| Assuming other significant systemic, neurologic, and ocular injuries have
been addressed adequately, the primary consideration in the management
of orbital fractures is to determine which fractures require surgery
and when such intervention should be undertaken. It should be kept
in mind that the range of severity of orbital fractures can vary from
simple linear or circular blow-out fractures to more complex injuries
in which several orbital walls and the orbital rims are involved as part
of a more widespread craniofacial injury. Clearly not all isolated
internal orbital fractures require surgical treatment; however, more severe
orbital fractures, particularly those that are a component of more
widespread craniofacial injury, benefit from repair as soon as possible (provided
the patient is otherwise stable) in order to achieve the
most satisfactory aesthetic and functional results. Surgery itself carries
some risks, including failure of the procedure to correct the deformity, the
possibility of worsening diplopia, the rare risk of visual
loss, and the systemic complications of general anesthesia.36 Therefore, in the group of patients with relatively less severe orbital
fractures, the risk-benefit ratio of surgery must be weighed against
the corresponding risk-benefit ratio of more conservative treatment. Controversy has surrounded the management of orbital blow-out fractures. Throughout
the past 50 years, opinions have ranged from suggestions
that all orbital blow-out fractures should be repaired to the suggestion that none should be repaired primarily. The historical perspective on the management
of blow-out fractures has been concisely summarized in a recent review.1 With the improved assessment of orbital injuries afforded by CT scanning, greater
consensus has developed regarding the indications for early
surgery (i.e., surgery performed within the first 2 weeks after injury).1,2 There is widespread agreement that early repair of blow-out fractures
is indicated for the following: Symptomatic, clinically significant diplopia with positive forced duction
test and/or CT evidence of muscle entrapment showing no improvement
over 1 to 2 weeks Early enophthalmos of at least 3 mm or significant hypo-ophthalmos
A large (greater than 50%) orbital wall defect likely to produce late enophthalmos
Associated displaced orbital rim or facial fractures
Observation (conservative treatment) is indicated for patients demonstrating
the following: Minimal diplopia with good motility that shows evidence of clinical improvement
over several weeks and no CT evidence of muscle entrapment
Absence of significant clinical enophthalmos or hypo-ophthalmos and small
orbital wall fractures that are not likely to produce late enophthalmos
Even with this general consensus, it is obvious that gray areas invariably
occur. In such situations, informed discussion with the patient is
particularly important regarding the relative merits and risks of primary
surgical intervention versus a more protracted period of observation. It is clear that as soon as appropriate indications for surgery are confirmed, it
is appropriate to proceed. Early surgery is technically easier
before soft tissue scarring and bony malunion progress. In cases with
obvious clinical and CT evidence of frank muscle entrapment, expeditious
repair is advised (within 48 hours if possible) to minimize ischemic
muscle injury and fibrosis, which may otherwise limit final outcome. Similarly, in
cases where early enophthalmos is clinically obvious, a
large (greater than 50%) orbital blow-out fracture is noted on CT
scan (which is likely to result in late enophthalmos), or displaced orbital
rim fractures or more complex orbital/craniofacial fractures are
present, surgery can be scheduled within several days after the injury
if the patient is otherwise stable. Nonophthalmic indications for repair
of orbital fractures involving other parts of the craniofacial skeleton
include facial deformity, malocclusion or trismus due to bony
impingement on the muscles of mastication, or an unstable facial skeleton (which
can be seen with panfacial fractures). In such cases, the internal
orbital fractures can be explored in the same operative setting
when necessary. In cases of small or isolated blow-out fractures causing
diplopia, but without frank extraocular muscle entrapment on CT scan, a
period of observation (1 to 2 weeks) is appropriate, during which
time serial examination and quantitation of diplopia and extraocular
muscle limitation is performed. Forced duction and force-generation
testing may be performed during this time to assess the etiology and severity
of strabismus. However, the previously mentioned caveat holds: forced
duction testing may be positive after the initial injury as a
result of edema or hemorrhage, thus limiting the utility of this test
early on. In this subset of patients, ocular motility is assessed more
reliably after the orbital edema subsides. Some authors have recommended a short course of corticosteroids to speed
resolution of edema,37 but this practice is not widely followed. A course of oral antibiotics (e.g., amoxicillin/clavulanate [Augmentin] or cefaclor) and nasal
decongestants (e.g., pseudoephedrine) is recommended by some to minimize the risk of sinusitis. This
is certainly most useful for those cases in which the maxillary
sinus is completely opacified or when the patient is immunocompromised. During
this period, all patients are instructed to refrain from
strenuous activities and sports and to avoid nose-blowing, which may
cause orbital emphysema. If restrictive strabismus causing diplopia or
clinically significant enophthalmos or hypo-ophthalmos is noted after
orbital edema subsides, it is preferable to proceed with surgery while
one still has an early “window of opportunity.” The decision
to perform surgery will obviously be based on the patient's
functional and occupational needs and aesthetic concerns. Based on review
of the literature, it is reasonable to tell the patient that diplopia
will typically stay the same or improve with time, whereas enophthalmos
will stay the same or worsen with time (i.e., 3 to 6 months). Although secondary orbital, eyelid, or strabismus surgery
can be performed many months after the initial injury, there is widespread
agreement that the results of such surgery are not as successful
as primary orbital fracture repair. SURGICAL REPAIR: OVERVIEW The surgical approach to orbital fracture repair is dictated by anatomic
location and severity of the orbital fractures and associated injuries. Most
orbital fracture repairs are performed under general anesthesia. Isolated
fractures of the internal orbit may be directly repaired, whereas
fractures of the orbital rims should be repaired before those
involving the internal orbit in order to establish proper spatial relations
and provide anterior support. Orbital fractures are usually repaired
in conjunction with other associated fractures of the craniofacial
skeleton. Subperiosteal exposure is required for all fractures requiring
repair.38 The inferior and lower medial orbit can be approached via an eyelid incision (infraciliary
or transconjunctival approach). In cases where additional
exposure of the more superior medial orbit is required, this
can be facilitated by extension of the conjunctival incision through the
caruncle (taking care to avoid injury to the lacrimal apparatus and
inferior oblique muscle) or through a skin incision (Lynch's incision) in
the medial canthus. Exposure of the medial orbit may also be
accomplished via a coronal approach. The lateral orbit can be exposed
by extension of the lower eyelid incision (either transcutaneous or transconjunctival) into
the lateral canthus. This incision is usually angled
slightly inferiorly along a “crow's foot” pattern. Lateral
cantholysis, which consists of lysis of the inferior crus (and
occasionally also the superior crus) of the lateral canthal tendon, is
helpful to improve exposure. Isolated exposure of a fracture at
