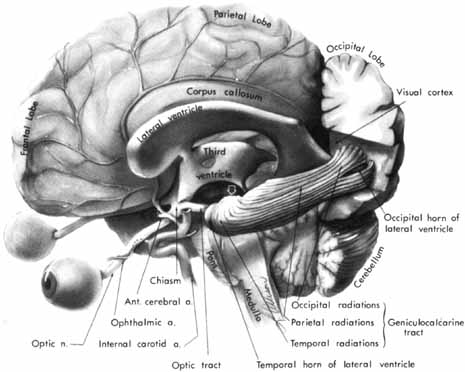
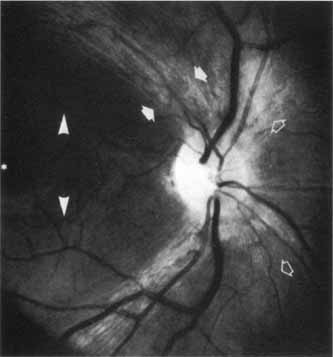
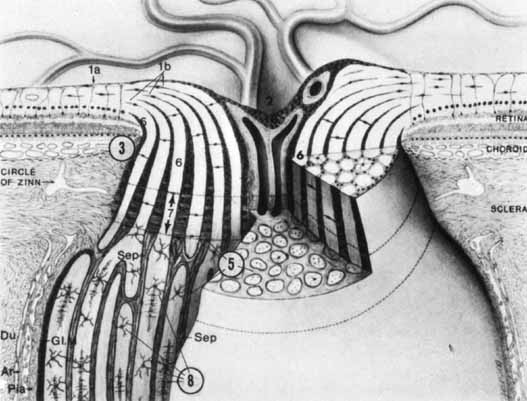
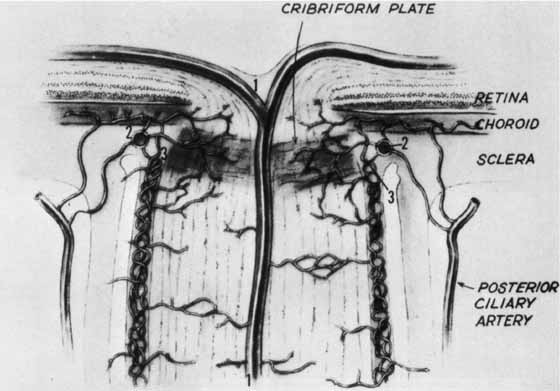
1. Buesch SR, Arey LB: The number of myelinated and unmyelinated fibers in the optic nerve of
vertebrates. J Comp Neurol 77:631, 1942 2. Lemire R. Loeser J, Leech R, et al: Normal and abnormal development of the human nervous system. In: The Optic System. New York, Harper & Row, 1975:196–205 3. Provis JM, van Driel D, Billson FA, et al: Human fetal optic nerve overproduction and elimination of retinal axons
during development. J Comp Neurol 238:92, 1985 4. Frost DO: Orderly anomalous retinal projections to the medical geniculate, vetrobasal
and lateral posterior nucleus of the hamster. J Comp Neurol 203:227, 1981 5. Rakic P: Prenatal development of the visual system in the rhesus monkey. Philos Trans R Soc Lond [Biol] 278:245, 1977 6. Godement P: Development of retinal projections in the mouse. In Stone J, Dreher B, Rapaport DH (eds): Development of Visual Pathways in Mammals. New York: Alan R. Liss, 1984 7. Livingstone M, Hubel D: Segregation of form, color, movement, and depth: Anatomy, physiology, and
perception. Science 240:740, 1988 8. Rodieck RW: The primate retina. In Steklis HD, Erwin J (eds): Comparative Primate Biology, Vol. 4, Neurosciences. New York: Alan R. Liss, 1988:203–278 9. Honrobia FM, Elliot JH: Efferent innervation of the retina: Morphological study of the human retina. Arch Ophthalmol 80:98, 1968 10. Panda S, Provencio I, Tu DC, et al: Melanopsin is required for non-image-forming photic responses
in blind mice. Science 25:301(5632):525–527. 11. Hattar S, Lucas RJ, Mrosovsky N, et al: Melanopsin and rod-cone photoreceptive systems account for all major
accessory visual functions in mice. Nature 424(6944):75–81, 3 Jul 2003 12. Bernson DM: Strange vision: Ganglion cells as circadian photoreceptors. Trends Neurosci 26(6):314–320, Jun 2003 13. Menaker M: Circadian rhythms: Circadian photoreception. Science 299(5604):213–214, 10 Jan 2003 14. Lubkin V, Beizai P, Sadun AA: The eye as metronome of the body. Surv Ophthalmol 47(1):17–26, 2002 15. Osterberg G: Topography of the layer of rods and cones in the human retina. Acta Ophthalmol (Suppl) 6:1, 1935 16. Van Buren JM: The retinal ganglion cell layer. Springfield, IL: Charles C Thomas, 1963:130 17. Glaser JS: The nasal visual field. Arch Ophthalmol 77:358, 1967 18. Hendrickson AK, Floren I, Patterson R, et al: Neurotransmitter localization in the Macaca monkey retina. Invest Ophthalmol Vis Sci (Suppl) 20:237, 1981 19. Frederick JM, Rayborn ME, Laties AM, et al: Dopaminergic neurons in the human retina. J Comp Neurol 210:65, 1982 20. Ogden TE: Nerve fiber layer of the primate retina: Morphometric analysis. Invest Ophthalmol Vis Sci 25:19, 1984 21. Bishop GH: Fiber groups in the optic nerve. Am J Physiol 106:460, 1933 22. Polyak S: The retina. Chicago: University of Chicago Press, 1941 23. Perry VH, Oehler R, Dowey A: Retinal ganglion cells that project to the dorsal lateral geniculate nucleus
