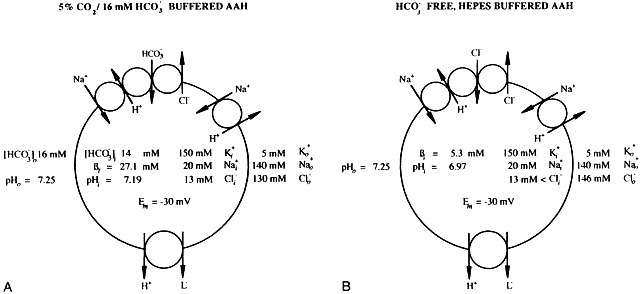
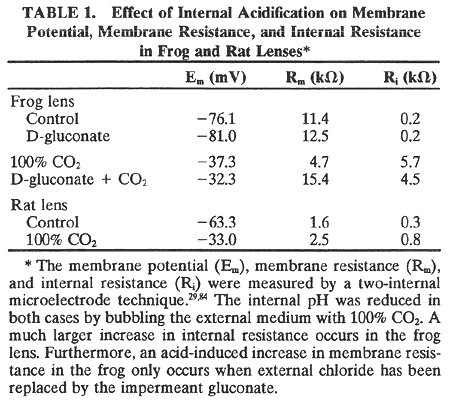
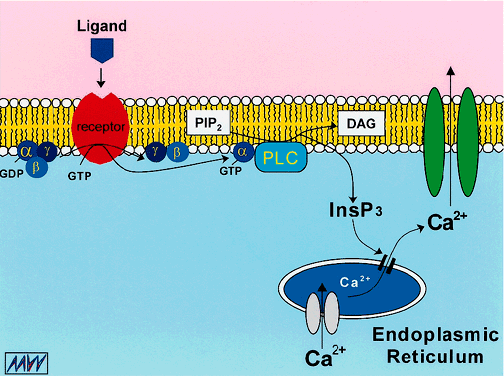
1. Duncan G: Brewster's contribution to the study of the lens of the
eye. In Morrison-Low AD, Christie JRR (eds): Martyr of Science: Sir David
Brewster 1781-1868. Edinburgh: Royal Scottish Museum, 1984:101 2. Brewster D: On the anatomical and optical structure of the crystalline lenses of animals, particularly
that of the cod. Phil Trans Roy Soc Lond 123:323, 1833 3. Brewster D: On the cause and cure of cataract. Trans Roy Soc Edin 24: 11, 1865 4. Bassnett S, Missey H, Vucemilo I: Molecular architecture of the lens fiber-cell basal membrane complex. J Cell Sci 112:2155, 1999 5. Mathias RT, Rae JL, Balado GJ: Physiological properties of the normal lens. Physiol Revs 77:21, 1997 6. White TW, Goodenough DA, Paul DL: Targeted ablation of connexin 50 in mice results in microphthalmia and
zonular pulverulent cataracts. J Cell Biol 143:815, 1998 7. Duncan G, Wormstone IM, Davies PD: The aging human lens: Structure, growth and physiological behaviour. Br J Ophthalmol 81:907, 1997 8. Reid TW: Growth control of cornea and lens epithelial cells. In Osborne
N, Chader GJ (eds): Retinal and Eye Research. Vol 13. Oxford, UK: Elsevier, 1994:507 9. Liu CSC, Wormstone IM, Duncan G et al: A study of human lens cell growth in vitro: A model for posterior capsule
opacification. Invest Ophthalmol Vis Sci 37:906, 1996 10. Wormstone IM, Liu CSC, Rakic JM et al: Human lens epithelial cell proliferation in a protein-free medium. Invest Ophthalmol Vis Sci 38:396, 1997 11. Kinsey VE, Reddy DVN: Studies of the crystalline lens xi. The relative role of the epithelium
and capsule in transport. Invest Ophthalmol Vis Sci 4:104, 1965 12. Candia OA, Bentley PJ, Mills CD: Short-circuit current and active Na transport across isolated lens of the
toad. Am J Physiol 220:539, 1971 13. Parmelee JT: Measurement of steady currents around the frog lens. Exp Eye Res 42:433, 1986 14. Patterson JW: Characterisation of the equatorial current of the lens. Ophthalmol Res 20:139, 1988 15. Duncan G: The site of the ion restricting membranes in the toad lens. Exp Eye Res 8:406, 1969 16. Duncan G, Stewart S, Prescott AR et al: Membrane and junctional properties of the isolated frog lens epithelium. J Membrane Biol 102:195, 1988 17. Prescott A, Duncan G, van Marle J et al: The correlated study of metabolic cell communication and gap junction distribution
in adult frog lens. Exp Eye Res 58:737, 1994 18. Duncan G, Juett JR, Croghan PC: A simple chamber for measuring lens asymmetry potentials. Exp Eye Res 25:391, 1977 19. Musil LS, Beyer EC, Goodenough DA: Expression of the gap junction protein connexin 43 in embryonic chick lens: Molecular
cloning, ultrastructural localization and post-translational
phosphorylation. J Membrane Biol 116:163, 1990 20. Bassnett S, Kuszak JR, Reinisch HG et al: Intercellular communication between epithelial and fiber cells of the eye
lens. J Cell Sci 107:799, 1994 21. Rae JL, Bartling J, Rae J et al: Dye transfer between cells of the lens. J Membrane Biol 150:89, 1996 22. Jacob TJC, Duncan G: Electrical coupling between fiber cells in amphibian and cephalopod lenses. Nature 290:704, 1981 23. Eisenberg RS, Rae JL: Current voltage relationships in the crystalline lens. J Physiol 262:285, 1976 24. Mathias RT, Rae JL, Eisenberg RS: The lens as a nonuniform syncytium. Biophys J 34:61, 1981 25. Thomas GR, Duncan G, Sanderson J: Acetylcholine-induced membrane potential oscillations in the intact lens. Invest Ophthalmol Vis Sci 39:111, 1998 26. Alvarez LJ, Candia OA, Zamudio AC: Acetylcholine modulation of the short-circuit current across the rabbit