the zygomatico-frontal suture can also be performed via an upper eyelid
crease incision or small incision paralleling the orbital rim. The superior
orbit can be approached extracranially via an extended upper eyelid
crease incision, an incision just below the eyebrow cilia or via
a coronal approach. When necessary, the orbital roof may also be exposed
intracranially (via craniotomy) with the assistance of a neurosurgeon. In
complex orbital fractures involving the maxilla or zygoma, a buccal
sulcus incision can be used to expose fractures involving the maxillary
buttresses and lower nasoethmoid-orbital region, if necessary. Internal Orbital Fractures Internal orbital fractures may involve any wall of the orbit, but most
typically they involve the orbital floor (79%) and medial wall (30%).39 Repair of internal orbital fractures necessitates that they be completely
exposed and that all herniated or entrapped orbital soft tissues be
liberated. Reduction of displaced segments of the orbital walls is occasionally
possible; however, because the walls of the internal orbit
are thin and frequently comminuted, it is usually necessary to reconstruct
the internal orbital defect with an orbital implant to prevent orbital
volume deficiencies and recurrent herniation of the orbital soft
tissues. The options for orbital implants include autogenous grafts (e.g., bone, cartilage, fascia) and alloplastic materials (either permanent or absorbable). The relative merits of these materials
are widely debated.40 Among autogenous implants, bone grafts are most typically used. These
offer the advantage of excellent biocompatibility but require a second
procedure to harvest the graft, with attendant donor-site morbidity and
increased operating time. Variable resorption (or conversely graft
take) may produce late undercorrection of enophthalmos/hypo-ophthalmos. The
typical sites for harvesting bone grafts include the cranium, rib, and
iliac crest. Cranial bone is favored by many because it is membranous bone and undergoes relatively less resorption than endochondral bone (e.g., rib, iliac crest). The anterior wall of the maxillary antrum is also
a source of membranous bone, but the amount of bone available for grafting
is limited to only about 1 × 1.5 cm in adults. Numerous types of alloplastic implants are available for orbital reconstruction
and can be categorized as nonporous, porous, and absorbable. Nonporous implants include metallic implants (usually composed of titanium or vitallium) such
as miniplates, microplates, and grids or mesh. Additional nonporous
orbital implants include silicone, Supramid, and Teflon, among many
others. Recent interest has increased in the use of porous implants that allow fibrous ingrowth. Fibrovascular ingrowth has at least two potential
advantages: (1) it anchors the implant to the surrounding orbital
tissues, rendering the implant less likely to migrate or extrude; and (2) it
is able to resist late infection. Commercially available porous
orbital implants include hydroxyapatite and high-density porous
polyethylene. Hydroxyapatite is hard and brittle, limiting its usefulness
as an orbital floor implant. High-density polyethylene (Medpor) can
be fabricated to be thin and malleable enough to be fitted into the
orbit while still providing good structural support. Absorbable implants, such as Gelfilm and polygalactin (Vicryl) as well as newer compounds are
also available for orbital fractures, but they are generally limited
to smaller fractures that do not require a great deal of rigid support. The advantages of alloplastic orbital implants include decreased operating
time, lack of donor-site morbidity, absence of early resorption, and
increased ease of handling and contouring. Complications associated
with alloplastic implants have recently been reviewed and include infection
and complications related to migration or extrusion. The exact
incidence of such complications is difficult to determine, but overall
it is believed to be low.41 Complications associated with orbital implants depend not just on the
type of implant, but also on the surgical technique employed. Proper positioning
and stable fixation of alloplastic orbital implants can be
expected to decrease complications markedly.41,42 Ultimately the choice of implant material is determined by the experience
of the surgeon. ORBITAL BLOW-OUT FRACTURE. Repair of the typical orbital blow-out fracture involving the orbital
floor (with or without involvement of the lower medial wall) proceeds
as follows. Forced-duction testing is performed immediately before the
start of surgery to assess the degree of restriction. The inferior orbital
rim can be approached via a transcutaneous eyelid incision placed
just below the eyelashes (infraciliary), the lower eyelid crease, or
the orbital margin. In these cases, dissection proceeds in a submuscular
plane, just anterior to the orbital septum down to the inferior orbital
rim. Alternatively, a transconjunctival approach may be used. This
approach is believed to reduce the risk of postoperative eyelid retraction
or ectropion.43,44 If a transconjunctival approach is used, it is helpful to perform a lateral
canthotomy and cantholysis to improve exposure. The incision through
the conjunctiva and lower lid retractors can be made through the
deeper fornix, going through the orbital fat to the inferior orbital rim. Alternatively, an
incision can be made through the conjunctiva and
lower lid retractors approximately 3 to 4 mm below the inferior tarsal
border, continuing the dissection in a plane anterior to the orbital
septum down to the orbital rim. This latter approach has the advantage
of minimizing fat prolapse into the field. Once the inferior orbital rim is reached, an incision is made in the periosteum
with a scalpel or sharp edge of a periosteal elevator. It is
important that this periosteal incision remain at the edge of the orbital
rim rather than deviating posteriorly, particularly near the medial
aspect of the inferior orbital rim where the inferior oblique muscle
is located. Once the periosteum is incised, it can then be bluntly elevated
with the periosteal elevator extending the dissection in a sweeping
fashion more posteriorly to expose the anterior edge of the fracture. It
is helpful to expose as much of the more anterior portion of the
fracture as possible before attempting to extract the orbital soft
tissues from within the fracture itself. The orbital soft tissues should
be handled with blunt dissection and gently removed from within the
fracture using blunt periosteal elevators and malleable retractors in
a hand-over-hand technique (Fig. 5). In some cases, fibrosis (occurring even within 1 to 2 weeks after injury) can
make release of the entrapped orbital soft tissues difficult. The
temptation to use sharp dissection should be avoided, however. In
some cases, it is helpful to remove a small amount of the more anterior
orbital floor using rongeurs to allow better leverage to get beneath
and elevate the prolapsed tissues. 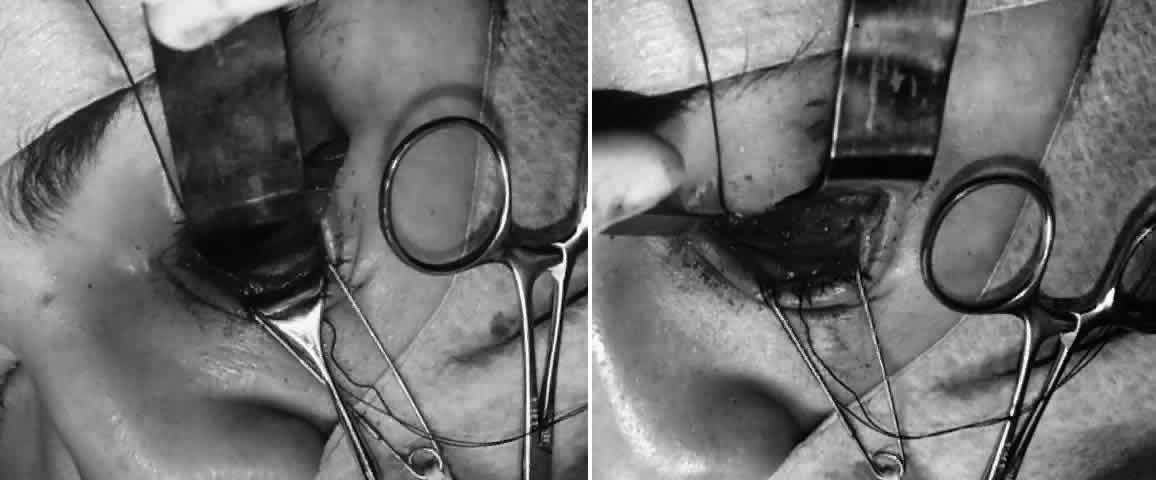 Fig. 5. Orbital floor blow-out fracture repair via a transconjunctival approach
with lateral cantholysis. A. The periosteum is incised and then blunt dissection used to expose the
fracture site, thus releasing all entrapped orbital soft tissues. B. After the 360° perimeter of fracture is exposed and all prolapsed
tissue placed back in the orbit, the orbital implant is placed. In this
case, a porous polyethylene sheet was used to cover the defect and was
secured with two microscrews. Fig. 5. Orbital floor blow-out fracture repair via a transconjunctival approach
with lateral cantholysis. A. The periosteum is incised and then blunt dissection used to expose the
fracture site, thus releasing all entrapped orbital soft tissues. B. After the 360° perimeter of fracture is exposed and all prolapsed
tissue placed back in the orbit, the orbital implant is placed. In this
case, a porous polyethylene sheet was used to cover the defect and was
secured with two microscrews.
