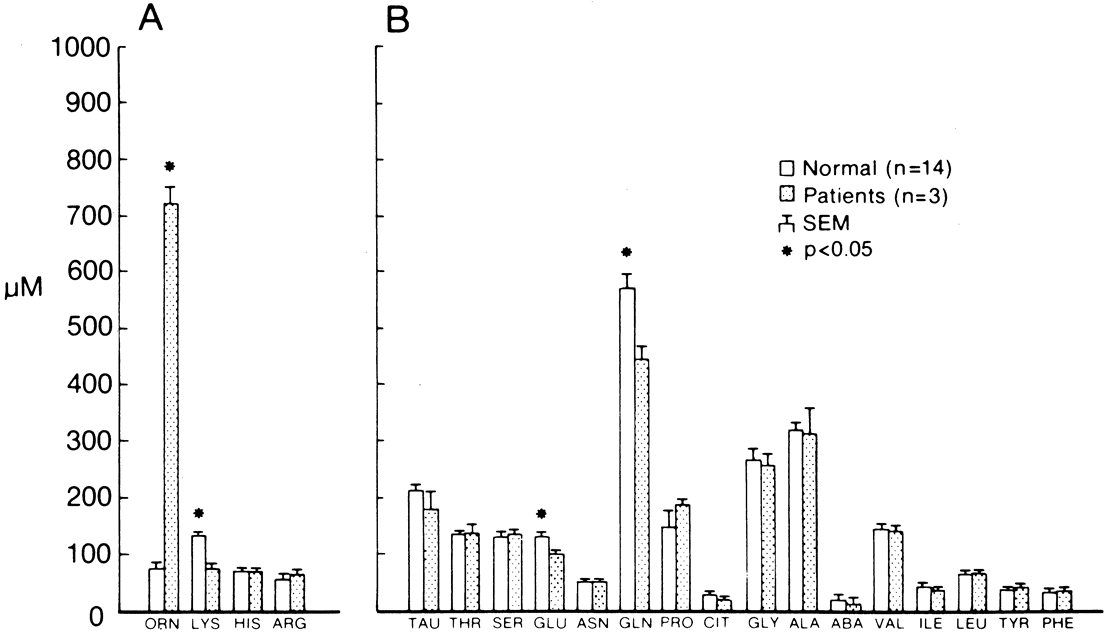
Although ocular involvement is the most clinically significant manifestation of GA, other organs may be affected. On systemic examination, ornithine levels about 10 to 20 times normal values (fasting morning plasma ornithine = 400 to 1400 μM [normal = 40 to 120 μM, mean = 60 to 80 μM]) were observed.8 Hyperornithinemia is an essential component of GA and was the finding that proved GA to be an inborn error of metabolism, with the eye as the most apparent target organ of the metabolic derangement. McCulloch and Marliss34 demonstrated that ornithine was being released from many organ systems and suggested multisystem involvement. The following organs and tissues have so far been found to be affected in GA:
Brain: Takki22 first
reported that abnormal electroencephalographic (EEG) recordings and borderline
low intellectual function were common in GA. McCulloch and coworkers11,34
confirmed these findings and stated that they do not necessarily coexist
in the same patient. Kaiser-Kupfer and associates35
also reported EEG abnormalities in GA. Using brain magnetic resonance
imaging (MRI), Valtonen and colleagues36
found degenerative lesions in the white matter of 50% of 23 GA patients
studied and premature atrophic changes in 70%, with a striking increase
in the number of Virchow spaces. In agreement with the above-mentioned
studies,11,22,32,35
EEG recordings revealed abnormal slow background activity, focal lesions,
or high-amplitude B rhythms (greater than 50 mV) in 58% of
33 GA patients tested. There was no correlation between EEG and MRI results
or the age or sex of the patients. The MRI and EEG results did not differ
between untreated and creatine (Cr)-supplemented GA patients. The authors
concluded that early degenerative and atrophic brain changes and abnormal
EEG findings are additional clinical features of GA. Nanto-Salonen and
colleagues37 analyzed the proton magnetic
resonance (MR) spectra of the basal ganglia in 20 GA patients. They found
reduced brain Cr stores in GA, which was partially corrected by low-dose
Cr supplementation and an arginine-restricted diet. They concluded that
these results support the hypothesis that hyperornithinemia in GA results
in chronic Cr depletion and decreased phospho-Cr (PCr) stores in the retina,
central nervous system, and muscle, and may contribute to the observed
pathology in those organs.