lens. Exp Eye Res 61:129, 1995 27. Gandolfi SA, Duncan G, Tomlinson J et al: Mammalian lens inter-fiber resistance is modulated by calcium and calmodulin. Curr Eye Res 9:533, 1990 28. Mathias RT, Riquellme G, Rae JL: Cell to cell communication and pH in the frog lens. J Gen Physiol 98:1085, 1991 29. Bassnett S, Duncan G: The influence of pH on membrane conductance and intercellular resistance
in the rat lens.J Physiol 398:507, 1988 30. Shestopalor VI, Bassnett S: Expression of autofluorescent proteins reveals a novel protein permeable
pathway between cells in the lens core. J Cell Sci 113:1913, 2000 31. Duncan G: Role of membranes in controlling ion and water movements in the
lens. In The Human Lens in Relation to Cataract. Ciba Symposium 19. London: Pitman, 1973:99 32. Broekhuyse RM: Biochemistry of membranes. In Duncan G (ed): Mechanisms
of Cataract Formation in the Human Lens. London: Academic Press, 1981:151 33. Duncan G, Jacob TJC: Calcium and the physiology of cataract. In Human Cataract
Formation. Ciba Foundation Symposium 106. London: Pitman, 1984:132 34. Rae JL, Kuszak JR: The electrical coupling of epithelium and fibers in the frog lens. Exp Eye Res 36:317, 1983 35. Menko AS, Klunks KA, Tai-Feng L et al: Junctions between lens cells in differentiating cultures: structure, formation, intercellular
permeability and junctional protein expression. Dev Biol 123:307, 1987 36. Fitzgerald PG, Goodenough DA: Rat lens cultures: MIP expression and domains of intercellular coupling. Invest Ophthalmol Vis Sci 27:755, 1986 37. Miller TM, Goodenough DA: Evidence for two physiologically distinct gap junctions expressed by the
chick lens epithelial cell. J Cell Biol 102:194, 1986 38. Vrensen G, van Marle J, van Veen H et al: Membrane architecture as a function of lens fiber maturation: A freeze
fracture and scanning electron microscopic study of the human lens. Exp Eye Res 54:433, 1992 39. Rae JL, Shepard AR: Molecular biology electrophysiology of calcium-activated potassium channels
from lens epithelium. Curr Eye Res 17:264, 1998 40. Shepard AR, Rae JL: Ion transporters and receptors in cDNA libraries from lens and cornea epithelia. Curr Eye Res 17:708, 1998 41. Stewart S, Duncan G, Marcantonio JM et al: Membrane and communication properties of tissue cultured human lens epithelial
cells. Invest Ophthalmol Vis Sci 29:1713, 1988 42. Duncan G, Bushell AR: Ion analysis of human cataractous lenses. Exp Eye Res 20:223, 1975 43. Duncan G, Williams MR, Riach RA: Calcium, cell sig-nalling and cataract. In
Osborne NN, Chader GJ (eds):Progress in Retina and Eye Research. Oxford, UK:Pergamon 1994:623 44. Goldman DE: Potential, impedance and rectification in membranes. J Gen Physiol 27:37, 1943 45. Duncan G: Relative permeability of the lens membranes to sodium and potassium. Exp Eye Res 8:315, 1969 46. Tomlinson J, Bannister SC, Croghan PC et al: Analysis of rat lens45Ca2+ fluxes: Evidence for Na+ -Ca2+ exchange. Exp Eye Res 52:619, 1991 47. Delamere NA, Dean WL: Distribution of lens sodium-potassium-adenosine triphosphatase. Invest Ophthalmol Vis Sci 34:2159, 1993 48. Sweadner KJ: Isozymes of the Na+ /K+ ATPase. Biochim Biophys Acta 988:185, 1989 49. Moseley AE, Dean WL, Delamere NA: Isoforms of NaK ATPase in rat lens epithelium and fiber cells. Invest Ophthalmol Vis Sci 37:1502, 1996 50. Garner MH, Horwitz J: Catalytic subunit isoforms of mammalian lens NaK ATPase. Curr Eye Res 13:65, 1994 51. Delamere NA, Dean WL, Stidam JM et al: Differential expression of sodium pump catalytic subunits in the lens epithelium
and fibers. Ophthalmic Res 28:73, 1996 52. Delamere NA, Manning RE, Liu Lx et al: Na,K-ATPase polypeptide upregulation responses in lens epithelium. Invest Ophthalmol Vis Sci 39:763, 1998 53. Okafor MC, Dean WL, Delamere NA: Thrombin inhibits active sodium-potassium transport in porcine lens. Invest Ophthalmol Vis Sci 40:2033, 1999 54. Duncan G, Hightower KR, Gandolfi SA et al: Human lens membrane cation permeability increases with age. Invest Ophthalmol Vis Sci 30:1855, 1989 55. Jacob TJC, Bangham JA, Duncan G: Characterization of a cation channel on the apical surface of the frog