|
The location of the infraorbital neurovascular bundle should be determined
during the course of dissection. The typical blow-out fracture is
usually medial to the course of the infraorbital neurovascular bundle. The
neurovascular bundle can be seen as a faint shadow extending beneath
the thin bone of the orbital floor, or it may be directly visible
within the fracture. A small blood vessel is frequently seen extending
superiorly from the infraorbital artery into the orbit. It is frequently
necessary to lyse this small branch during dissection. The infraorbital
neurovascular bundle itself should be preserved. In some cases, the
orbital soft tissues are adherent to the infraorbital neurovascular
bundle, and they must be gently teased off in order to reposit the tissues
and allow placement of an orbital implant. The entire 360° perimeter
of the fracture should be exposed and all prolapsed tissue replaced
back into the orbit. It is particularly important that the posterior
extent of the fracture be established. Usually the more posterior
bone of the orbital floor remains intact. This posterior ledge can
be used to support the orbital implant. Once all entrapped tissues are released and the fracture site has been
completely isolated, a decision is made regarding placement of an orbital
implant. If the fracture is relatively small, no orbital implant may
be needed once the entrapped tissues are released. Alternatively, a
thin, absorbable implant (e.g., Gelfilm) may be placed over the small fracture site as an added precaution
against recurrent entrapment. For larger defects, an autogenous
graft or alloplastic implant is necessary. The implant must be sufficiently
sized to bridge the entire defect on all sides, and it must be able
to support the orbital soft tissues. If the implant is sufficiently
malleable, it can be contoured for optimal coverage of the defect and
reconstitution of the orbital shape. After the orbital implant is placed, forced-duction
testing should be repeated to ensure that all entrapment
has been released. The implant should be well supported and stable to prevent migration or
slippage, which can result in undercorrection of enophthalmos or other
sequelae related to orbital implant malposition. Fixation of the orbital
implant to an intact orbital rim by various means can reduce the
potential for implant migration and takes little additional operating
time. Methods to secure the implant include gluing, placing a “tab” at
the anterior edge (for thin, synthetic implants), or direct
fixation to the rim with a suture, wire, screws, or miniplates. Direct fixation is probably the most reliable technique. To fixate the implant with a
suture or wire, two holes are drilled through the inferior orbital rim, and
then the suture or wire is passed in a mattress fashion through
two corresponding holes placed in the implant and tied. The knot should
be placed in a location where it will not be palpable or irritate the
globe. Usually the knot can be buried near the junction between the
implant and the orbital floor. The orbital implant may also be secured
relatively easily with metallic screws (from a miniplate or microplate
set; see Orbital Rim Fractures section), placed through the implant
just inside the orbital rim (see Fig. 5). It is important that the implant not project anteriorly past the orbital
rim so that it will not be palpable. Synthetic implants and bone
grafts of sufficient thickness and tensile strength can also be secured
with a miniplate cantilevered over the orbital rim and into the internal
orbit. This technique of fixation can be particularly helpful for
large internal fractures, where the miniplate provides additional support
and rigid fixation. When more extensive disruption of two or more walls is present within the
internal orbit, rigid fixation is essential. Metallic grids or mesh implants are available, which can
be custom shaped to span a large portion or the entire defect and then
fixed to the orbital rim (Fig. 6). This type of implant can then be used as a platform for additional alloplastic
implants or bone grafts to augment the internal orbital reconstruction
and volume. Many surgeons routinely place a bone graft or
other implant over these metallic grid or mesh implants, whereas others
report that placement of the metallic orbital implant alone can be successful. After
the orbital implant is secured, the periosteum is closed. After
this, the eyelid incisions (either conjunctival or transcutaneous) are
closed, usually in a single layer. It is important to avoid
catching the orbital septum in the closure because this places the patient
at risk of postoperative lower eyelid malposition. If a lateral
canthotomy and cantholysis has been performed, the lateral canthus should
be reconstituted by aligning the natural eyelid margin landmarks (lash
line). The lateral canthal tendon may be reapproximated with absorbable
suture (e.g., 5-0 Vicryl) just inside the lateral rim to the periosteum. Care should
be taken to avoid overtightening this lateral canthal suture or placing
the suture too anteriorly, which can result in an unnatural, acutely
angled appearance. 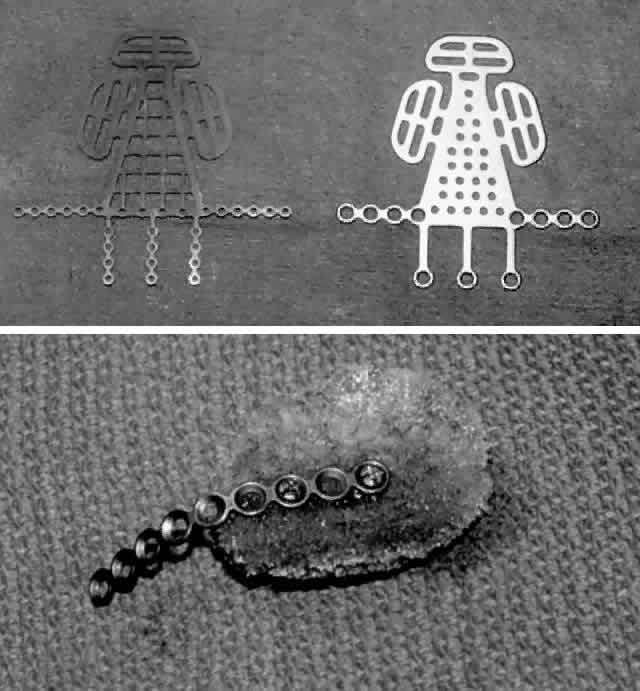 Fig. 6. A. Metallic grid implants are used for rigid internal orbital fixation when
extensive disruption of two or more walls is present. B. Synthetic implants or bone grafts may also be rigidly fixed with a miniplate, which
is then “cantilevered” over the orbital rim. Fig. 6. A. Metallic grid implants are used for rigid internal orbital fixation when
extensive disruption of two or more walls is present. B. Synthetic implants or bone grafts may also be rigidly fixed with a miniplate, which
is then “cantilevered” over the orbital rim.