Peripheral nervous system: Peltola and colleagues38 used neurography, quantitative sensory threshold testing, and evoked potential
testing to evaluate peripheral nervous system involvement in 40 patients
with GA. All the patients tested were homozygotes or compound
heterozygotes for one single OAT mutation (L402P). Fifty-three
percent of the patients had at least two abnormal findings
on neurography, prolonged F-latency being the earliest detectable
change characteristic of sensorimotor axonal distal neuropathy. None
of the patients studied had disabling neuropathy, 40% of the
patients had mild and asymptomatic neuropathy, and 10% had symptomatic
peripheral neuropathy. The neurography findings correlated
with the severity of GA fundus changes, and patients with signs of neuropathy
had the most severe ophthalmologic GA stage; Cr supplements had
no effect on neuropathy prevalence.
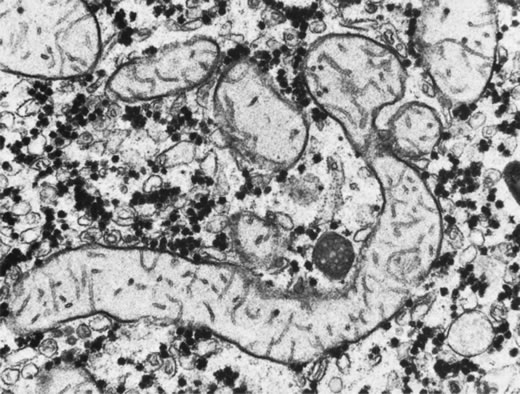
Skeletal muscle: McCulloch and Marliss34
were the first to report atrophy of, and the presence of tubular aggregates
in, type 2 skeletal muscle fibers of one patient with GA (Fig.
3), a finding later confirmed by various other investigators.35,38–40
All reported abnormalities have been confined to type 2 fibers, type 1
fibers being normal in appearance and number in all examined biopsy specimens
reported to date. Similar histologic muscle changes were noted in the
iris dilator muscle of one patient.41 Tubular
aggregates appear to be more common in GA than in any other disease, and
aside from patients with GA, they are rarely observed in female patients.
They have been noted in male patients in periodic paralysis, hyperthyroidism,
porphyria cutanea tarda, acromegaly, myasthenia gravis, polymyositis,
myotonic dystrophy, muscular viral infections, denervations, and drug
and alcohol abuse, and in some apparently normal persons. The reason that
female patients with these disorders do not have tubular aggregates is
unknown. Tubular aggregates are thought to represent a detoxifying mechanism
or a response to injury and may perhaps be a result of muscle degeneration,
eventually disappearing with the affected fiber. Consistent with this
hypothesis is the finding by Sipila and colleagues39
of nonspecifically abnormal electromyograms in 16 of 17 deltoid muscle
recordings in GA patients, indicative of a mild to moderate skeletal muscle
myopathy in GA. The progression of the myopathy is slower than that of
the chorioretinopathy, but muscle is nevertheless fairly severely affected
by the underlying metabolic defect. Approximately 10% of GA patients
demonstrate mild proximal muscle weakness.8
Kaiser-Kupfer and co-workers,35 who found
tubular aggregates in skeletal muscle biopsies of three of four patients,
grew the muscle cells of these patients in tissue culture. The addition
of 20 mmol/L ornithine to the culture media was lethal to GA muscle cells
within 48 to 72 hours, but had no effect on cultured normal muscle cells.
In addition to type 2 muscle fiber atrophy and tubular aggregates, Valtonen
and colleagues43 found computed tomography
and MRI changes in the thigh muscles of seven GA patients, although relaxation
time measurements gave little additional muscle metabolism insights. Heinanen
and colleagues,44 in conjunction with studying
brain Cr,36,37
studied the creatine phosphate (CrP) levels in skeletal muscle of GA patients.
