Amblyopia primarily affects spatial or form vision, although abnormalities of the light sense may also be found in at least some cases. Subsequent discussion considers several specific aspects of amblyopic vision, with emphasis on points that either are important in clinical practice or seem to shed light on the pathophysiology or anatomic localization of the underlying visual system disturbance.
VISUAL ACUITY
Amblyopic patients are deficient in their ability to resolve closely spaced contours and recognize the patterns they form. Visual acuity measured with conventional tests, which rely on both these functions, is always subnormal. Clinical diagnosis of amblyopia is in fact usually based on the observation of reduced acuity in association with a history of abnormal visual experience and a lack of any other abnormality on examination that can account for the acuity deficit.
Measurement of visual acuity is a major concern for the clinician in relation to the amblyopic child because, although reliable assessment of acuity may be quite difficult with young patients, amblyopia is most effectively and efficiently treated in childhood. Children old enough to identify block letters are usually tested with standard letter optotypes, either projected, wall mounted, or computer generated. Today's sophisticated youngster, exposed to educational television programs from infancy, is sometimes able to name letters even before age 3 years. For the less verbal child, testing must be modified to permit the use of manual pointing responses.
Currently, the nonverbal Snellen equivalents most widely used in North America are the tum-bling E test and the HOTV test (a simplificationof the older Sheridan-Gardiner test).19 The former relies on a child's ability to indicate with fingers the direction of the legs of a letter E that is rotated to point up, down, left or right; the latter involves matching each test letter to one of the four letters H, O, T, and V printed on a card that can be held in the child's hands. For most 3-year-olds and nearly all developmentally normal 4-year-olds, fairly reliable visual acuity measurements can be obtained with either of these tests after a minute or two ofinstruction (and confirmation of the patient's competence in responding) using large demonstration letters. Some children do better with one test than the other, both should therefore be available to the clinician. An advantage of the HOTV test is that there is no need to discriminate left/right mirror image figures, which may be difficult for the young patient. This problem can be eliminated with the E test by giving credit for correct identification of horizontal orientation to either a right or left response.
Various acuity tests have been devised that substitute pictures for letters. The most widely used test of this type in North America remains the set of cards developed by Allen, each of which displays a figure composed of dark and light elements having dimensions that correspond to those of a 30-foot(9 meter) Snellen letter. The examiner presents the cards in random order at progressively greater distances as the child identifies each pictured object by name (any consistently used word or phrase is acceptable) until recognition is no longer possible. The greatest distance at which correct responses are obtained is recorded in feet as the Snellen numerator over denominator 30.
Some small children who will not point to identify letters respond well to testing with pictures, but the shy child may actually be more difficult to test in this way because of the requirement for verbal responses. (It is sometimes helpful to have the child whisper answers to a parent.) Lack of familiarity with the pictured objects or inability to recognize the stylized images may be a problem for some children; testing should begin with a review of the cards up close, and any that are not readily identified should be eliminated (three or four remaining different pictures are sufficient for testing). Cluesderived from the overall shape of the Allen cardimages may undermine the accuracy of acuity measured. In general, this form of testing should be the last resort after failure with letter testing at each examination. On the occasion when a switch is made from Allen pictures to letters (or any significant change in the method of acuity assessment is made), results from both tests should be recorded to ensure comparability of measurements over time.
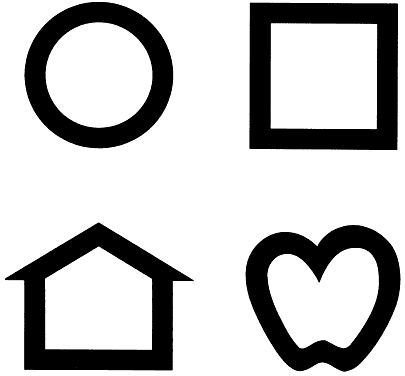
A newer approach to pictographic vision testing with significant advantages employs the set of 4 Lea symbols (Fig. 1). These simple forms are easy for most toddlers to deal with, they are more similar in configuration to Snellen letters than the Allen pictures and have been carefully calibrated and assessed for reliability.20 Several testing devices using Lea symbols are now available (Precision Vision, Lasalle, IL). Matching to shapes on a handheld card, as in the HOTV test, is an option.