lens epithelium. Q J Exp Physiol 70:403, 1985 56. Rae JL, Mathias RT, Cooper K et al: Divalent cation effects on lens conductance and stretch-activated action
channels. Exp Eye Res 55:135, 1992 57. Weale RA: Physical changes due to age and cataract. In Duncan G (ed): Mechanisms
of Cataract Formation in the Human Lens. London: Academic Press, 1981:47 58. Sanderson J, Duncan G: pCMPS-induced changes in lens membrane permeability and transparency. Invest Ophthalmol Vis Sci 34:2518, 1993 59. Harding JJ, Crabbe MJC: The lens: Development, proteins, metabolism and
cataract. In Davison H (ed): The Eye. Vol 1B. New York: Academic Press, 1984:207 60. Lucas VA, Duncan G, Davies PD: Membrane permeability characteristics of perifused human senile cataractous
lenses. Exp Eye Res 42:151, 1986 61. Jacob TJC, Duncan G: Calcium controls both sodium and potassium permeability of lens membranes. Exp Eye Res 33:85, 1981 62. Duncan G, Patmore L, Pynsent PB: Impedance of the amphibian lens. J Physiol 312:17, 1981 63. Duncan G, Emptage NJ, Hightower KR: p-Chloro-mercuriphenylsulphonate activates a quinine-sensitive potassium
conductance in frog lens. J Physiol 404:637, 1988 64. Lucas VA, Bassnett S, Duncan G et al: Membrane conductance and potassium permeability of the rat lens. Q J Exp Physiol 72:81, 1987 65. Ballabeni A, Trevisi A, Chiaponi C et al: Molecular characterization of
the potassium channels of transparent and cataractous human lenses (submitted) 66. Duncan G, Dart C, Croghan PC et al: Evidence for a Na+ -Cl--H+ -HCO3- exchanger system in the mammalian lens. Exp Eye Res 54:941, 1992 67. Duncan G: Movement of sodium and chloride across amphibian lens membranes. Exp Eye Res 10:117, 1970 68. Williams MR, Duncan G, Croghan PC et al: pH regulation in tissue-cultured bovine lens epithelial cells. J Membrane Biol 129:179, 1992 69. Diecke FPJ, Beyer-Mears A: A mechanism for regulatory volume decrease in cultured lens epithelial
cells. Curr Eye Res 16:279, 1997 70. Zhang JJ, Jacob TJC: ATP-activated chloride channel inhibited by an antibody to p-glycoprotein. Am J Physiol 36:C1095, 1994 71. Duncan G: Permeability of amphibian lens membranes to water. Exp Eye Res 9:188, 1970 72. Duncan G, Croghan PC: Mechanisms for the regulation of cell volume with particular reference
to the lens. Exp Eye Res 8:421, 1969 73. Hasegawa H, Lian SC, Finkberner WE et al: Extrarenal tissue distribution of CHIP 28 water channels: In situhybridization
and antibody staining. Am J Physiol 266:C893, 1994 74. Kushmerick C, Rice SJ, Baldo GJ et al: Ion, water and neutral solute transport in Xenopus oocytes expressing frog
lens MIP. Exp Eye Res 61:351, 1995 75. Wolosin JM, Alvarez LJ, Candia OA: Cellular pH andNa+ -H+ exchange activity in lens epithelium of Bufo marinus toad. Am J Physiol 255:C595, 1988 76. Bassnett S: Intracellular pH regulation in the embryonic chicken lens epithelium. J Physiol 431:445, 1990 77. Williams MR: pH and Calcium Regulation in Lens Epithelial Cells: A Fluorimetric
Dye Study. PhD Thesis, University of East Anglia, Norwich, UK, 1993 78. Thomas RC: Experimental displacement of intracellular pH and mechanisms of its subsequent
recovery. J Physiol 354:3P, 1984 79. Thomas RC: Bicarbonate and pHi response. Nature 337:601, 1989 80. Jentsch TK, von der Haar B, Keller SK et al: Response of the intracellular potentials of cultured bovine lens cells
to ions and inhibitors. Exp Eye Res 41:131, 1985 81. Turin L, Warner AE: Intracellular pH in early Xenopus embryos: Its effect on current flow between
blastomeres.J Physiol 300:489, 1980 82. Spray DC, Harris AL, Bennett MVL: Gap junctional conductance as a simple and sensitive function of intracellular