Abnormal 31P-MR spectra were found in resting
calf muscle of GA patients, with decreased ratios of PCr/adenosine triphosphate
(ATP) and PCr/Pi, proposed to reflect a decreased CrP concentration in
calf muscle of GA patients. However, these decreases did not reflect the
clinical stage of the disease, degree of muscle destruction, or ornithine
concentrations. The researchers proposed that disturbed cellular energy
production resulting from intracellular ornithine-induced inhibition of
Cr synthesis, and decreased PCr muscle stores may partially explain the
pathogenesis of GA. Daily oral supplementation with physiological levels
of Cr (1.5–2 g/day) for 8 to 15 years led to almost normalized
PCr/P and PCr/ATP ratios in the calf muscle of GA patients, likely by
increasing intracellular PCr.45
|
|
Other: Francois48 reported an association of Alder's anomaly (the presence of numerous azurophilic granules in the cytoplasm of neutrophils, which stain dark violet by Pappenheim's technique) with GA in one patient. However, Alder's anomaly has generally been associated with Hurler syndrome and has not been reported in association with GA by other investigators. In a study performed at the U.S. National Eye Institute, and therefore not expected to deal with a homogenous genetic population (as one might find with Finnish GA patients), an increased occurrence of thyroid disease in patients with GA compared with control patients was found by Whitcup and colleagues.49 Seven of 34 patients with GA had thyroid disease, resulting in an estimated odds ratio for thyroid disease in GA patients of 12.7 that of normal controls. Similar but less pronounced results were found in retinitis pigmentosa patients, with an estimated odds ratio of 6.2 compared with normal controls.
ANIMAL DATA
Hepatic mitochondrial changes similar to those seen in patients with GA have been produced in normal rats maintained on high-ornithine diets.50 A case of an adult male cat with retinal degeneration, extreme hyperornithinemia, and absence of OAT—an apparent replica of human GA—was reported by Valle and associates.51 Attempts to breed the cat were unsuccessful, and it later died. Histopathologic examination of the cat's eyes demonstrated extensive neuroretinal and RPE damage and cell loss. There was a decrease in the number of small choroidal vessels, but not the large ones. In cats with other retinal degenerations, such loss of RPE and choriocapillaris is uncommon. This “GA cat” did not have cataracts.
Kuwabara and associates52 demonstrated selective destruction of RPE cells in rats and monkeys after intravitreal injections of ornithine. Secondary destruction of overlying photoreceptors and underlying choroid developed later. Based on these data, they suggested that the RPE may be the primary target organ in GA. A more recent attempt by Daune-Anglard and colleagues53 to mimic the damage of GA with induced ornithine toxicity in mice and chickens using 5-fluoromethylornithine (5FMOrn) administered orally for 53 days showed no ocular pathologic changes in these animals despite a tenfold increase in tissue ornithine. However, 10% to 20% of tissue OAT is refractory to inactivation by 5FMOrn, so these results only show that mice and chickens will not develop GA as a result of sustained hyperornithinemia due to incomplete blockage of OAT for this period of time.
Subsequently, Wang and colleagues54 successfully created a mouse model of GA that closely mimics GA in human patients. Using targeted disruption of the murine OAT gene, they produced OAT-deficient mice that exhibit chronic hyperornithinemia, ten to 15 times normal. These OAT-deficient mice developed slowly progressive retinal degeneration over the first 12 months of life. The RPE cells were the initial site of damage. Using this mouse model of GA, Wang and colleagues55 further evaluated the effect of long-term reduction in ornithine on prevention of retinal degeneration. OAT-deficient mice fed an arginine-restricted diet for 12 months had significantly reduced plasma ornithine levels. Importantly, retinal degeneration, as measured by ERG and retinal histologic and ultrastructural studies, was prevented. The researchers concluded that ornithine accumulation, and not OAT activity in the retina and RPE, is a necessary factor in the pathophysiology of retinal degeneration in GA.