Whether letters or pictures are used, considerable patience, effort, and experience on the part of the examiner are required to ensure reliable measurement of the amblyopic child's visual acuity. It must always be kept in mind that a child's short attention span and lack of familiarity with a recognition task may be critical factors in acuity testing. Even when vision in the two eyes is equal, some young patients do better with the second eye tested as a result of practice provided by testing the first eye, or worse, because of fatigue and waning interest. Several brief sessions, beginning sometimes with the right eye and sometimes with the left, may need to be conducted before acuity assessment can be considered complete. It is worthwhile to record observations relating to the child's general behavior during testing as well as the numeric result. Because normal preschool children often test no better than 20/30 to 20/40 (although actual acuity may exceed this level), mild amblyopia may escape detection in children at an early age unless differences in the speed and confidence with which the patient responds are noted.
When amblyopia is present, its severity may be overestimated if the child is reluctant to identify distinguishable but distorted figures seen by the amblyopic eye. Even adults with amblyopia often begin to have difficulty and make errors in letter identification two lines or more above the actual visual acuity level. Testing should continue until incorrect responses are obtained for most letters on a given level; the level immediately above this is taken as the eye's acuity. A good deal of prodding is sometimes necessary to ensure continuation of responses to the point at which recognition becomes impossible. In approaching this limit it may be helpful at times briefly to back up to larger, “easier” letters to recover momentum.
The acuity tester must also be constantly alert to peeking and memorization or guessing on the part of the child. The eye not being tested should be occluded by a broad opaque object or an adhesive patch, not the fingers of a hand, and movement that allows glimpses around the occluder must be prevented. It is best to observe the child constantly during acuity measurement. This is facilitated by placing a mirror on the wall behind the patient, in which letters displayed at the opposite end of the room can be viewed without the need for head turning. If the acuity of an amblyopic eye abruptly “improves” to equality with the opposite better eye in the course of amblyopia treatment, peeking must always be considered as a possible explanation.
To avoid memorization of letter sequences on a standard chart (readily accomplished by many children), an eye with known or suspected amblyopia should be tested before the child has an opportunity to view the chart with the better eye. Charts on which a small number of different figures are repeated in random order (such as those designed for E and HOTV matching) make memorization more difficult; their use may thus be advantageous even for older patients who know the entire alphabet.
Some poker-faced children can mislead even an experienced examiner by guessing. When four different figures are used, the probability of guessing correctly at three out of four characters on a given acuity level is greater than 0.05; with a single character the probability is of course 0.25, or higher, if the patient remembers the chart from previous testing sessions. It is therefore important to avoid reliance on very short sequences of letters. This is especially a concern at low acuity levels (20/100 or less), for which there are usually four or fewer letters (and frequently one or two) on standard charts. Strategies that enable the examiner to minimize the effect of guessing by increasing the number of responses required include presentation of figures on cards that can be shuffled (such as the Allen pictures or the 150-foot letters that are provided for demonstration of HOTV matching) at increasing distances, repeat testing with the child standing half the usual distance from the chart (doubling each Snellen denominator), and confirmation of distance measurement with a near test that involves similar figures and presentation. Newer devices such as the B-VAT vision tester that employ a computer to generate endless random sequences of characters provide an excellent way to avoid the pitfalls created by memorization and guessing.
The use of preferential looking devices, visual evoked potential (VEP) recording, and observation of fixation behavior to assess amblyopic visual loss in infants and children too young or uncooperative for any form of conventional acuity testing is discussed in later sections of this chapter. Each of these approaches can provide valuable information to the clinician when used appropriately. None should be considered an adequate substitute for actual measurement of acuity when it can be performed reliably using the techniques already described, however great the effort required.