|
Postoperative care includes light (or no) dressings and ice, as tolerated, for 12 to 24 hours
after surgery. If a synthetic orbital implant is
used, prophylactic antibiotics (e.g., IV cefazolin [Ancef]) are given perioperatively and a course
of oral antibiotics (e.g., Augmentin, cefaclor) can be continued for 7 to 10 days after surgery. Pressure
patching of the operative eye is avoided during the immediate
postoperative period so that any change in the orbital status can be
detected (e.g., visual loss, orbital hemorrhage). The patient should be instructed to
contact the physician should they notice any such signs. A transient
increase in orbital edema as a result of surgery can be expected in the
immediate postoperative period. Some generalized limitation of ocular
motility can be expected when orbital edema is prominent. In the absence
of any contraindications, some surgeons advocate the use of parenteral
or oral corticosteroids perioperatively in order to minimize postoperative
edema. The postoperative result is appreciated after resolution
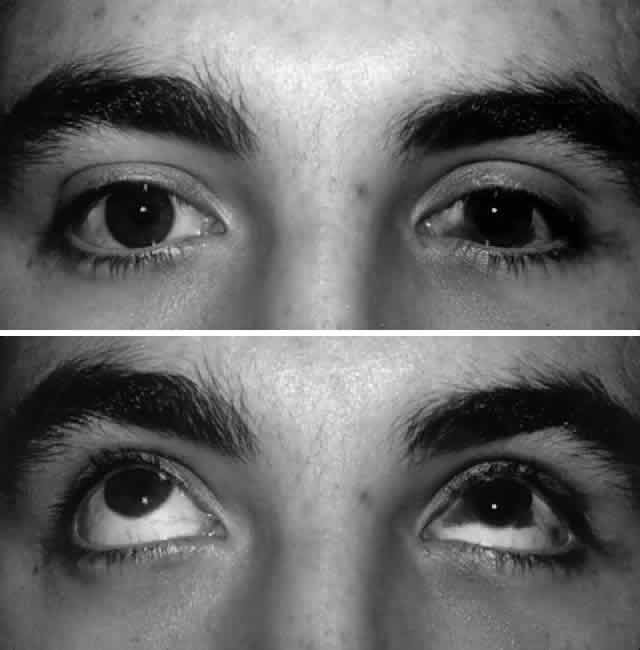
of edema (Fig. 7). 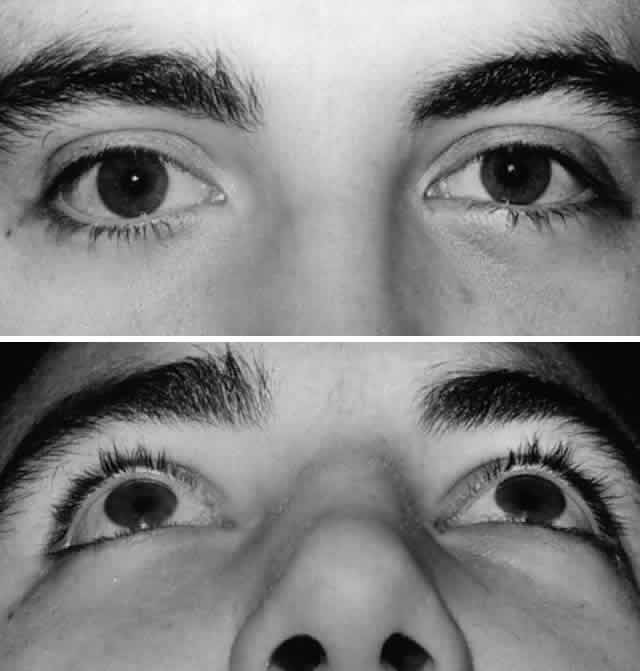 Fig. 7. A. Postoperative appearance of patient in Figure 3 one month after left orbital blow-out fracture repair. B. Note good globe position with no evidence of enophthalmos. Ocular motility
returned to normal. Fig. 7. A. Postoperative appearance of patient in Figure 3 one month after left orbital blow-out fracture repair. B. Note good globe position with no evidence of enophthalmos. Ocular motility
returned to normal.
|
Orbital Rim Fractures Orbital rim fractures occur with various degrees of displacement and comminution, typically
proportional to the direction and energy of the impact. Extensive
orbital rim fractures almost invariably include damage
to the internal walls of the orbit. Reduction and fixation of unstable
orbital rim fractures should precede repair of the internal orbit. The
aim of orbital reconstruction is to restore normal orbital anatomy
by accurately repositioning the displaced fracture segments followed
by stable fixation.1–3,38,39 The orbital rims determine the circumference of the orbital aperture and
provide a supportive anterior framework for the walls of the orbit
as well as the attachment of the canthal tendons. Although repair of isolated
nondisplaced rim fractures is not required, open reduction with
fixation is indicated for rim fractures with significant displacement, comminution, or
bone loss. Orbital rim fractures may be a component
of a more complex craniofacial fracture pattern, including nasoethmoid
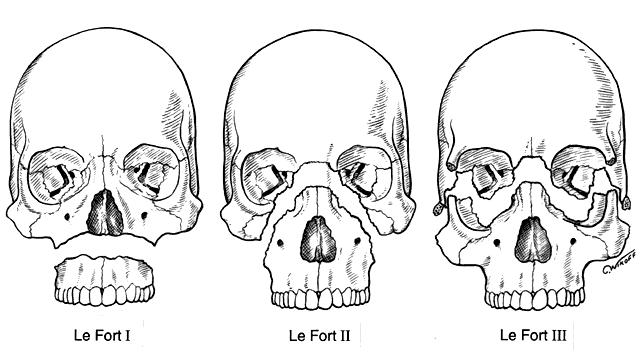
orbital fractures,45–47 zygomatico-orbital (tripod or trimalar fractures),48–54 cra-nio-fron-tal fractures and maxillary fractures including Le Fort II
and III fractures (see Classification section).38,39 When multiple orbital rims are disrupted, the order of reconstruction
depends on the nature of associated craniofacial fractures. When severe
panfacial fractures are present, the order of repair in the orbital
region (after reduction and fixation of the lower face and mandible) generally
begins superiorly with the cranial base/frontal region (which
includes the superior orbital rim and orbital roof), followed by reconstruction
of the medial nasoethmoid-orbital region and the lateral orbit/zygoma, followed
by repair of the inferior orbital rim and maxillary
buttresses. Because these repairs frequently involve a multidisciplinary
team, it is essential that a clear surgical plan be established
beforehand, including the order in which repair of the involved anatomic
regions will proceed. Methods of stabilization include interosseous wiring and miniplate fixation. Interosseous wiring is generally sufficient for repair of isolated orbital fractures resulting
from low-energy injuries. Higher energy injuries, which result in
multiple orbital rim fractures and comminution, benefit from rigid miniplate
fixation to maintain a stable three-dimensional reconstruction
and improve osteosynthesis (Figs. 8, 9, and 10). Miniplate fixation allows bridging of areas of extensive comminution. Miniplates come in
various sizes (i.e., plate thickness [“profile”] and screw size) and
shape. Miniplates generally have a profile of at least 1 mm and utilize screws equal to
or greater than 1.5 mm, whereas microplates have profiles on the order of 0.5 mm and screw sizes of approximately 1 mm. Plates
and screws of intermediate size are also available, and these
appear to be ideal for orbital reconstruction. Most miniplates (and
screws) are composed of corrosion-resistant metals, such as titanium
or vitallium (an alloy of cobalt, chromium, and molybdenum). Because
these metals are nonmagnetic, postoperative MRI is not contraindicated
after reconstruction with these materials. These materials do produce
some artifact on CT scan; however, this artifact is less than that noted
with stainless steel, with titanium having the least artifact. 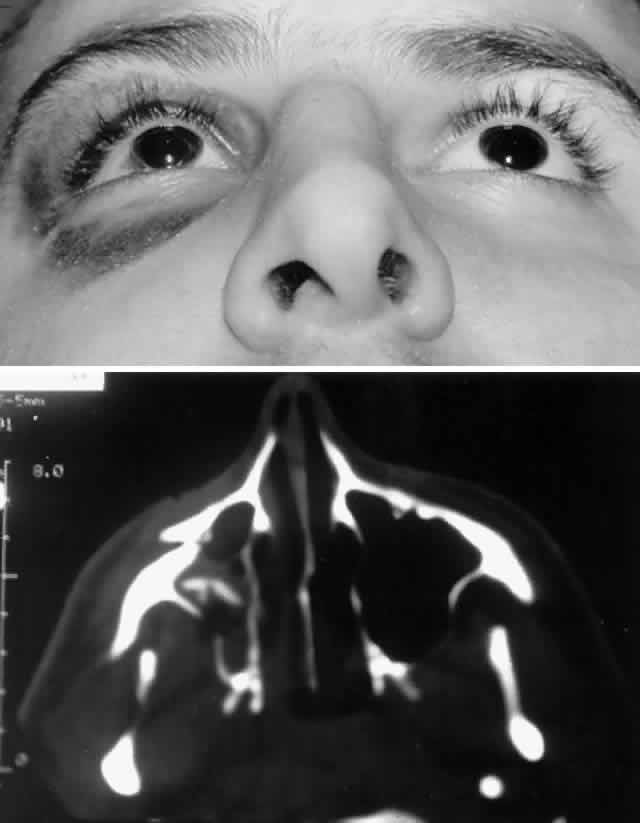 Fig. 8. A. Patient with right zygomatico-orbital fracture. Note flattening of right
malar eminence and slight right lateral canthal dystopia. B. Axial CT scan shows displaced right zygomatico-orbital fracture. Fig. 8. A. Patient with right zygomatico-orbital fracture. Note flattening of right
malar eminence and slight right lateral canthal dystopia. B. Axial CT scan shows displaced right zygomatico-orbital fracture.