DIFFERENTIAL DIAGNOSIS
Ocular
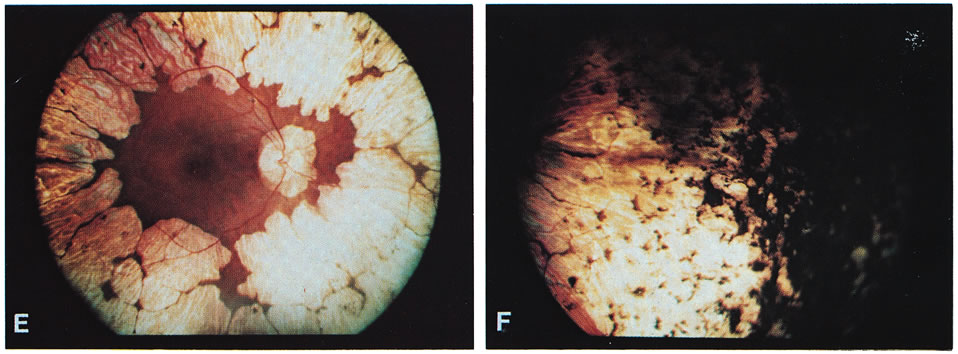
Although cases of GA have been mislabeled as many other things, usually “atypical retinitis pigmentosa,” and patients referred as having GA often turn out to be suffering from some other disorder, the diagnosis is actually quite easy to make if the five main features (see previous Diagnosis section) can be recalled (Fig. 5). Confusion in diagnosis is primarily due to the rarity of the disease. It is extremely unlikely that any primary care ophthalmologist would have the opportunity to make this diagnosis more than once in a career, since the incidence of GA appears to be less than one in 1,000,000 everywhere except in Finland, where it occurs in about one in 50,000 individuals.8
|
|
Almost invariably, the main ophthalmic features of high myopic astigmatism, posterior subcapsular cataracts, and typical retinal appearance are obvious to the ophthalmologist. One can be quite certain that if the patient does not have the typical fundus picture, as well as extreme hyperornithinemia, GA is not the correct diagnosis. The autosomal recessive inheritance pattern can usually be quickly ascertained with a brief family history asking about affected relatives and parental consanguinity (see Fig. 5), even before confirmatory laboratory biochemical and genetic testing is undertaken.
Non-GA patients with extremely high myopia (usually more than –15 D) may occasionally have posterior polar, peripapillary, or peripheral clusters of round, full-thickness, chorioretinal atrophic lesions, often causing significant reduction in visual acuity.56 This, however, is not the pattern of the lesions seen in GA.