SPATIAL INTERACTIONS
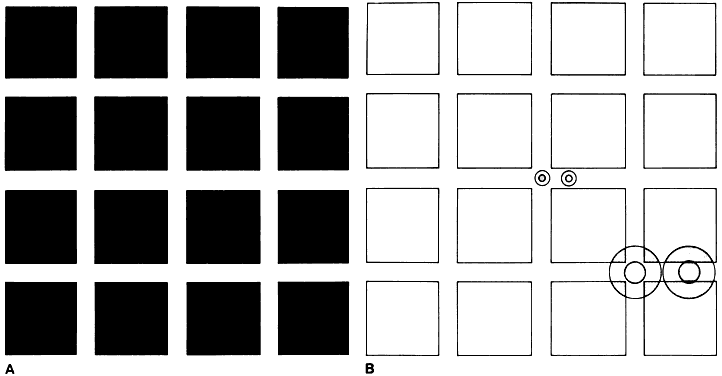
It is well established that many persons with amblyopia have increased difficulty identifying test let-ters when they are presented in a linear or two-dimensional array rather than as isolated characters. A similar effect can be produced by placing interactive bars around a single letter (Fig. 2). This observation, sometimes described as the “crowding phenomenon” or “separation difficulty,” is an example of the effect of contour interaction on visual acuity. It can be demonstrated in normal and organically diseased as well as in amblyopic eyes when figures near the limit of resolution are surrounded by other closely spaced forms. In the normal fovea, contour interaction occurs when forms are separated by a distance of 1 to 3 minutes of arc (0.2 to 0.6 times the overall size of a 20-ft [6-m] Snellen letter); in the normal periphery, its extent is much greater. In the amblyopic fovea, contour interaction typically extends over an increased distance, to a degree that is roughly proportional to the reduction in acuity.21
|
Other conditions that decrease visual acuity may produce similar extension of contour interaction.22 What appears to be unique to amblyopia is the magnitude of the effect on measured acuity that may result from this phenomenon. Occasionally, an amblyopic eye that can resolve a 20/20 letter in isolation drops to as low as 20/100 in the presence of maximal contour interaction. Such a large discrepancy sometimes develops in the course of treatment as acuity measured with isolated letters improves more rapidly than interactive acuity. Thus, amblyopia cannot be considered cured until interactive acuity has become normal.
Several difficulties are encountered in attempting to quantify the effect of contour interaction with linear or multiline presentation of characters on traditional Snellen charts. Preschool children tend to be confused by a collection of letters and may not read them in the appropriate left to right, top to bottom order, or may be reluctant to respond at all. When using a wall-mounted chart, the examiner can help by physically pointing to each letter in turn, but this approach does not work well with projected charts because they are used in a darkened room. Spacing between characters on most charts is not in uniform proportion to their size from line to line, and with projected charts, separation between the letters and the edge of the light rectangle that surrounds them varies considerably. Contour interaction can be produced by this edge even when only a single letter is projected.
Given these concerns, the examiner should not hesitate to rely on isolated letter presentation for assessment of visual acuity in appropriate circumstances. In particular, when the goal of acuity measurement is initial detection of amblyopia or documentation of an interval change during the early stages of treatment there is no real disadvantage to the use of isolated letters. When concern about the effect of contour interaction increases in the late stages of treatment, effort should be made to obtain responses with multicharacter presentation. An alternative that should also be considered is the use of single letters surrounded by bars (drawn on cards for hand-held presentation, or computer-generated by a device such as the B-VAT vision tester). This approach permits measurement of interactive acuity with considerably greater ease and precision than is possible with standard charts.