pH. Science 211:712, 1981 83. Schutze SM, Goodenough DA: Dye transfer between cells of the embryonic chick lens becomes less sensitive
to CO2 treatment with development. J Cell Biol 92:694, 1982 84. Emptage NJ, Duncan G, Croghan PC: Internal acidification modulates membrane and junctional resistance in
the isolated lens of the frog Rana pipiens. Exp Eye Res 54:33, 1992 85. Baldo GJ, Mathias RT: Spatial variations in membrane properties in the intact rat lens. Biophys J 63:518, 1992 86. Kuszak JR, Shek YH, Carney KC et al: A correlative freeze-etch and electrophysiological study of communicating
junctions in crystalline lenses. Curr Eye Res 4:1145, 1985 87. Kuszak JR, Macsai MS, Bloom KL et al: Cell to cell fusion of lens fiber cells in situ: Correlative light, scanning
electron microscopic and freeze-fracture studies. J Ultrastruct Res 93:144, 1985 88. Marcantonio JM, Duncan G, Rink H: Calcium-induced opacification and loss of protein in the organ-cultured
bovine lens. Exp Eye Res 42:617, 1986 89. David LL, Shearer TR: Role of proteolysis in lenses: A review. Lens Eye Toxic Res 6:725, 1989 90. Sanderson J, Marcantonio JM, Duncan G: A human lens model of cortical cataract: Calcium induces protein loss, vimentin
cleavage and opacification in cultured human lens. Invest Ophthalmol Vis Sci 41:2251, 2000 91. Srivastava SK, Wang L-F, Ansari NH et al: Calcium homeostasis of isolated single cortical fibers of rat lens. Invest Ophthalmol Vis Sci 38:2300, 1997 92. Williams MR, Duncan G, Riach RA et al: Acetylcholine receptors are coupled to mobilization of intracellular calcium
in cultured human lens cells. Exp Eye Res 57:381, 1993 93. Riach RA, Duncan G, Williams MR et al: Histamine and ATP mobilize calcium by activation of H1 and P2u receptors in human lens epithelial cells. J Physiol 486:273, 1995 94. Churchill GC, Louis CF: Roles of Ca2+ inositol trisphosphate and cyclic ADP-ribose in mediating intercellular
Ca2+ signalling in sheep lens cells. J Cell Sci 111:1217, 1998 95. Shaffer RN, Hethrington J: Anticholinesterase drugs and cataract. Am J Ophthalmol 64:613, 1966 96. Fraser PJ, Duncan G, Tomlinson J: Effects of a cholinesterase inhibitor on salmon lens: A possible cause
for the increased incidence of cataract in salmon Salmo solar. Exp Eye Res 49:293, 1989 97. Duncan G, Riach RA, Williams MR et al: Calcium mobilization modulates growth of lens cells. Cell Calcium 19:83, 1996 98. Duncan G, Wormstone IM, Liu CSC et al: Thapsigargin-coated intraocular lenses inhibit lens cell growth. Nat Med 3:1026, 1997 99. Berridge MJ: Elementary and global aspects of calcium signalling. J Physiol 499:291, 1997 100. Thomas GR, Sanderson J, Duncan G: Thapsigargin inhibits a potassium conductance and stimulates calcium influx
in the intact rat lens. J Physiol 516:191, 1999 101. Heath JK: Growth Factors. Oxford, UK: Oxford University Press, 1993 102. Nicola NN: Cytokines and Their Receptors. Oxford, UK: Oxford University
Press, 1994 103. Burgess WH, McCiag T: Heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem 58:575, 1989 104. Partanen J, Vainikka S, Alitalo K: Structural and functional specificity of FGF receptors. Phil Trans R Soc Lond 340:297, 1993 105. Lovicu FJ, McAvoy JW: Localization of acidic fibroblast growth factor, basic fibroblast growth
factor, and heparan sulphate proteoglycan in rat lens: Implications
for polarity. Invest Ophthalmol Vis Sci 34:3355, 1993 106. Robinson ML, Overbeek PA, Verran DJ et al: Extracellular FGF-1 acts as a lens differentiation factor in transgenic
mice. Development 121:505, 1995 107. Chow RL, Roux GD, Roghani M et al: FGF suppresses apoptosis and induces differentiation of fibre cells in
the mouse lens. Development 121:4383, 1995 108. Stolen CM, Jackson MW, Griep AE: Overexpression of FGF-2 modulates fiber differentiation and survival in