|
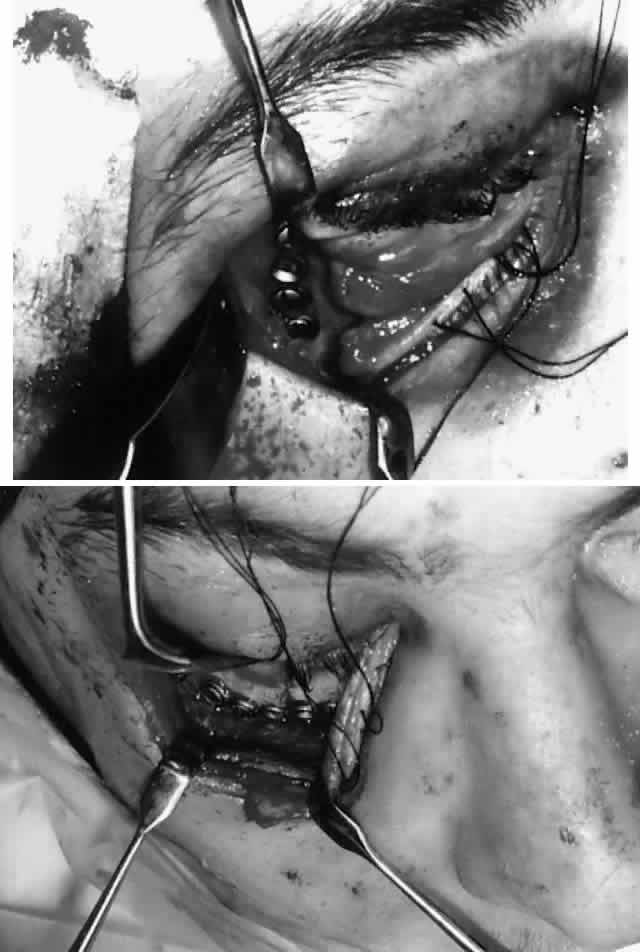 Fig. 9. Repair of zygomatico-orbital fracture with rigid miniplate fixation, approached
via a single transconjunctival incision with extended lateral
canthotomy/cantholysis. A. Exposure and fixation of lateral (frontozygomatic) orbital rim fracture. B. Reduction and fixation of inferior orbital rim fracture. Orbital floor
can also be explored via the same approach. Fig. 9. Repair of zygomatico-orbital fracture with rigid miniplate fixation, approached
via a single transconjunctival incision with extended lateral
canthotomy/cantholysis. A. Exposure and fixation of lateral (frontozygomatic) orbital rim fracture. B. Reduction and fixation of inferior orbital rim fracture. Orbital floor
can also be explored via the same approach.
|
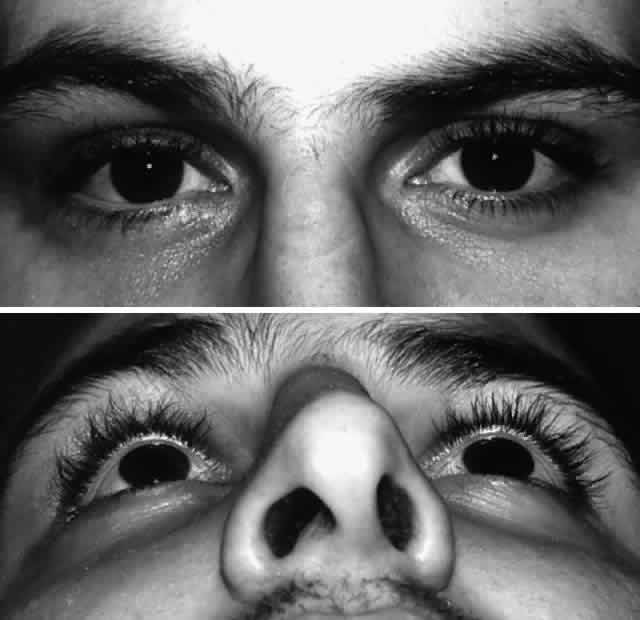 Fig. 10. A. Postoperative appearance of patient in Figure 8 showing excellent position of the globe and eyelid. B. Normal malar contour has been re-established. Fig. 10. A. Postoperative appearance of patient in Figure 8 showing excellent position of the globe and eyelid. B. Normal malar contour has been re-established.
|
The general application of these devices is relatively straightforward. Reduction
and alignment of the displaced bone fragments is performed
first. In some cases, particularly when multiple fracture sites are present
within a given anatomic region, initial alignment using interosseous
wiring may be helpful to link the segments together before the miniplates
are applied. A miniplate is then sized and contoured to span
the fracture. Using special instruments provided in the set, the miniplate
can be customized to fit. The miniplate should be placed in such
a manner that will be virtually impalpable while still affording stable
alignment and fixation. With the typical straight or curved miniplate, at
least two screws are placed on either side of the fracture line
to prevent late rotation of the fracture segments. Low-speed drills with
continuous cooling are used to minimize bony necrosis. Most sets now
supply self-taping screws. Emergency screws, which have a slightly larger
diameter shaft, are available if stripping occurs during placement
of the standard screw. Miniplates have revolutionized the management
of orbital/craniofacial fractures, but it is important to remember that
they are alloplastic devices and may be associated with complications
such as infection, soft tissue irritation, and exposure. Fortunately
these complications appear to be relatively uncommon in the orbital
region. The following sections include special considerations that apply
to the management of specific regional orbital rim fractures. SUPERIOR ORBITAL RIM. The superior orbital rim is contiguous with the frontal bone and cranium. This
portion of the cranium is divided into a central (frontal sinus) area
and two lateral (frontotemporal orbital) areas. Most fractures
involving the superior orbital rim and frontal skull are linear fractures
with little displacement.18–20,38,39 Extensive frontal bone injuries usually produce stellate fracture patterns
extending within one or two areas of the frontal portion of the skull
frequently involving the superior orbit. Open reduction and stabilization
is required for these displaced fractures. Rigid fixation of
the supraorbital regions (“frontal bar”) stabilizes forehead
projection. The temporal bones and anterior cranial base can be used
to confirm anatomic alignment in cases of more extensive fractures. Usually
a single curved miniplate can be used to repair a fracture of
the superior orbital rim itself. Bony defects are repaired with placement
of carefully contoured bone grafts. Fractures in this region may involve the frontal sinus, including the anterior
or posterior wall, or both. Simple fractures of the anterior wall
can be managed by direct reduction and fixation with preservation
of the sinus. Fractures involving the posterior wall and nasofrontal duct
require a more complex repair. The potential for dural laceration
in displaced posterior wall fractures necessitates either obliteration
or cranialization of the frontal sinus, usually with the assistance of
a neurosurgeon or otolaryngologist. Severe disruption of the orbital roof requires stabilization with bone
grafts or alloplastic implants with rigid intracranial fixation to the
frontal bar. Reconstruction of the superorbital rim (frontal bar) establishes
the proper width of the orbit as well as a stable reference point
for realignment of the nasal ethmoid and lateral orbit in the case
of more extensive upper midfacial and panfacial fractures. When exposing
the superior orbital rim and orbital roof, caution should be observed
medially to avoid injury to the trochlea and the supraorbital neurovascular
bundle. MEDIAL ORBITAL RIM (NASOETHMOID-ORBITAL REGION). The bones of the medial orbital rim represent the lateral aspect of the
nasoethmoid complex, and fractures of the medial orbital rim are most
typically the component of a more generalized nasoethmoid orbital injury. Nasoethmoid
orbital fractures may be isolated or associated with
other more extensive craniofacial fractures (e.g., Le Fort II and III). Successful management of nasoethmoid orbital injury
requires consideration of both the bony and soft tissue injury.38,39,45–47 The most important soft tissue structure in the nasoethmoid-orbital region
is the medial canthal tendon. The medial canthal tendon has medial
and posterior insertions to the anterior and posterior lacrimal crest
in the anterior portion of the medial orbit.16 Disruption of the medial canthal tendon or the bony segment containing
the insertion of the medial canthal tendon can result in telecanthus. Because
the lacrimal drainage system is closely tied with this area, it
is at risk for injury from the original trauma as well as during surgical
repair. Nasoethmoid orbital injuries may also be associated with fracture extension
into the anterior cranial fossa (adjacent to the cribriform plate), which
may result in cerebrospinal fluid rhinorrhea, pneumocephalus, olfactory
nerve disruption, and potential frontal lobe injury. Markowitz
and colleagues45 have proposed a classification scheme for nasoethmoid orbital fractures
that is related to the condition and position of what they term the central fragment (the portion of the frontal process of the maxilla providing the bony
insertion of the medial canthal tendon). Three types of fractures are
outlined: Type 1: A nasoethmoid orbital fracture that results in the central fragment's
being fractured as a single segment (either nondisplaced or displaced)
Type 2: A fracture involving comminution of the bony central fragment, but not
involving insertion of the medial canthal tendon
Type 3: A fracture involving comminution of the central fragment extending through
the insertion of the medial canthal tendon
Reconstruction is based on the degree of disruption of the central fragment
of the medial orbital rim. Displaced type 1 injuries can be repaired
by direct reduction and fixation of the central fragment using microplates