Contour interaction is related to two other features of visual processing, spatial summation and lateral inhibition. Spatial summation refers to the reduction in the brightness required for detection of a small spot of light as its area is increased. Lateral inhibition is observed when the threshold for detection of a small test light is increased by illumination of the surrounding retina. Like contour interaction, spatial summation and lateral inhibition occur over very short distances in the normal fovea (about3-min arc for spatial summation and 10-min arc for lateral inhibition), and greater distances in the normal periphery. Spatial summation is responsible for the increased visibility of larger test spots in perimetry. Lateral inhibition can be demonstrated with a familiar optical illusion called the Hermann grid, which consists of black squares separated by white stripes (Fig. 3). The intersections of the stripes appear darker than the stripes themselves because the greater area of white surrounding points in the intersections produces greater lateral inhibition. The effect is less near the point of fixation because the smaller foveal zones of inhibition fall entirely within the width of a stripe. Spatial summation and lateral inhibition have both been found to extend over much greater distances than normal in the amblyopic fovea.23,24
A probable explanation for contour interaction, spatial summation, and lateral inhibition is provided by data from electrophysiologic experiments inanimals.25 Studies employing microelectrode recordings from individual cells have shown that each responds to light within a small area of the field of vision known as its receptive field. This area is subdivided into zones within which light either excites or inhibits activity in the cell. In retinal and lateral geniculate neurons, the inhibitory zone forms an annulus around a central excitatory zone, or the reverse (Fig. 4). Cortical neurons generally respond best to oblong stimuli with a particular orientation, but they too have receptive fields of limited size divided into excitatory and inhibitory zones. The dimensions of excitatory zones appear to determine the extent of spatial summation at a particular location in the visual field; the dimensions of inhibitory zones determine the extent of lateral inhibition. The increased distance over which spatial interactions occur in the amblyopic fovea suggests that neurons serving foveal vision have enlarged receptive fields. The observed abnormalities could occur at any level from the retina to the visual cortex.
VISIBILITY OF GRATINGS
Patterns of alternating light and dark stripes known as gratings have been used extensively to analyze form vision in recent years. Gratings simplify acuity measurement (in a psychophysical sense) by substituting a task of resolution or detection alone for the combination of resolution and recognition involved in testing with Snellen letters. Their application to the study of amblyopia has led to a number of interesting and useful findings.
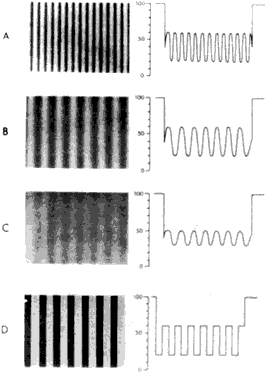
A grating pattern is characterized by the orientation of its stripes, its spatial frequency (the number of light/dark pairs or cycles per degree of visual angle), and the contrast between its light and dark elements (Fig. 5). A square-wave grating consists of uniform light and dark stripes with sharp edges. A sinusoidal grating has a sine wave luminance profile with gradual transitions from light to dark. Sinusoidal gratings are particularly important and widely used in vision research because any complex luminance profile can be resolved into sine wave components of different frequency by the mathematical technique of Fourier analysis.26