in the macaque monkey. Neuroscience 12:1101, 1984 24. Enroth-Cugell C, Robson GJ: The contrast sensitivity of retinal ganglion cells of the cat. J Physiol 187:517–552, 1966 25. Boycott BB, Wassle H: The morphological types of ganglion cells of the domestic cat's retina. J Physiol Lond 240:397, 1974 26. Rodieck RW: The vertebrate retina. San Francisco: WH Freeman, 1973 27. Stone J: Parallel processing in the visual system. New York: Plenum Press, 1983:3397 28. Leventhal AG, Rodieck RW, Dreher B: Retinal ganglion cell classes in the Old World monkey: Morphology and central
projections. Science 213:1139, 1981 29. Livingstone MS, Hubel DH: Psychophysical evidence for separate channels for the perception of form, color
movement, and depth. J Neurosci 7:3416, 1987 30. Rodieck RW, Binmuoeller KF, Dineen J: Parasol and midget ganglion cells of human retina. J Comp Neurol 233:115, 1985 31. Watanabe J, Rodieck RW: Parasol and midget ganglion cells of the primate retina. J Comp Neurol 289:434, 1989 32. Leventhal AG, Ault SJ, Vitek DJ: The nasotemporal division in primate retina: The neural bases of macular
sparing and splitting. Science 240:66, 1988 33. Ogden TE: Nerve fiber layer of the owl monkey retina: Retinotopic organization. Invest Ophthalmol Vis Sci 24:265, 1983 34. Hu EH, Bloomfield SA: Gap junctional coupling underlies the short-latency spike synchrony
of retinal alpha ganglion cells. J Neurosci . 23(17):6768–77, 30 Jul 2003 35. Lauglin SB: Retinal function: Coupling cones clarifies vision. Curr Biol . 12(24):R833–834, 2002 36. Cusato K, Bosco A, Rozental R, et al: Gap junctions mediate bystander cell death in developing retina. J Neurosci 23(16):6413–22, 23 Jul 2003 37. Jonas JB, Gusek GC, Naumann GOH: Optic disc, cup, and neuroretinal rim size, configuration and correlations
in normal eyes. Invest Ophthalmol Vis Sci 29:1151, 1988 38. Jonas JB, Schmidt AM, Muller-Bergli JA, et al: Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci 33:2012, 1992 39. Quigley HA, Brown AE, Morrison JP, et al: The size and shape of the optic disc in normal human eyes. Arch Ophthalmol 108:51, 1990 40. Hernandez MR, Igoe F. Neufeld AH, et al: Extracellular matrix of the human optic nerve head. Am J Ophthalmol 102:139, 1986 41. Minckler DS: Correlations between anatomic features and axonal transport in primate
optic nerve head. Trans Am Ophthalmol Soc 84:429, 1986 42. Anderson DR, Hoyt WF: Ultrastructure of intraorbital portion of human and monkey optic nerve. Arch Ophthalmol 82:506, 1969 43. Anderson DR: Ultrastructure of meningeal sheaths: Normal human and monkey optic nerves. Arch Ophthalmol 82:659, 1969 44. Anderson DR: Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol 82:800, 1969 45. Anderson DR: Ultrastructure of the optic nerve head. Arch Ophthalmol 83:63, 1970 46. Ding L, Yamada K, Takayama C, et al: Development of astrocytes in the lamina cribrosa sclerae of the mouse optic