An entity of “central gyrate atrophy” has been described,57 but this is probably equivalent to end-stage serpiginous choroidopathy rather than a separate inherited chorioretinopathy.58,59
Large areas of paving stone degeneration may superficially resemble GA, albeit on a much smaller scale. Paving stone degeneration is usually found in the inferior quadrants peripherally, whereas GA is not segmental and involves all 360 degrees of the fundus.60 Occasional reports of atypical GA appear in the literature.61–64 Review of these cases usually reveals either that the retina did not quite have the typical appearance of GA, myopia and cataracts were not present, and/or hyperornithinemia was absent. It is interesting to speculate as to why other disorders have retinal lesions similar in appearance to GA and whether or not some final common pathway to chorioretinal destruction exists, especially in cases where some similar systemic involvement may coexist, as in muscular dystrophy. However, labeling such cases with similar lesions as “atypical gyrate atrophy” can be misleading, especially to the majority of practitioners who have never encountered GA, and may lead to corruption of research efforts and reports by the inclusion of different etiologic disorders in supposedly homogeneous study groups.65
Metabolic
Hyperornithinemia is not unique to GA.11,46 It was reported in two other conditions. In the late 1960s, Kekomaki and colleagues66 and Bickel and associates67 described two siblings who presented with failure to thrive, prolonged neonatal jaundice, atypical hepatic cirrhosis, renal tubular dysfunction, and mental retardation. Hyperornithinemia (about three times normal), renal glycosuria, generalized aminoaciduria, and mild hyperammonemia were present. Hepatic OAT levels were about one-sixth those of normal patients. Garnica and