The highest spatial frequency at which the stripes in a grating can be resolved with maximum contrast is called the cut-off frequency or grating acuity. For the normal human eye, this is 30 to 40 cycles per degree (for both square-wave and sinusoidal gratings), comparable with the minimum separation of elements required for recognition of conventional Snellen letters. In most disorders that affect visual resolution, grating acuity and Snellen acuity are reduced to a similar extent.
In amblyopia, grating acuity is generally reduced, but often by considerably less than Snellen acuity. Significant discrepancy between grating and Snellen acuity occurs much more frequently in strabismic than in anisometropic amblyopia.27,28 Some strabismic amblyopic patients actually have normal grating acuities. Although they may experience no difficulty detecting the presence of a grating pattern near the cut-off frequency, these patients report that they observe marked distortion of the stripes.29
The spatial distortion that seems to account for this difference between grating and recognition acuity (along with the related phenomenon of spatial uncertainty, a lack of precision in spatial localization) has long been noted by clinicians to be characteristic of amblyopic vision.30 It interferes with the performance of various visual tasks, including bisection of a line segment and counting a numberof closely spaced objects (such as the letters on aSnellen line). Laboratory investigators have developed refined and quantitative approaches to assess spatial distortion and have found it to be a feature primarily of strabismic amblyopia.31
Distortion of visual space also appears to under-lie the effect of amblyopia on ability to detect off-set between contiguous line segments, known as Vernier acuity. This function too is much more severely affected in strabismic than in anisometropic amblyopia.32,33 The physiologic basis for spatial distortion and its consequences in strabismic amblyopia remains a matter of controversy, but recent evidence suggests that processing beyond the level of the primary visual cortex is involved.34,35
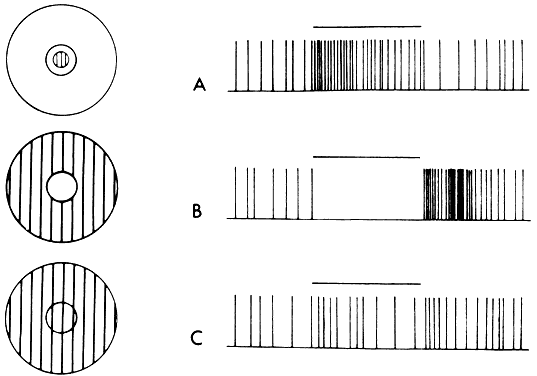
These considerations imply that measurement of grating acuity in amblyopic patients (and other tests based on detection rather than recognition, such as pointing to or picking up a minute object), is likely to underestimate the degree of visual loss, especially when strabismus is present. In some situations, grating acuity testing may nevertheless be of value to the clinician. It is well established that grating acuity can be measured reliably in very young patients using devices based on the technique of preferential looking.36,37 Infants naturally prefer to look at stripes rather than blank surfaces. In preferential looking tests, a trained examiner determines the patient's ability to see high contrast gratings of various spatial frequencies by noting the consistency with which the infant looks toward the pattern in a controlled setting (Fig. 6). At least one such test, the Teller acuity card system (Vistech Consultants, Dayton, OH), has now proved suitable for use in clinical practice, although the testing process remains somewhat cumbersome and time consuming (about 15 minutes per patient under optimal conditions).
|