nerve, with special reference to myelin formation. Okajimas Folia Anat Jpn 79(5):143–58, 2002 47. Morcos Y, Chan-Ling T: Concentration of astrocytic filaments at the retinal optic nerve junction
is coincident with the absence of intra-retinal myelination: comparative
and developmental evidence. J Neurocytol . 29(9):665–78, 2000 48. Kurosawa H, Kurosawa A: Scanning electron microscopic study of pial septa of the optic nerve in
humans. Am J Ophthalmol 99:490, 1985 49. Anderson DR: Vascular supply of the optic nerve of primates. Am J Ophthalmol 70:341, 1970 50. Hayreh SS: Anatomy and physiology of the optic nerve head. Trans Am Acad Ophthalmol Otolaryngol 78:240, 1974 51. Sadun AA, Carelli V, Bose S, Ross-Cisneros F, Barboni P, Ahrens ET: First application of extremely-high resolution magnetic resonance
imaging to study microscopic features of normal and LHON human optic
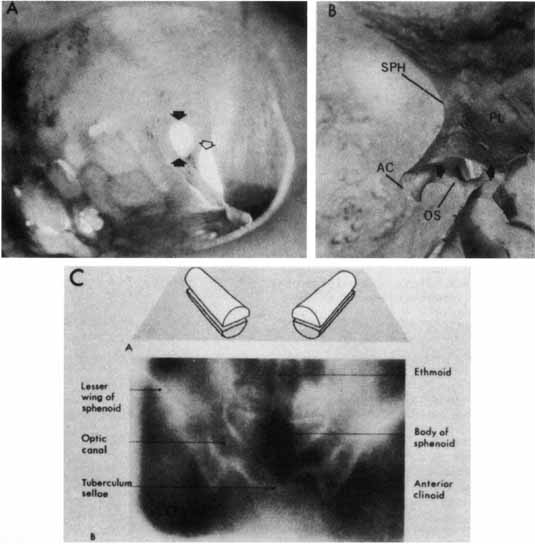
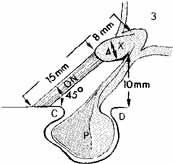
nerve. Ophthalmol 109(6):1085–91, 2002 52. Fujii K, Chambers SM, Rhoton AL: Neurovascular relationships of the spheroid sinus: A microsurgical study. J Neurosurg 50:31, 1979 53. Walker AK: The neurosurgical evaluation of the chiasmal syndromes. Am J Ophthalmol 54:563, 1962 54. Bergland RM, Ray BS, Torack RM: Anatomical variations in the pituitary gland and adjacent structures in 2252 human
autopsy cases. J Neurosurg 28:93, 1968 55. Rucker CW: The concept of a semidecussation of the optic nerves. Arch Ophthalmol 59:159, 1958 56. Daniels DL, Haughton VM, Williams AC, et al: Computed tomography of the optic chiasm. Radiology 137:123, 1980 57. Parravano JG, Toledo A, Kucharczyk W: Dimensions of the optic nerves, chiasm, and tracts. MR quantitative comparison
between patients with optic atrophy and controls. J Comput Assist Tomogr 17:688, 1993 58. Barber AN, Ronstrom GN, Mueeling RJ: Development of the visual pathway: Optic chiasm. Arch Ophthalmol 52:447, 1954 59. Kupfer C, Chumbley L, Downer J, De CC: Quantitative histology of optic nerve, optic tract and lateral geniculate
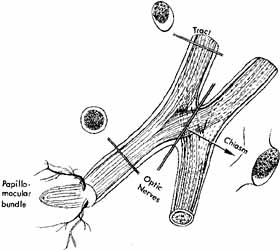
nucleus of man. J Anat 101:393, 1967 60. Jacobson H, Hirose G: Origin of the retina from both sides of the embryonic brain: A contribution
to the problem of crossing at the optic chiasm. Science 202:637, 1978 61. Oster SF, Sretavan DW: Connecting the eye to the brain: The molecular basis of ganglion cell axon
guidance. Br J Ophthalmolo 87(5):639–45, 2003 62. Shrivan A, Kimron M, Holdengreber V, et al: Anti-semaphorin 3A antibodies rescue retinal ganglion cells from
cell death following optic nerve axotomy. J Biol Chem 277(51):49799–807, 2002 63. Silver J, Sapiro J: Axonal guidance during development of the optic nerve: The role of pigmented
epithelia and other extrinsic factors. J Comp Neurol 202:521, 1981 64. Strongin AC, Guillery RW: The distribution of melanin in the developing optic cup and stalk and its
relation to cellular degeneration. J Neurosci 1:1193, 1981 65. Recordon E, Griffith SO: A case of primary bilateral anophthalmia. Br J Ophthalmol 22:253, 1938 66. Rogalski T: The visual path in a case of unilateral anophthalmia with special reference