or miniplates, with superior fixation to the frontal bone, and
inferior fixation to the adjacent inferior orbital rim/maxillary buttress. It
is important that the medial canthal tendon be left attached
to the central fragment; thus, subperiosteal dissection underlying the
medial canthal tendon insertion is avoided. Type 2 fractures, because of bony comminution, generally require transnasal
wiring to stabilize the central fracture segment containing the medial
canthal tendon and to minimize the risk of postoperative telecanthus. The
canthal tendon-bearing portion of the central fragment is isolated
by subperiosteal dissection, except for the area of medial canthal
tendon insertion, which is not detached. Transnasal (28-gauge) wires
are passed through drill holes placed superior and posterior to the
lacrimal fossa (and medial canthal tendon) and on the central fragment. These
wires are then passed across the nose in a trans-septal fashion. If
a bilateral nasoethmoid fracture is present, the two central fragments
can be linked together. With unilateral fractures, the transnasal
wire extends to the intact contralateral nasal dorsal bone. All other
nasal and orbital bone segments are first linked by wires and then
fixed to the frontal bone and inferior orbital rim/maxillary buttress
with junctional plate and screw fixation. Tightening of the transnasal
wires produces central fragment reduction and creates proper intercanthal
dimensions. The intercanthal soft tissue distance is rarely overcorrected, and
it is more frequently undercorrected. Therefore, transnasal
reduction should deliberately minimize the bony interorbital distance
between the medial orbital rims to obtain a satisfactory result. Type 3 comminuted fractures rarely avulse the medial canthal tendon; however, the
central fragment is frequently too small in such fractures
to utilize in reconstruction. In such cases, the medial canthal tendon
is detached and transnasal reduction of the medial orbital rim segments
is performed, followed by direct transnasal wiring of the medial canthal
tendon itself. The medial canthal tendon may be attached to the
transnasal wire with a smaller permanent monofilament or braided suture. It
is important to pass the transnasal wire posterior and slightly
superior to the lacrimal sac fossa in order to achieve proper eyelid-globe
apposition and impart a natural appearance to the medial canthus. Care
should be taken to preserve the lacrimal system during transnasal
wiring; this can be facilitated by placement of lacrimal probes within
the canaliculi. Other techniques for securing the medial canthal tendon in the setting
of nasoethmoid orbital fractures have been described. Shore and associates47 described repair of telecanthus using a miniplate cantilevered from the
lateral aspect of the nose and directed posteriorly into the orbit to
provide a stable fixation point for the medial canthal tendon. This
technique is probably most applicable for cases of unilateral traumatic
telecanthus, in which poor bony support for transnasal wires is suggested
on preoperative CT. After fracture reduction with transnasal wiring
or medial canthal tendon fixation, soft padded nasal bolsters may
be placed to help minimize edema and hematoma as well as to adapt the
skin to the nasal bones. Although these external bolsters play no role
in the reduction or stabilization of the medial orbital rims, some authors
believe they may help mold the bones of the nose and may minimize
the scarring and thickening of the medial canthal tissues. These bolsters
are secured with an additional transnasal wire, which is removed 7 to 10 days
after surgery. Adequate aesthetic repair of extensive fractures
in this region can be challenging. LATERAL ORBITAL RIM (ZYGOMATICO-ORBITAL). The lateral orbital rim is composed primary of zygomatic bone. The large
zygomatic bone (zygoma) also establishes the malar projection and midfacial
width, thus playing a prominent role in facial aesthetics. The
zygoma has articulations with the temporal, frontal, maxillary, and
sphenoid bones oriented in three different planes. Isolated lateral rim
fractures are uncommon, more typically occurring with disruption of
the zygoma as a unit.48–54 As previously noted, various names have been used to describe zygomatic
bone fracture, including “tripod” and “trimalar” fracture. More
recently there has been a trend to refer to such fractures
as zygomatico-orbital fractures (or conversely “orbitozygomatic” fractures), which more accurately
reflects the prominent orbital component of such fractures.48 Indeed, the main sequelae of zygomatico-orbital fractures are ophthalmic
in nature and include enophthalmos, diplopia, infraorbital nerve dysfunction, and
lateral canthal dystopia. In the pathogenesis of a zygomatico-orbital fracture, disruption occurs
with various degrees of displacement at each of the articulations of
the zygomatic bone. Unlike other portions of the orbit, significant dynamic
forces act on the zygoma, primarily due to the masseter and to a
lesser extent the temporalis muscle. Significant displacement of the
zygomatic segment of the orbit can occur. The zygoma may be rotated inwardly
toward the orbit, causing direct damage or functional impairment
of the globe, extraocular muscles, or optic nerve. More typically, however, it
rotates outward, creating orbital volume expansion and the
potential for enophthalmos. Classification schemes focusing on the different possible anatomic positions (rotations) of
the displaced zygoma have been described, but these
have not proved particularly helpful for guiding fracture management.51 A classification scheme based on the degree of comminution and displacement
is more useful for guiding the intensity of treatment. The classification
scheme of Manson and co-workers6 (see the Classification section) is particularly applicable to zygomatic
fractures. Low-energy zygomatico-orbital fractures demonstrate little
or no displacement. Frequently the fracture is incomplete through at
least one articulation with stability provided at this point (typically
the zygomaticofrontal suture). Zygomatico-orbital fractures with minimal
degrees of displacement do not require reduction. Conservative
treatment consists of a soft diet and protection of the malar eminence
for several weeks. These patients should nevertheless be followed closely
in the initial weeks after injury in order to detect any early zygomatic
displacement due to dynamic traction. Zygomatico-orbital fractures
with significant displacement are best managed with open reduction
and internal fixation (see Figs. 8, 9, and 10). The number of fracture sites requiring fixation varies with the severity
of the injury. For middle-energy zygomatico-orbital fractures, which
constitute the vast majority of such injuries, mild to moderate displacement
is seen, with a range of comminution. For simple, displaced zygomatico-orbital fractures with no major comminution, a
diversity of opinion exists as to the ideal approach and number
and location of points requiring direct visual alignment and fixation. In