coworkers68 later confirmed these findings. Kekomaki and coworkers66 studied the enzyme kinetics of the residual OAT in the hepatocytes of one patient and found them to be normal, suggesting that the problem in this disorder is one of decreased enzyme synthesis or excessive degradation. Both siblings, at ages 15 and 9, were severely retarded, had normal ocular examinations, normal plasma ornithine, normal liver function tests, generalized aminoaciduria, elevated serum creatinine, and hypertension. The etiology of this syndrome remains unknown.8
The second disorder with associated hyperornithinemia is HHH syndrome, a rare autosomal recessive disorder that is about one-third as common as GA. HHH patients exhibit hyperornithinemia about one-half as elevated as that of GA patients, but there is considerable overlap. In the late 1960s to early 1970s, Shih and colleagues69,70 and later (1975) Gatfield and colleagues71 described patients presenting in infancy with feeding difficulty, mental retardation, and seizures, in which the biochemical triad of hyperornithinemia, hyperammonemia, and homocitrullinuria was observed. Abnormal hepatic mitochondria, not unlike those seen in GA, have been identified in this disease. The biochemical defect was hypothesized by Fell and coworkers72 to reside in defective transport of ornithine across the inner mitochondrial membrane into the mitochondrial matrix, and the biochemical and clinical evidence supports this hypothesis.8,73–82 The ornithine/citrulline transporter was identified and confirmed as the defect in the HHH syndrome.83–85 The gene has been labeled ORNT1 and mapped to 13q14.86 HHH patients may voluntarily restrict their protein intake to avoid having symptoms. The symptoms are thought to be due to