to the problem of crossed and uncrossed visual fibers. J Anat 80:153, 1946 67. Sadun AA, Schaechter JD, Smith LEH: A retinohypothalamic pathway in man: Light mediation of circadian rhythms. Brain Res 302:371, 1984 68. Sadun AA, Johnson BM, Schaechter JD: Neuroanatomy of the human visual system: III. Three retinal projections
to the hypothalamus. Neuro-ophthalmology 6:371, 1986 69. Stephan FK, Sucker I: Circadian rhythms in drinking behavior and locomotor activity are eliminated
by hypothalamic lesions. Proc Natl Acad Sci USA 69:1583, 1982 70. Schaechter JD, Sadun AA: A second hypothalamic nucleus receiving retinal input in man: The paraventricular
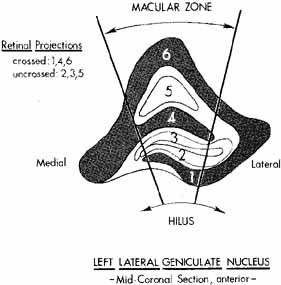
nucleus. Brain Res 340:243, 1985 71. Chacko LW: The laminar pattern of the lateral geniculate body in primates. J Neurol Neurosurg Psychiatry 11:211, 1948 72. Hubel DH, Wiesel TN, LeVay S: Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond [Biol] 278:131, 1977 73. Kupfer C: The projection of the macula in the lateral geniculate nucleus of man. Am J Ophthalmol 54:597, 1962 74. Bishop PO, Kozak W, Levick WR, Vakkur GJ: The determination of the projection of the visual field on the lateral
geniculate nucleus in the cat. J Physiol Lond 163:503, 1962 75. Kaas JH, Guillery RW, Allman JM: Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav Evol 6:253, 1972 76. Lin CS, Kass JH: Projections from cortical visual areas 17, 18, and MT onto the dorsal lateral
geniculate nucleus in owl monkeys. J Comp Neurol 173:457, 1977 77. Zehi SM: Representation of central visual fields in prestriate cortex of monkeys. Brain Res 14:271, 1969 78. Lachica EA, Casagrande VA: The morphology and collicular axons ending on small relay (W-like) cells
of the primate lateral geniculate nucleus. Vis Neurosci 10:403, 1993 79. Lund JS, Boothe RG: Intralaminar connections and pyramidal neuron organization in the visual
cortex, area 17, of the macaque monkey. J Comp Neurol 159:305, 1975 80. Szentagothi J: Neuronal and synaptic architecture of the lateral geniculate nucleus. In Jung R (ed): Handbook of sensory physiology, Vol. VII/3B. Berlin: Springer-Verlag, 1973:141–176 81. Freund J-H: Neuronal mechanisms of the lateral genicuIate body. In Jung R (ed): Handbook of sensory physiology, Vol. VII/3B. Berlin: Springer-Verlag, 1973:177–246 82. Matthews MR: Transneuronal cell degeneration in the lateral geniculate nucleus of the
macaque monkey. J Anat 94:145–169, 1960 83. Beatty B, Sadun A, Smith L, Richardson E: Direct demonstration of transsynaptic degeneration in the human visual
system: A comparison of retrograde and anterograde changes. J Neurol Neurosurg Psychiatry 45:143, 1982 84. Sadun AA: The neuroanatomy of the human visual system: 1.Retinal projections to the