the era in which interosseous wires were the primary means of fixation, experimental
and clinical studies suggested that two-point interfragmentary
wiring of the lateral and inferior orbital rims, while
the most common method of internal fixation, still permitted rotational
displacement of the zygoma about an axis between these two fixation
points caused by the continuous traction and force of the masseter muscle. Three-point
wire fixation was subsequently advocated by many surgeons, even
for simple displaced, noncomminuted zygomatico-orbital fractures. The
application of osteosynthesis technology (i.e., rigid miniplates and screws) has led to an evolution in the management
of zygomatico-orbital fractures. Miniplates provide greater stability
and are more resistant to rotational forces. Most authorities now agree
that two-point miniplate application (at two of the following locations: zygomatico
frontal suture, inferior orbital rim, or zygomaticomaxillary
buttress) provides stable fixation of most noncomminuted displaced
zygomatico-orbital fractures.48,49 Indeed, some surgeons have even suggested that one-point miniplate fixation
of zygomatic fractures may be sufficient, provided that appropriate
measures be taken to ensure three-point alignment.51,52 Advocates of one-point fixation caution that such techniques are appropriate
only for simple, noncomminuted zygomatico-orbital fractures as
assessed by preoperative CT. For middle-energy zygomatico-orbital fractures with comminution, however, at least a two-point fixation is clearly recommended. The
surgical approach to middle-energy zygomatico-orbital fractures
is dictated by the degree of exposure and number of fixation points
required. The inferior orbital rim fracture site and lateral orbital
rim (i.e., zygomaticofrontal suture) fracture sites can be approached quite practically
by a single eyelid incision (either transcutaneous or transconjunctival/lateral
cantholysis), by extending the incision laterally from
the lateral canthal angle. This approach also allows exploration of
the orbital floor and confirmation of an adequate reduction by direct
inspection of the articulation of the zygomatic bone with the more posterior
sphenoid bone, which helps ensure adequate anatomic alignment. Further
exposure of the zygomaticomaxillary buttress, if desired, can
be obtained via a gingivobuccal sulcus incision. Reduction of the zygoma
can be accomplished with an elevator or towel clip. A Girard screw, placed
in the malar eminence, may also facilitate reduction. After reduction
is performed, the articulation sites at the lateral and inferior
orbital rims (and lateral and inferior orbital walls) should be checked
to ensure that no soft tissue entrapment is present. Miniplate fixation
is performed; after this, any additional repair of the internal
orbit can be accomplished. High-energy zygomatico-orbital fractures are characterized by associated
comminution of the greater wing of the sphenoid (in the lateral orbit) and
by lateral displacement and posterior segmentation of the zygomatic
arch. The external angular process of the frontal bone is also frequently
comminuted. These more severe types of zygomatico-orbital fractures
are infrequently observed as isolated injuries, being associated
more typically with Le Fort and panfacial fractures. The extensive lateral
and posterior displacement of the arch results in loss of support
for the malar eminence, causing the cheek to be depressed posteriorly
and inferiorly. Midfacial width and orbital volume are significantly
increased. These high-energy zygomatico-orbital fractures require (1) coronal
exposure with reduction and fixation of the zygomatic arch in
order to correct facial width and to align and stabilize the forward
projection of the malar eminence6,38,53,54; and (2) rigid fixation of at least two other points of zygomatic articulation, as
previously discussed. INFERIOR ORBITAL RIM. Isolated fractures of the inferior orbital rim are relatively uncommon. Forces
sufficient to fracture the inferior orbital rim usually result
in internal orbital fractures as well. An inferior orbital rim fracture
may be a component of a zygomatico-orbital fracture or a nasoethmoid
orbital fracture. With high-energy injuries, extensive comminution
of the inferior orbital rim with bone loss may occur, typically as a component
of a panfacial fracture. In this setting, inferior orbital rim
fracture repair is facilitated by initial reconstruction of the superior, medial, and
lateral orbital rims to establish the vertical and horizontal
dimensions of the orbital aperture and to allow stabilization
of the inferior orbital rim segment(s) by rigid fixation to the zygoma
laterally and the nasomaxillary buttress medially. If bone loss (greater
than 5 mm) has occurred, bone grafts can be used to fill the defect
and are secured by rigid fixation. Inferior orbital rim fractures
may extend farther inferiorly through the maxilla. In such cases, attention
is directed toward stabilization and rigid fixation of the maxillary
buttresses. The thinner bone of the anterior maxillary antrum may
be comminuted; in most cases, however, primary repair of this portion
of the maxilla is not necessary. Postoperative Complications Complications after orbital fracture repair can of course be minimized
by good surgical technique. However, because of the severity of such injuries, including
the associated soft tissue damage, postoperative complications
certainly occur, and the physician should be familiar with
their management. Some complications are common to all types of orbital
surgery, some are specific to malposition of the orbital rims, and
others are related to disruption of the internal orbital walls.36 The most serious complication of orbital surgery is visual loss, which fortunately is rare. Visual loss may be caused by intraoperative
manipulation of the globe or optic nerve or by a postoperative orbital
hemorrhage that produces central retinal artery occlusion or compressive
optic neuropathy. Early detection of visual loss is essential, which
is why occlusive dressings are avoided and the patient is followed
closely after surgery. Pupil examination to check for an afferent pupillary
defect is a reliable, simple way to detect optic neuropathy. Orbital
hemorrhage in such cases is usually obvious; when it is found to
be causing impairment, it is treated as previously discussed with lateral
canthotomy, cantholysis, and other adjunctive medical and surgical
treatment.2 Repeat orbital CT scanning is appropriate, provided that treatment is
not significantly delayed. The following are among the complications related to malposition of the
orbital rims: persistent step-off fracture, bony malunion or resorption
creating contour deformity, and frank dislocation of a portion of the
orbital rim in the early postoperative period, which is caused by improper
alignment or fixation and in late cases by tractional forces acting
on an inadequately fixated segment. These complications are more