mouse lens. Development 124:4009, 1997 109. Lovicu FJ, Overbeek PA: Overlapping effects of different members of the FGF family on lens fiber
differentiation in transgenic mice. Development 125:3365, 1998 110. De Iongh RU, Lovicu FJ, Chamberlain CG et al: Differential expression of fibroblast growth factor receptors during rat
lens morphogenesis and growth. Invest Ophthalmol Vis Sci 38:1688, 1997 111. Del Rio-Tsonis K, Jung JC, Chiu I-M et al: Conservation of fibroblast growth factor function in lens regeneration. Proc Natl Acad Sci U S A 94:13701, 1997 112. Del Rio-Tsonis K, Trombley MT, McMahon G et al: Regulation of lens regeneration by fibroblast growth factor 1. Dev Dynamics 213:140, 1998 113. Ohuchi H, Koyama E, Myokai F et al: Expression pattern of two fibroblast growth factor receptor genes during
chick eye development. Exp Eye Res 58:649, 1994 114. Wormstone IM, Duncan G, Liu CSC et al: Persistent lens cell activity throughout long-term culture of human capsular
bags in protein-free medium. Invest Ophthalmol Vis Sci 39:S212, 1998 115. Wormstone IM, Del Rio-Tsonis K, McMahon G et al: Autocrine regulation and age-related expression of FGF in human lens cells. Invest Ophthalmol Vis Sci 41:S188, 2000 116. Marunouchi T, Hosoya H, Morioku T et al: Up-regulation of fibroblast growth factor receptor-1 in lens epithelial
cells paralleled by growth stimulation. Exp Eye Res 67:611, 1998 117. Weng J, Liang Q, Mohan RR et al: Hepatocyte growth factor, Keratinocyte growth factor, and other growth
factor-receptor systems in the lens. Invest Ophthalmol Vis Sci 38:1543, 1997 118. McAvoy JW, Chamberlain CG: Fibroblast growth factor (FGF) induces different responses in lens epithelial
cells depending on its concentration. Development 107:221, 1989 119. Chamberlain CG, McAvoy JW: Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr Eye Res 6:1165, 1987 120. Ibaraki N, Lin LR, Reddy VN: Effects of growth-factors on proliferation and differentiation in human
lens epithelial-cells in early subculture. Invest Ophthal Vis Sci 36:2304, 1995 121. Schweigerer L, Ferrara N, Haaparanta T et al: Basic fibroblast growth factor: Expression in cultured cells derived from
corneal endothelium and lens epithelium. Exp Eye Res 46:71, 1988 122. Carpenter G, Wahl MI: The epidermal growth factor family. Handb Exp Pharmacol 95:69, 1990 123. Massague J, Pandiella A: Membrane-anchored growth factors. Annu Rev Biochem 62:515, 1993 124. Dernyck R: The physiology of transforming growth factor alpha. Adv Cancer Res 94:191, 1992 125. Tripathi RC, Borisuth NSC, Tripathi BJ et al: Radioimmunoassay of epidermal growth factor in lenses at various stages
of development of cataract. Exp Eye Res 53:759, 1991 126. Majima K: Human lens epithelial cells proliferate in response to exogenous EGF and
have EGF and EGF receptor. Ophthalmic Res 27:356, 1995 127. Lee EH, Joo C: Role of transforming growth factor-β in transdifferentiation and fibrosis
of lens epithelial cells. Invest Ophthalmol Vis Sci 40:2025, 1999 128. Majima K, Kojima Y, Ouhashi F: Cell biological analysis with respect to cause of fibrous opacification
of the anterior capsule after cataract extraction. Ophthalmologica 212:364, 1998 129. Fu SCJ, Zheng DR, Wang SL et al: Immunocytochemical localization of epidermal growth-factor (EGF) and its
receptor (EGF-R) in rat lens epithelium. Invest Ophthalmol Vis Sci 34:1371, 1993 130. Hongo M, Itoi M, Yamamura Y et al: Distribution of epidermal growth-factor receptors in rabbit lens epithelial-cells. Invest Ophthalmol Vis Sci 34:401, 1993 131. Fleming TP, Song Z, Andley UP: Expression of growth control and differentiation genes in human lens epithelial
cells with extended life span. Invest Ophthalmol Vis Sci 39:1387, 1998 132. Hollenberg MD: Receptors for insulin and epidermal growth factor: Relation to synthesis