LGN and PT as demonstrated with a new stain. Neuro-ophthalmology 6:353, 1986 85. Polyak S: The vertebrate visual system. Chicago: University of Chicago Press, 1957 86. Hoblen AL: The central visual pathways. In Davson H (ed): The eye, Vol. 2A, Visual function in man. New York: Academic Press, 1976 87. Lund JS: Anatomical organization of macaque monkey striate visual cortex. Ann Rev Neurosci 11:253, 1988 88. Sadun AA: Parallel processing in the human visual system: A new perspective. Neuro-ophthalmology 6:351, 1986 89. Schiller PH: The role of the monkey superior colliculus in eye movement and vision. Invest Ophthalmol Vis Sci 11:451, 1972 90. ,Sadun AA, Johnson BM, Smith LEH: Neuroanatomy of the human visual system: 11.Retinal projections to the
superior colliculus and pulvinar. Neuro-ophthalmology 6:363, 1986 91. Fredericks CA, Giolli RA, Blanks RH, Sadun AA: The human accessory optic system. Brain Res 454:116, 1988 92. Adams JE, Rutkin BB: Visual responses to subcortical stimulation in the visual and limbic systems. Confin Neurol 32:158, 1970 93. Nashold BS: Phosphenes resulting from stimulation of the midbrain in man. Arch Ophthalmol 84:433, 1970 94. Brindley GS, Gautier-Smith PC, Lewin W: Cortical blindness and the functions of the non-geniculate fibers
of the optic tracts. J Neurol Neurosurg Psychiatry 32:259, 1969 95. Weiskrantz L: Blindsight: A case study in implications. Oxford Psychological Series #10. Oxford, England: Clarendon Press, 1986 96. Sadun AA. The afferent visual system: Anatomy and physiology. In Yanoff M, Duker JS (eds), Ophthalmology. London: Mosby, Chapter 11, 2.1, 1999 97. Hoyt WF, Luis O: Visual fiber anatomy in the infrageniculate pathway of the primate: Uncrossed
and crossed retinal quadrant fiber projections studied with Nauta
silver stain. Arch Ophthalmol 68:94, 1962 98. Hoyt WF, Luis O: The primate chiasm: Details of visual fiber organization studies by silver
impregnation techniques. Arch Ophthalmol 70:69, 1963 99. Hoyt WF, Tudor RC: The course of parapapillary temporal retinal axons through the anterior
optic nerve: A Nauta de-generation study in the primate. Arch Ophthalmol 69:503, 1963 100. Potts AM, Hodges D, Shelman CB, et al: Morphology of the primate optic nerve: I–III. Invest Ophthalmol Vis Sci 11:980, 1972 101. Spalding JMK: Wounds of the visual pathway: I. The visual radiation. J Neurol Neurosurg Psychiatry 15:99, 1952 102. Van Buren JM, Baldwin M: The architecture of the optic radiation in the temporal lobe of man. Brain 81:15, 1958 103. Holmes G: A contribution to the cortical representation of vision. Brain 54:470, 1931 104. Spalding JMK: Wounds of the visual pathway: II. The striate cortex. J Neurol Neurosurg Psychiatry 15:169, 1952 105. Stensaas MA, Eddington DK, Dobelle WH: The topography and variability of the primary visual cortex in man. J Neurosurg 40:747, 1974 106. Horton JC, Hoyt WF: The representation of the visual field in human striate cortex. Arch Ophthalmol 109:816, 1991 107. Brindley GS: Sensory effects of electrical stimulation of the visual and paravisual