Teller cards or an alternative preferential looking device provide a reliable means for detecting amblyopia, estimating its severity, and monitoring the progress of treatment in the patient with anisometropia or media opacity who is too young for any form of recognition testing. For patients with strabismus, preferential looking assessment that indicates subnormal grating acuity confirms the presence of amblyopia, but it cannot be ruled out on the basis of normal responses. Because observation of fixation preference is available to the clinician as a rapid and highly sensitive means of detecting strabismic amblyopia, preferential looking tests are not usually helpful with such patients.
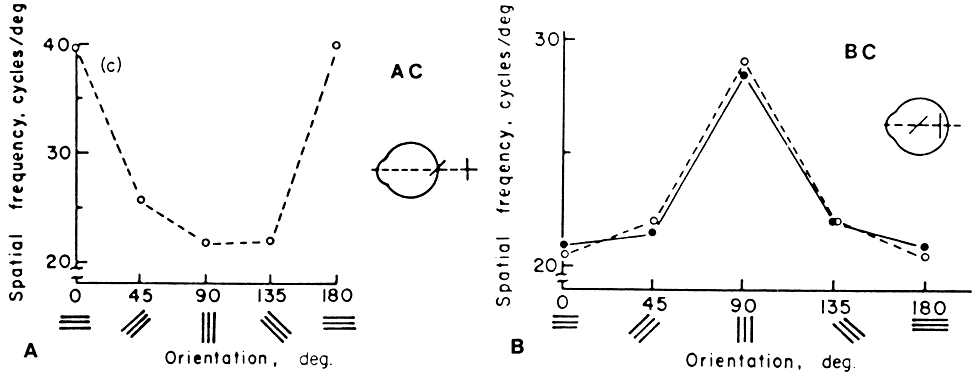
Gratings of different orientation are applicable to the assessment of meridional variation in acuity. Reduction in grating acuity that is limited to the more ametropic meridians is seen in patients with optically corrected astigmatism and meridional amblyopia (Fig. 7).38 Some patients with strabismic amblyopia (esotropic or exotropic) who do not have significant astigmatism show greater reduction of acuity for vertical gratings than for horizontal gratings. This is thought to reflect the greater impact of horizontal image displacement in the deviating eye on cortical neurons selective for vertical orientation.39
The visual system can respond to sinusoidal gratings over a broad range of spatial frequencies below the acuity cutoff. At different frequencies, varying amounts of contrast are required for detection of the grating pattern. Plotting contrast sensitivity (the reciprocal of threshold contrast) against spatial frequency yields a curve called the contrast sensitivity function. Normally, contrast sensitivity peaks at about three cycles per degree and falls off gradually at lower frequencies.26 Low frequency contrast sensitivity is a measure of ability to detect gradual transitions from light to dark. This is an important aspect of form vision (conveying information about the shape and position of large objects) that seems to be handled differently by the visual system from input concerning fine detail that is carried by higher spatial frequencies.40
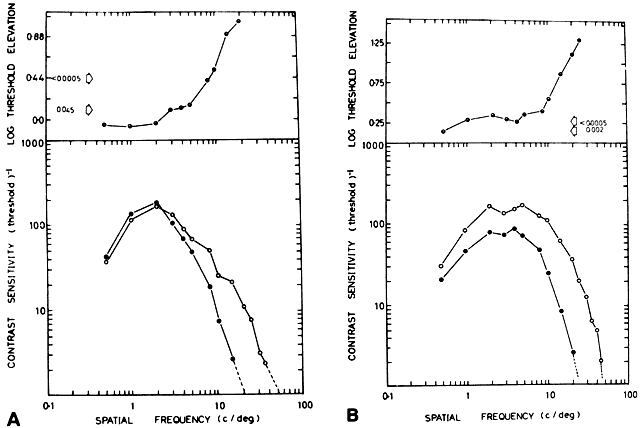
Investigation of contrast sensitivity functions in amblyopia has revealed that some affected persons have reduced sensitivity only at high spatial frequencies, whereas others show reduction at all frequencies (Fig. 8).41 No consistent relationship has been established between contrast sensitivity profile and etiologic category or any other clinically significant feature of amblyopia, however. Although several devices that permit contrast sensitivitytesting in the clinical setting are now available,there is no generally agreed on indication for theirapplication to the care of amblyopic patients at present.
VISUAL FIELD
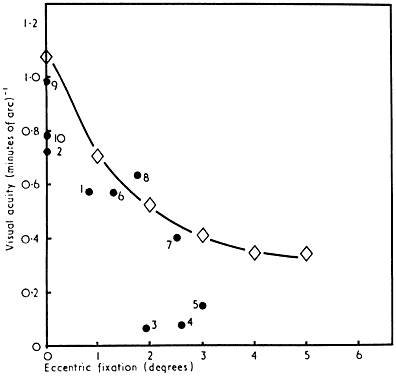
Amblyopia affects primarily foveal vision, but demonstration of a central scotoma by conventional means is difficult because of the difficulty that many amblyopic patients have in maintaining central fixation and the subtlety of the defect itself. Wald and Burian measured absolute light sensitivity in dark-adapted amblyopic patients and found no difference from normal across the visual field.42 In the dark-adapted state, however, both normal and amblyopic eyes show a relative reduction in light sensitivity in the rod-free fovea. When the visual field of an amblyopic eye is plotted in the light-adapted state, a relative central scotoma can be demonstrated if very small test targets are employed.23 If larger test spots are used, increased spatial summation of the amblyopic fovea eliminates the scotoma (Fig. 9). Recent observations made with automated perimetry suggest that there is slight reduction in light sensitivity of amblyopic eyes compared with their fellow normal eyes that extends well into the peripheral field even when acuity reduction is moderate.43
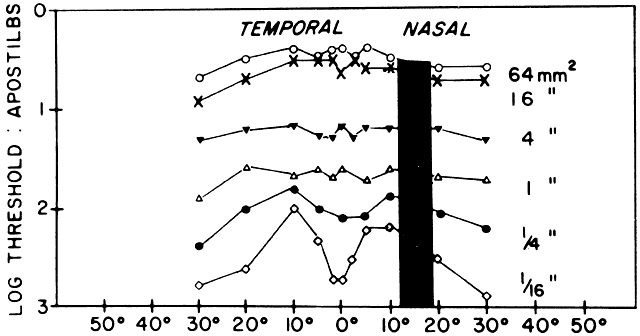