of DNA in cultured rabbit lens epithelium. Arch Biochem Biophys 171:371, 1975 133. Reddan JR, Wilsond-Ziedzic D: Insulin growth-factor and epidermal growth-factor trigger mitosis in lenses
cultured in a serum-free medium. Invest Ophthalmol Vis Sci 24:409, 1983 134. Ohguro N, Fukuda M, Sasabe T et al: Concentration dependent effects of hydrogen peroxide on lens epithelial
cells. Br J Ophthalmol 83:1064, 1999 135. Majima K, Kojima Y, Ouhashi F: Cell biological analysis with respect to cause of fibrous opacification
of the anterior capsule after cataract extraction. Ophthalmologica 212:364, 1998 136. Miyazawa K, Tsubouchi H, Naka D et al: Molecular-cloning and sequence-analysis of cDNA for human hepatocyte growth-factor. Biochem Bioph Res Com 163:967, 1989 137. Nakamura T, Nishizawa T, Hagiya M et al: Molecular-cloning and expression of human hepatocyte growth-factor. Nature 342:440, 1989 138. Weidner KM, Arakaki N, Hartmann G et al: Evidence for the identity of human scatter factor and human hepatocyte
growth-factor. Proc Natl Acad Sci USA 88:7001, 1991 139. Rubin JS, Chan AML, Bottaro DP et al: A broad-spectrum human lung fibroblast-derived mitogen is a variant of
hepatocyte growth-factor. Proc Natl Acad Sci USA 88:415, 1991 140. Naka D, Ishii T, Yoshiyama Y et al: Activation of hepatocyte growth-factor by proteolytic conversion of a single
chain form to a heterodimer. J Biol Chem 267:20114, 1992 141. Naldini L, Tamagnone L, Vigna E et al: Extracellular proteolytic cleavage by urokinase is required for activationof
hepatocyte growth-factor scatter factor. EMBO J 11:4825, 1992 142. Wormstone IM, Tamiya S, Marcantonio JM et al: C-met expression and hepatocyte
growth factor function in human lens epithelial cells. Invest Ophthalmol
Vis Sci S562, 1999 143. Smith DL, Reddan JR, Cooper KE: The effects of hepatocyte growth factor
on lens epithelial wound healing. Invest Ophthalmol Vis Sci S201, 1997 144. Reneker LW, Overbeek PA: Lens-specific expression of PDGF-A alters lens growth and development. Dev Biol 180:554, 1996 145. Potts JD, Bassnett S, Kornacker S et al: Expression of platelet-derived growth-factor receptors in the developing
chicken lens. Invest Ophthalmol Vis Sci 35:3413, 1994 146. Knorr M, Wunderlich K, Steuhl KP et al: Lens epithelial-cell response to isoforms of platelet-derived growth-factor. Graefes Arch Clin Exp Ophthalmol 231:424, 1993 147. Collison DJ, Coleman RA, James RS et al: Characterisation of muscarinic acetylcholine receptors in human lens cells. Invest Ophthalmol Vis Sci 41:2633, 2000 148. Brewitt B, Clark JI: growth and transparency in the lens, an epithelial tissue, stimulated by
pulses of PDGF. Science 242:777, 1988 149. Brewitt B, Clark JI: A new method for study of normal lens development invitro using pulsatile
delivery of PDGF or EGF in hl-1 serum-free medium. In Vitro Cell Dev Biol 26:305, 1990 150. Massague J: The transforming growth factor-beta family. Annu Rev Cell Biol 6:597, 1990 151. Roberts AB, Sporn MB: Physiological actions and clinical applications of transforming growth
factor-beta (TGF-beta). Growth Factors 8:1, 1993 152. Roberts AB, Sporn MB: Differential expression of the TGF-beta isoforms in embryogenesis suggests
specific roles in developing and adult tissues. Mol Reprod Dev 32:91, 1992 153. Yingling JM, Wang XF, Bassing CH: Signaling by the transforming growth-factor-beta receptors. BBA Rev Cancer 1242:115, 1995 154. Harpel JG, Metz CM, Kojima S et al: Control of transforming growth factor-beta activity: Latency vs. activation. Prog Growth Factor Res 4:321, 1992 155. Dennis PA, Rifkin DB: Cellular activation of latent transforming growth-factor-beta requires
binding to the cation-independent mannose 6-phosphate insulin-like growth-factor
type-II receptor. Proc Natl Acad Sci USA 88:580, 1991 156. Lee YS, Kay EP: TGF-beta stimulates synthesis of FGF-2 keratocytes and is inhibited by