cortex in man. In June R (ed): Handbook of sensory physiology, Vol. VII/3B. Berlin: Springer-Verlag, 1973:583–594 108. Karten HJ, Keyser KT, Brecha NC: Biochemical and morphological heterogeneity of retinal ganglion cells. In Cohen B, Bodis-Wollner I (eds): Vision and the brain. New York: Raven Press, 1984 109. Ehrlich D, Keyser A, Karten HJ: Distribution of substance P-like immunoreactive retinal ganglion cells
and their pattern of termination in the optic tectum of the chick (Gallus
gallus). J Comp Neurol 266:220, 1987 110. Zucker CL, Dowling JE: Centrifugal fibers synapse on interplexiform cells in the teleost retina. Nature 330:166, 1987 111. Schiller PH: The on and offchannels of the visual system. In Cohen B, Bodis-Wollner I (eds): Vision and the brain. New York: Raven Press, 1990 112. Reiter HO, Stryker MP: Neural plasticity without postsynaptic action potentials: Less active inputs
become dominant when kitten visual cortex cells are pharmacologically
inhibited. Proc Natl Acad Sci USA 85:3623, 1988 113. Livingstone M: Segregation of form, color, movement, and depth processing in the visual
system: Anatomy, physiology, art and illusion. In Cohen B, Bodis-Wollner I (eds): Vision and the brain. New York: Raven Press, 1990 114. Mikami A, Newsom WT, Wurtz RH: Motion selectivity in macaque visual cortex: I. Mechanisms of direction
and speed selectivity in extrastriate area MT. J Neurophysiol 55:1308, 1986 115. Sadun AA, Bassi C: Optic nerve damage in Alzheimer's disease. Ophthalmology 97:1, 9, 1990 116. Fitzke FW: Imaging the optic nerve and ganglion cell layer. Eye 14:450, 2000 117. Chen W, Kato T, Zhu XH, et al: Mapping of lateral geniculate nucleus activation during visual stimulation
in human brain using fMRI. Magn Reson Med 39:89, 1998 118. Fox PT, Fox JM, Raichle ME, Burde RM: The role of cerebral cortex in the generation of voluntary saccades: A
positron emission tomographic study. J Neurophysiol 54:348, 1985 119. David S, Aguayo AJ: Axonal elongation into peripheral nervous system “bridges” after
central nervous system injury in adult rats. Science 214:931–933, 1981 120. Keirstead SA, Rasminsky M, Fukuda Y, Carter DA, Aguayo AJ, Vidal-Sanz M: Electrophysiologic responses in hamster superior colliculus evoked by regenerating
retinal axons. Science 246:255–257, 1989 121. Davies SJ, Field PM, Raisman G: Regeneration of cut adult axons fails even in the presence of continuous
aligned glial pathways. Exp Neurol 142:203–216, 1996 122. McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE: Identification of myelin-associated glycoprotein as a major myelin-derived
inhibitor of neurite growth. Neuron 13:805–811, 1994 123. Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT: A novel role for myelin-associated glycoprotein as an inhibitor
of axonal regeneration. Neuron 13:757–767, 1994 124. Bergland RM, Ray BS: The arterial supply of the human optic chiasm. J Neurosurg 31:327, 1969 125. Smith CG, Richardson WFG: The course and distribution of the arteries supplying the visual (striate) cortex. Am J Ophthalmol 61:1391, 1966 |