hyperammonemia as they are similar to other hyperammonemia syndromes, and can be dramatically ameliorated with ornithine HC1, 0.5 to 1.0 mmol/kg/day dietary supplementation.78 The additional dietary ornithine is thought to elevate cytosolic ornithine levels, thereby driving it into the mitochondrial matrix, where it acts as the limiting substrate in the elimination of ammonia via the urea cycle. HHH syndrome, like GA, is inherited as an autosomal recessive disorder. Some patients do not respond to ornithine or arginine supplementation, suggesting heterogeneity in the mutation of the affected protein. Ocular examination has been normal in all patients examined.8
In neither of the above two metabolic syndromes have ocular abnormalities been noted. The senior author (SAA) repeatedly examined a currently 26-year-old patient and a teenage patient, both with HHH syndrome, as they underwent ornithine supplementation treatment over many years. Neither patient manifested any retinal or ERG changes (Steve A. Arshinoff, unpublished data, York Finch Eye Associates, Toronto, Ontario, Canada, 1985–2003). GA retinal lesions have not been observed in an infant. Hayasaka and coworkers19 noted that a 2-year-old patient with biochemical GA did not develop fundus lesions until age 4, and Stoppoloni and colleagues87 reported on a 13-year-old patient who had early fundus lesions. The senior author has followed a GA patient, who first presented at age 7 with well-defined peripheral GA lesions involving 360 degrees of her retina.

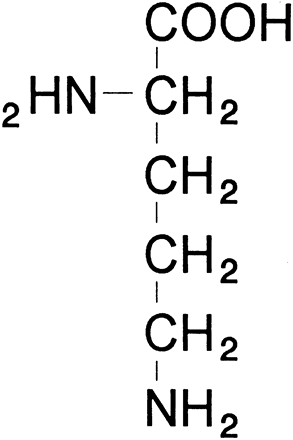
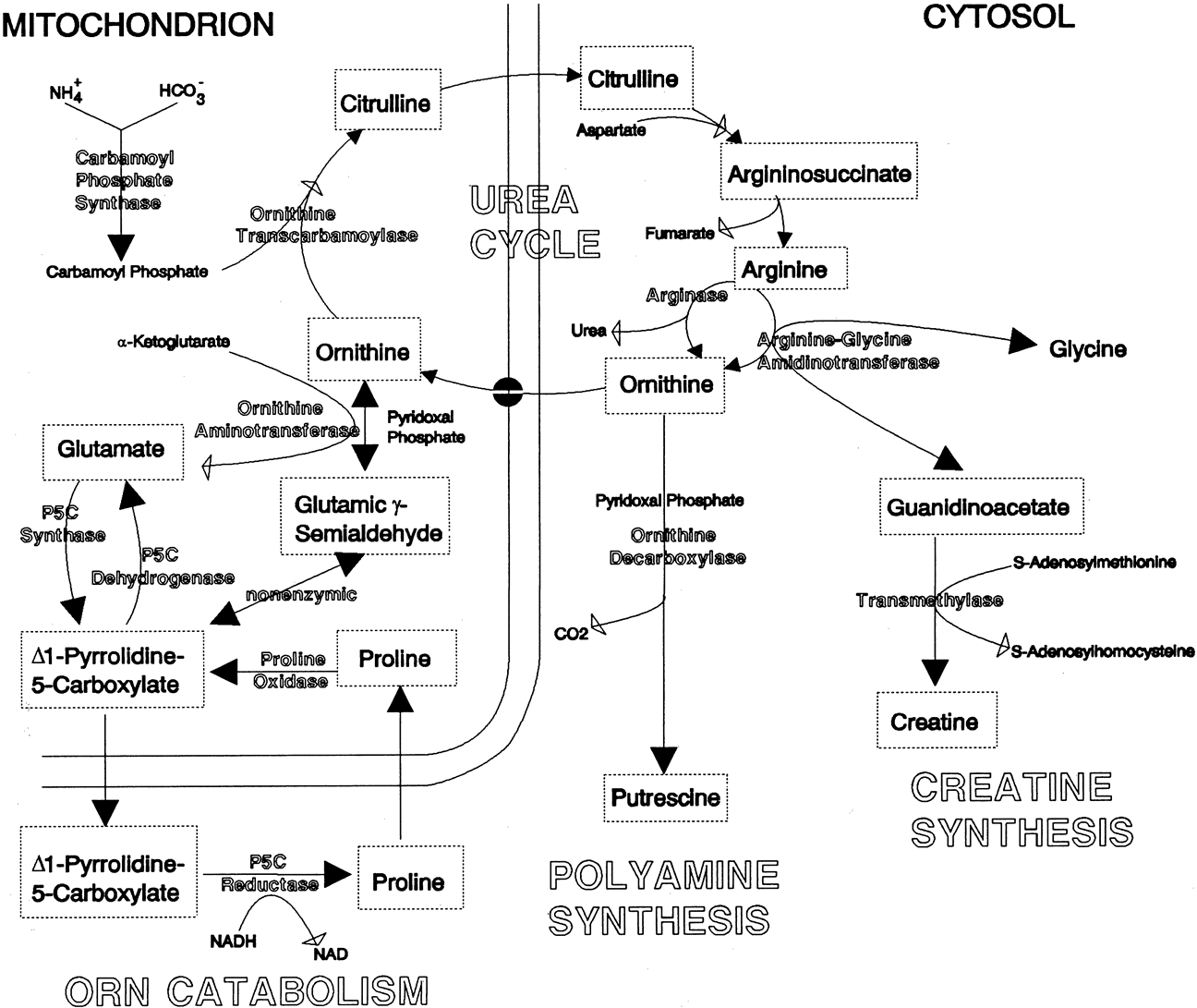
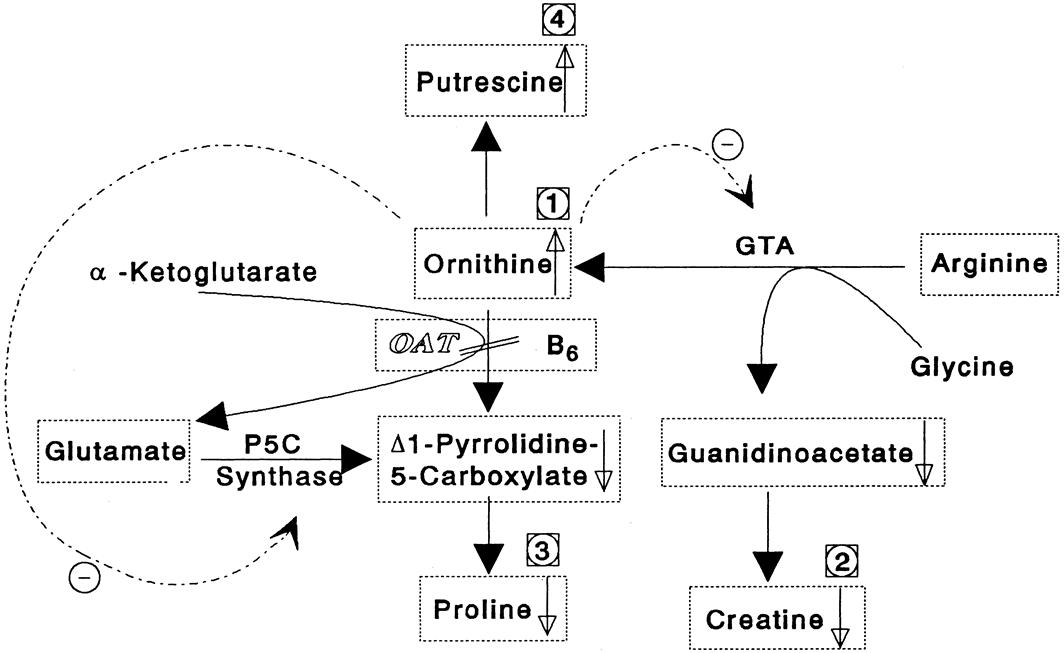
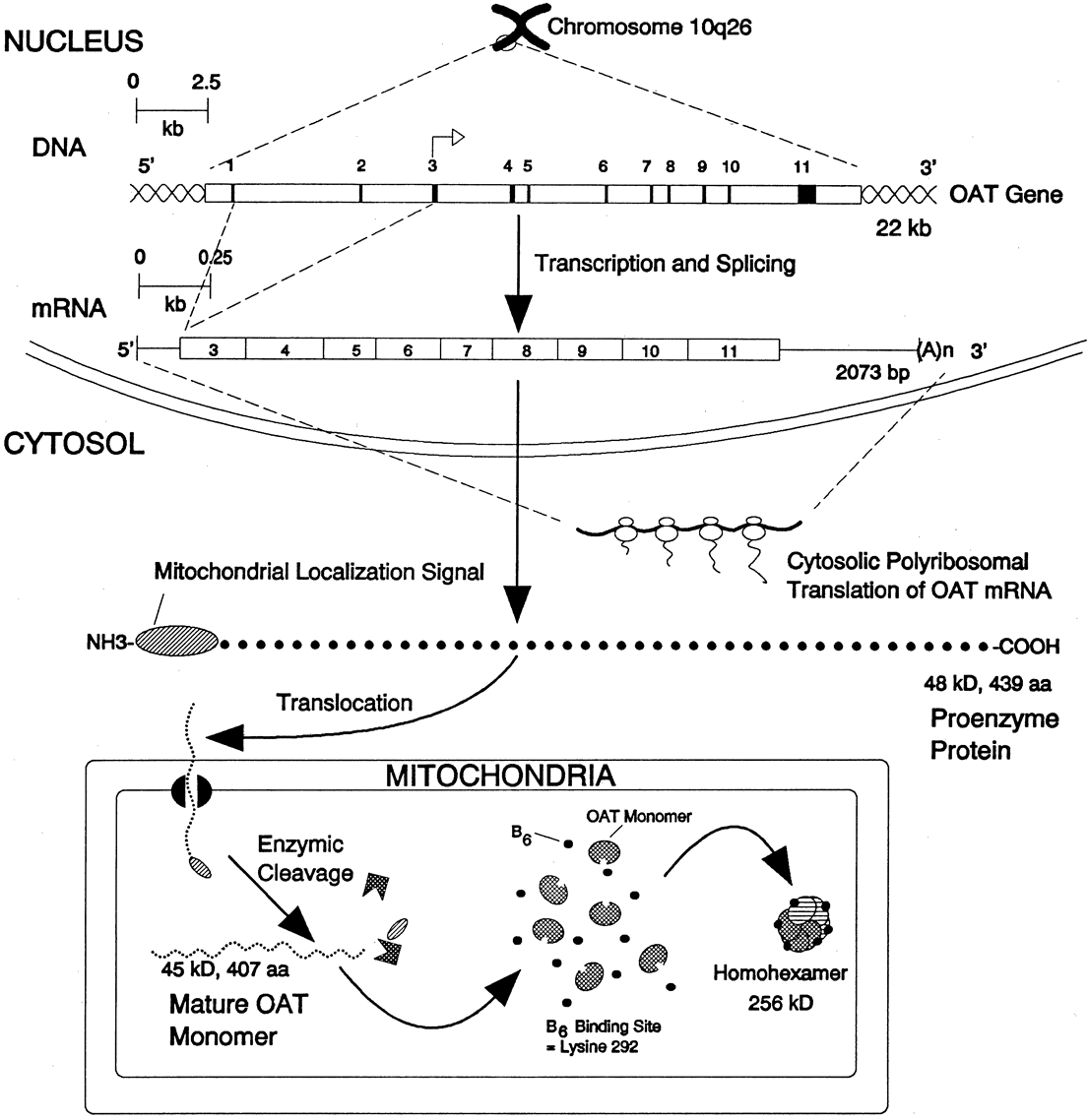
 glutamate + glutamic γ-semialdehyde
glutamate + glutamic γ-semialdehyde