mannose 6-phosphate. Invest Ophthalmol Vis Sci 38:S2317, 1997 157. James K: Interactions between cytokines and alpha-2-macroglobulin. Immunol Today 11:16, 1990 158. Schulz MW, Chamberlain CG, McAvoy JW: Inhibition of transforming growth factor-beta-induced cataractous changes
in lens explants by ocular media and alpha-2-macroglobulin. Invest Ophthalmol Vis Sci 37:1509, 1996 159. Pelton RW, Saxena B, Jones M et al: Immunohistochemical localization of TGF-beta-1, TGF-beta-2, and TGF-beta-3 in
the mouse embryo: Expression patterns suggest multiple roles during
embryonic-development. J Cell Biol 115:1091, 1991 160. Gordon-Thomson C, de Iongh RU, Hales AM et al: Differential cataractogenic potency of TGF-beta(1), -beta(2), and -beta(3) and
their expression in the postnatal rat eye. Invest Ophthalmol Vis Sci 39:1399, 1998 161. Richiert DM, Ireland ME: TGF-beta elicits fibronectin secretion and proliferation in cultured chick
lens epithelial cells. Curr Eye Res 18:62, 1999 162. Nishi O, Nishi K, Fujiwara T et al: Effects of the cytokines on the proliferation of and collagen synthesis
by human cataract lens epithelial cells. Br J Ophthalmol 80:63, 1996 163. Kurosaka D, Nagamoto T: Inhibitory effect of TGF-beta-2in human aqueous-humor on bovine lens epithelial-cell
proliferation. Invest Ophthalmol Vis Sci 35:3408, 1994 164. Wallentin N, Wickstrom K, Lundberg C: Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell
proliferation. Invest Ophthalmol Vis Sci 39:1410, 1998 165. Srinivasan Y, Lovicu FJ, Overbeek PA: Lens-specific expression of transforming growth factor beta 1 in transgenic
mice causes anterior subcapsular cataracts. J Clin Invest 101:625, 1998 166. Colitz CMH, Malarkey D, Dykstra MJ et al: Histologic and immunohistochemical characterization of lens capsular plaques
in dogs with cataracts. Am J Vet Res 61:139, 2000 167. Hales AM, Chamberlain CG, Murphy CR et al: Estrogen protects lenses against cataract induced by transforming growth
factor-beta (TGF beta). J Exp Med 185:273, 1997 168. Hales AM, Chamberlain CG, Dreher B et al: Intravitreal injection of TGF beta induces cataract in rats. Invest Ophthalmol Vis Sci 40:3231, 1999 169. Liu J, Hales AM, Chamberlain CG et al: Induction of cataract-like changes in rat lens epithelial explants by transforming
growth-factor-beta. Invest Ophthalmol Vis Sci 35:388, 1994 170. Reddan JR, Unaker N, Harding C et al: Induction of mitosis in the cultured rabbit lens initiated by the addition
of insulin to medium KEI-4. Exp Eye Res 20:45, 1975 171. Beebe DC, Silver MH, Belcher KS et al: Lentropin, a protein that controls lens fiber formation, is related functionally
and immunologically to the insulin-like growth-factors. Proc Natl Acad Sci USA 84:2327, 1987 172. Richardson NA, Chamberlain CG, McAvoy JW: IGF-1 enhancement of FGF-induced lens fiber differentiation in rats of
different ages. Invest Ophthalmol Vis Sci 34:3303, 1993 173. Liu J, Chamberlain CG, McAvoy JW: IGF enhancement of FGF-induced fibre differentiation and DNA synthesis
in lens explants. Exp Eye Res 63:621, 1996 174. Hutchings SE, Sato GH: Growth and maintenance of HeLa cells in serum-free medium supplemented
with hormones. Proc Natl Acad Sci USA 75:901, 1975 175. McGahan MC, Harned J, Goralska M et al: Transferrin secretion by lens epithelial cells in culture. Exp Eye Res 60:667, 1995 176. Davidson MG, Harned J, Grimes AM et al: Transferrin in after-cataract and as a survival factor for lens epithelium. Exp Eye Res 66:207, 1998 177. Tripathi RC, Borisuth NSC, Tripathi BJ: Detection, quantification, and significance of basic fibroblast growth-factor