Severe amblyopia, such as that produced by a unilateral congenital cataract, may profoundly degrade peripheral vision, and in some cases depression of the visual field all the way to its nasal termination can be demonstrated. A region that seems consistently to be spared, however, is the temporal monocular crescent.44 This part of an amblyopic eye's projection to higher visual centers is not subject to competition from the input of the other eye, and thus can be affected only by the amblyopiogenic mechanism that is strictly dependent on form vision deprivation. Because the far periphery is normally exposed to relatively degraded images, it may be resistant to this effect as well. The monocular temporal field is functionally important for mobility and personal safety. Its preservation implies that the total extent of the binocular field of vision is normal even in a severely amblyopic patient, unless the amblyopic eye is esodeviated or obstructed by a dense media opacity.45
EFFECTS OF CHANGING LIGHT LEVELS
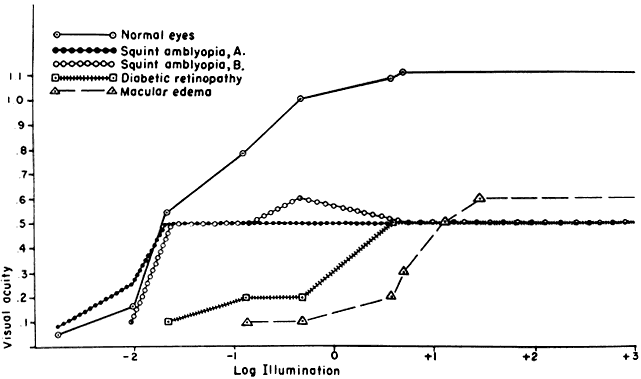
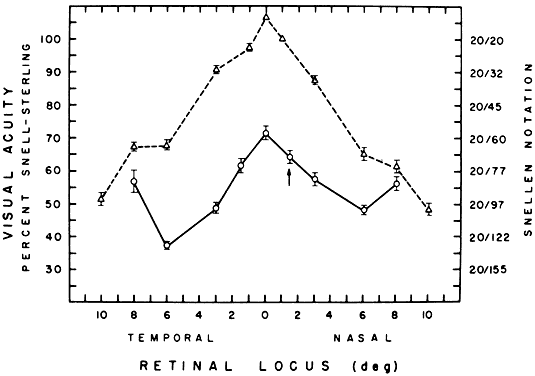
As already mentioned, the visual field of the amblyopic eye appears normal when plotted with very dim lights following dark adaptation. The difference between normal and amblyopic eyes with respect to other visual functions also tends to decrease when the light level is diminished. Von Noorden andBurian46 found that an amblyopic eye may show little or no reduction in acuity when viewing through neutral density filters that are dark enough to decrease the normal eye's acuity to the amblyopic eye's level. In contrast, performance of organically diseased eyes typically deteriorates more than normal with lowering of illumination (Fig. 10). Recent observations indicate that this characteristic behavior at reduced light levels is found only in strabismic amblyopic patients.47
Whether the process of dark adaptation is abnormal in amblyopia remains a matter of dispute. Wald and Burian observed normal dark adaptation in their classic study of amblyopic vision, but they tested this function in an extrafoveal area.42 Flynn found both a decreased rate of dark adaptation and residual threshold evaluation in some patients with amblyopia using a large central test field.48
These findings do not imply abnormality of the rods and cones in amblyopic eyes, for dark adaptation is a complex process involving functional changes in neurons as well as regeneration of visual pigments. Adjustment to varying light levels does occur primarily at lower levels of visual processing, however, and the effects of illumination on amblyopic vision may in fact have a subcortical origin.
COLOR VISION
In contrast to organic disease of the retina or optic nerve, amblyopia typically causes no major disturbance of color vision. Wald and Burian demonstrated normal spectral sensitivity in severely amblyopic patients in both the photopic and the scotopic states.42 Abnormalities have, however, been found in the increment threshold spectral sensitivity, which differs from the absolute sensitivity measured by Wald and Burian in that test lights are presented against a brightly illuminated white background.49 Like other aspects of amblyopic vision already considered, spectral sensitivity seems to be most disturbed at relatively high levels of overall illumination.
Mild to moderate amblyopia does not affect performance on clinical tests of color vision, but when acuity is severely reduced (20/200 or less) errors are common. The mistakes made by amblyopic patients appear random and are probably secondary to marked impairment of form vision rather than to a specific defect in color discrimination.