common with fractures of the inferior lateral orbital rims (i.e., zygomatico-orbital fractures) because of the tractional forces of the
masseter muscle. If early dislocation is noted, prompt repositioning
and stabilization (fixation) should be performed. In cases where this
complication is noted later, treatment may consist of onlay bone grafts
or synthetic implants (e.g., malar eminence). In more severe cases, however, osteotomy with repositioning
and fixation of the dislocated segment is necessary. Soft tissue disruption associated with orbital rim fractures may also produce
late postoperative sequelae, primarily consisting of displacement
of the lateral canthus (inferior dystopia) and medial canthus (typically
producing telecanthus). Mild lateral canthal dystopia may be managed
by lateral canthoplasty with repositioning of the lateral canthus
using a tarsal strip procedure, provided that the bony rim/malar position
is not significantly displaced. More severe forms of dystopia usually
necessitate osteotomy and repositioning of the displaced lateral
orbital rim/zygoma. Persistent telecanthus unfortunately is a frequent
complication of nasoethmoid orbital fractures (Fig. 11). Treatment is usually performed with medial canthopexy using transnasal
wiring or other medial canthal fixation techniques (Fig. 12). Excess scar tissue and displaced bone fragments should be removed from
the medial canthal region in order to achieve a satisfactory result. Damage
to the nasolacrimal drainage system may also be a sequela of
a nasoethmoid orbital or midfacial fracture. If injury to the nasolacrimal
drainage system is obvious at the patient's initial presentation, primary
repair can be performed. In most cases, the nasolacrimal
injury is not noted until later, typically manifesting as nasolacrimal
duct obstruction producing epiphora, with or without dacryocystitis. Treatment
is accomplished with dacryocystorhinostomy. 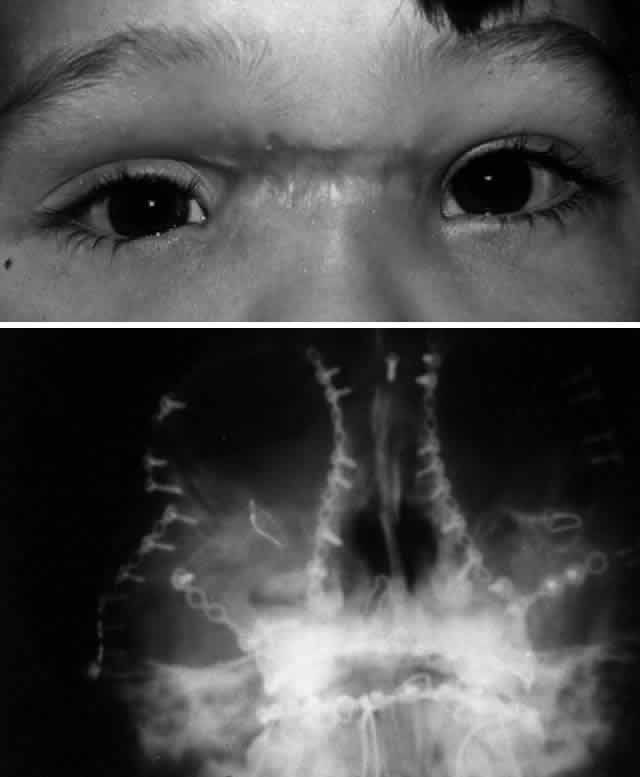 Fig. 11. Young boy shown after repair of panfacial fractures. A. Bilateral telecanthus and inferior medial canthal dystopia is noted, along
with a wide band of scar tissues across the nasal bridge. The patient
also had epiphora secondary to nasolacrimal duct obstruction. B. Postoperative facial x-ray shows extensive nature of injury and repair
with rigid miniplate fixation. Fig. 11. Young boy shown after repair of panfacial fractures. A. Bilateral telecanthus and inferior medial canthal dystopia is noted, along
with a wide band of scar tissues across the nasal bridge. The patient
also had epiphora secondary to nasolacrimal duct obstruction. B. Postoperative facial x-ray shows extensive nature of injury and repair
with rigid miniplate fixation.
|
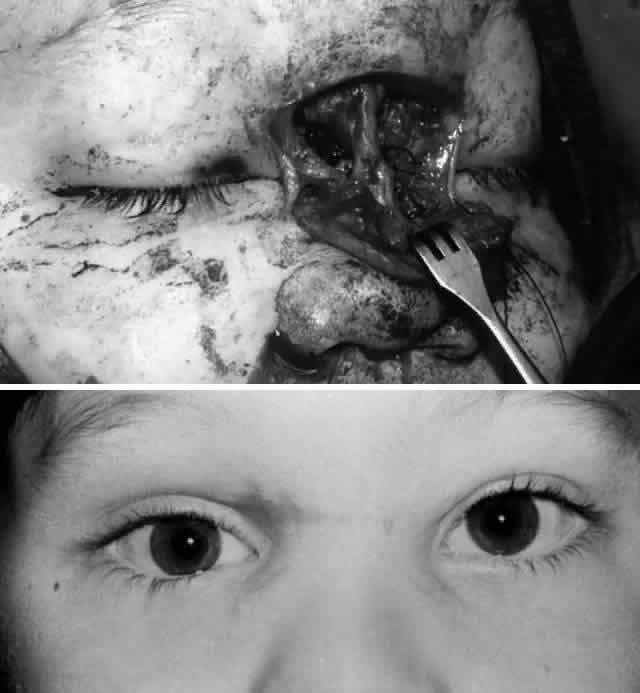 Fig. 12. A. Secondary, bilateral medial canthopexy was performed on the patient in Figure 11 with transnasal wiring. In this case an “open-sky technique” was
used and combined with scar revision. Bilateral dacryocystorhinostomy
was also performed in the same setting to treat coexistent nasolacrimal
duct obstruction. B. Postoperative appearance showing improved medial canthal position. Epiphora
was also successfully relieved. Fig. 12. A. Secondary, bilateral medial canthopexy was performed on the patient in Figure 11 with transnasal wiring. In this case an “open-sky technique” was
used and combined with scar revision. Bilateral dacryocystorhinostomy
was also performed in the same setting to treat coexistent nasolacrimal
duct obstruction. B. Postoperative appearance showing improved medial canthal position. Epiphora
was also successfully relieved.
|
Complications related to repair of internal orbital wall fractures include
predominantly persistent enophthalmos/hypo-ophthalmos and diplopia, as
well as those related to synthetic orbital implants or bone grafts
per se. Several of the potential postoperative complications related
to alloplastic implants and bone grafts (see Internal Orbital Fractures
section) include migration, extrusion, and infection. Such complications
are possible with alloplastic implants or bone grafts, but are more
common with alloplastic implants. Bone grafts have the potential complication
of variable degrees of resorption, which is believed to be greater with endochondral bone compared with membranous
bone grafts. Infection or extrusion generally requires removal of the implant or bone graft if prompt improvement
with antibiotic therapy is not seen. In the setting of infection, the
implant should not be replaced. In many cases, fibrous tissue
forms around alloplastic implants after several weeks or months, which
may allow enough orbital soft tissue support so that a replacement implant
is not required. In other cases, secondary repair with a suitable
alloplastic implant or graft must be performed after the infection is
adequately controlled. Migration of an alloplastic implant or bone graft, if minor and not causing significant
sequelae, may be observed. More marked implant migration causing
functional impairment necessitates removal of the alloplastic implant
or bone graft. Globe malpositions after blow-out fracture repair usually are caused by failure of surgery
to reconstruct the normal anatomic boundaries (walls) of the orbit. Soft
tissue scarring (and perhaps in a small number of cases, fat atrophy) may
also contribute to late enophthalmos. Enophthalmos may be corrected by repositioning the implant or by adding
additional alloplastic implant or bone graft to augment the volume deficiency. Camouflage
techniques such as blepharoplasty of the contralateral
upper eyelid or ptosis repair of the ipsilateral eyelid may also
be considered. Diplopia after blow-out fracture repair may be due to persistent orbital soft tissue
entrapment, but it may also be due to coincident extraocular muscle
injury or neurologic injury. Postoperative orbital edema may also be associated with generalized limitation of ocular motility; therefore, ocular
motility is most appropriately reassessed after the
edema resolves (usually in 2 to 3 weeks). Repeat CT scanning may also
be helpful. If the clinical examination and CT findings are suggestive
of persistent extraocular muscle entrapment in a patient with functionally
significant diplopia, then early orbital re-exploration is indicated. Persistent ocular motility deficits that are not amenable to orbital surgery
generally require strabismus surgery. Usually a period of 6 months
or more is allowed for spontaneous improvement, during which time serial
orthoptic measurements are taken. Within this waiting period, diplopia
may be symptomatically treated with Fresnel prisms, fogging (partial
occlusion), or patching (complete occlusion) of one eye. Young children
at risk for amblyopia require closer follow-up and appropriate
treatment should amblyopia develop. Most authorities consider blow-out
fracture repair successful if diplopia is relieved within the functional (30°) fields
of gaze. Reoperation is usually not indicated for
diplopia occurring in more eccentric fields of gaze, although each case
must be individualized. |