in the aqueous-humor of man, cat, dog and pig. Exp Eye Res 54:447, 1992 178. ArakiSasaki K, Danjo S, Kawaguchi S et al: Human hepatocyte growth factor (HGF) in the aqueous humor. Jpn J Ophthalmol 41:409, 1997 179. Ohashi Y, Motokura M, Kinoshita Y et al: Presence of epidermal growth-factor in human tears. Invest Ophthalmol Vis Sci 30:1879, 1989 180. Namiki M, Tagami Y, Yamamoto M et al: Presence of epidermal growth factor (hEGF), basic fibroblast growth factor (bFGF) in
human aqueous. Acta Soc Ophthalmol Jap 96:652, 1992 181. Cousins SW, McCabe MM, Danielpour D et al: Identification of transforming growth-factor-beta as an immunosuppressive
factor in aqueous-humor. Invest Ophthalmol Vis Sci 32:2201, 1991 182. Dernouchamps JP, Vaerman JP, Michiels J et al: La transferrine des liquides endoculaires chez le lapin. Ophthalmologica 170:72, 1975 183. Li J, Tripathi RC, Chalam KV et al: Expression of PDGF-Aand PDGF-B mRNAs by trabecular cells and lack of detectable
level of PDGF-BB in aqueous humor. Invest Ophthalmol Vis Sci 37:3792, 1996 184. Ishizaki Y, Voyvodic JT, Burne JF et al: Control of lens epithelial cell survival. J Cell Biol 121:899, 1993 185. Kohno T, Sorgente N, Ishibashi T et al: Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci 28:506, 1987 186. Iwata S: Physiological and biological characteristics of ocular lens capsule. IOL 2:13, 1988 187. Parmigiani CM, McAvoy JW: The roles of laminin and fibronectin in the development of the lens capsule. Curr Eye Res 10:501, 1991 188. Olivero DK, Furcht LT: Type IV collagen, laminin and fibronectin promote the adhesion and migration
of rabbit lens epithelial cells in vitro. Invest Ophthalmol Vis Sci 34:2825, 1993 189. Oharazawa H, Ibaraki N, Lin LR et al: The effects of extracellular matrix on cell attachment, proliferation and
migration in a human lens epithelial cell line. Exp Eye Res 69:603, 1999 190. Menko AS, Philip NJ: Beta(1) integrins in epithelial tissues: A unique distribution in the lens. Exp Cell Res 218:516, 1995 191. Nishi O, Nishi K, Akaishi T et al: Detection of cell adhesion molecules in lens epithelial cells of human
cataracts. Invest Ophthalmol Vis Sci 38:579, 1997 192. Walker JL, Menko AS: Alpha 6 integrin is regulated with lens cell differentiation by linkage
to the cytoskeleton and isoform switching. Dev Biol 210:497, 1999 193. Zhang XH, Ji J, Zhang H et al: Detection of integrins in cataract lens epithelial cells. J Cataract Refract Surg 26:287, 2000 194. Birkedal-Hansen H: Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol 7:728, 1995 195. Basbaum CB, Werb Z: Focalized proteolysis: Spatial and temporal regulation of extracellular
matrix degradation at the cell surface. Curr Opin Cell Biol 8:731, 1996 196. Smine A, Plantner JJ: Membrane type 1 metalloproteinase in human ocular tissues. Curr Eye Res 16:925, 1997 197. Butler GS, Butler MJ, Atkinson SJ et al: Membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation
of progelatinase A: A kinetic study. J Biol Chem 273:1998 198. Belien AT, Paganetti PA, Schwab ME: Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration
of glioma cells in central nervous system white matter. J Cell Biol 144:373, 1999 199. d'Ortho MP, Will H et al: Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum
proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem 250:751, 1997 200. Richiert DM, Ireland ME: Matrix metalloproteinase secretion is stimulated by TGF-β in cultured
lens epithelial cells. Curr Eye Res 19:269, 1999 | 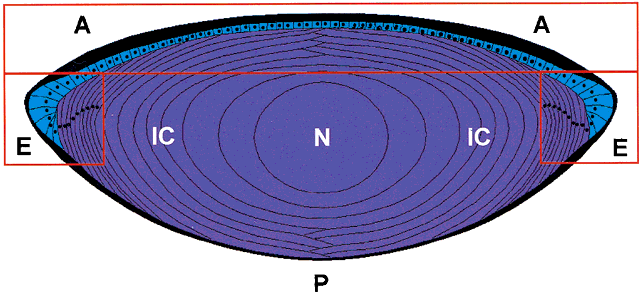
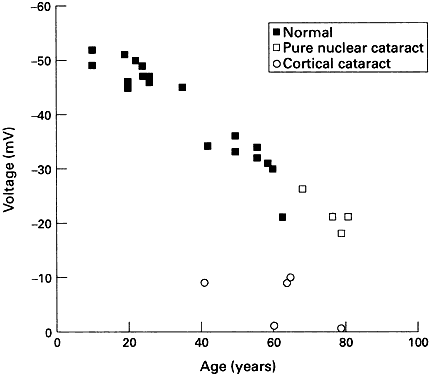
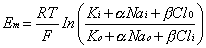
 PK. In the amphibian lens, the relative permeabilities obtained in this way
are in good agreement with the individual permeabilities calculated
from radioisotope flux data obtained for the individual ions.
PK. In the amphibian lens, the relative permeabilities obtained in this way
are in good agreement with the individual permeabilities calculated
from radioisotope flux data obtained for the individual ions.