1. Grant WM: Systemic drugs and adverse influence on ocular pressure. In Leopold IH (ed): Symposium on Ocular Therapym, Vol 3. St. Louis, CV Mosby, 1968. 2. Blackman HJ, Peck GL, Olsen TG et al: Blepharoconjunctivitis: A side effect of 13-cis-retinoic
acid therapy for determatologic diseases. Ophthalmology 86:753, 1979. 3. Giles CL, Soble AR: Intracranial hypertension and tetracycline therapy. Am J Ophthalmol 981, 1971. 4. Pearson MG, Littlwood SM, Bowden AN: Tetracycline and benign intracranial hypertension. Br Med J 1:282, 1981. 5. Saraux H, Biais B: Neurites optiques par le disulfirame. Ann Ocul 203:769, 1970. 6. Hirst LW, Sanborn G, Green WR et al: Amodiaquine ocular changes. Arch Ophthalmol 100:1300, 1982. 7. Easterbrook M: Ocular effects and safety of antimalarial agents. Am J Med 85:23, 1988. 8. Bovino JA, Marcus DF: The mechanism of transient myopia induced by sulfonamide therapy. Am J Ophthalmol 94:99, 1982. 9. Brinkley JRJr , Dubois EL, Ryan SJ: Long-term course of chloroquine retinopathy after cessation of medication. Am J Ophthalmol 88:1, 1979. 10. Graniewski-Wijnands HS, VanLith GHM, Vijfvinkel-Bruinenga S: Ophthalmological examination of patients taking chloroquine. Doc Ophthalmol 48:231, 1979. 11. Ogawa S, Kurumatasi N, Shibaike N et al: Progression of retinopathy long after cessation of chloroquine therapy. Lancet 1:1408, 1979. 12. Gottlieb NL, Major JC: Ocular chrysiasis correlated with gold concentrations in the crystalline
lens during cryo-therapy. Arthritis Rheum 21:704, 1978. 13. Bron AJ, McLendon BF, Camp AV: Epithelial deposition of gold in the cornea in patients receiving systemic
therapy. Am J Ophthalmol 88:354, 1979. 14. Camus JP, Crouzet J, Bach JF et al: Autoantibodies in $PI$vD-penicillamine treated rheumatoid arthritis. Agents Actions 6:351, 1976. 15. Atcheson SG, Ward JR: Ptosis and weakness after start of $PI$vD-penicillamine therapy. Ann Intern Med 89:939, 1978. 16. Bocanegra T, Espinoza LR, Vasey FB et al: Myasthenia gravis and penicillamine therapy of rheumatoid arthritis. JAMA 244:1822, 1980. 17. Podos SM, Canellos GP: Lens changes in chronic granulocytic leukemia. Possible relationship to
chemotherapy. Am J Ophthalmol 68:500, 1969. 18. Becher R, Schutt P, Osieka R et al: Peripheral neuropathy and ophthalmologic toxicity after treatment with
cis-dichlorodiaminoplatinum II. J Cancer Res Clin Oncol 96:219, 1980. 19. Caravella LPJr , Burns JA, Zangmeister M: Punctalcanalicular stenosis related to systemic fluorouracil therapy. Arch Ophthalmol 99:284, 1981. 20. Blanke RV, Sady JJ, Drummond DC et al: Tilorone in a corneal biopsy. J Anal Toxicol 9:206, 1979. 21. Kaiser-Kupfer MI, Lippman ME: Tamoxifen retinopathy. Cancer Treat Rep 62:315, 1978. 22. Kaiser-Kupfer MI, Kupfer C, Rodriques MM: Tamoxifen retinopathy. Ophthalmology 88:89, 1981. 23. Beck M, Mills PV: Ocular assessment of patients with tamoxifen. Cancer Treat Rep 63:1833, 1979. 24. Weiss JN, Ochs AL, Abedi S et al: Retinopathy after tilorone hydrochloride. Am J Ophthalmol 90:846, 1980. 25. Albert DM, Wong VG, Henderson ES: Ocular complications of vincristine therapy. Arch Ophthalmol 78:709, 1967. 26. Sanderson PA, Kuwabara T, Cogan DG: Optic neuropathy presumably caused by vincristine therapy. Am J Ophthalmol 81:146, 1976. 27. Byrd RL, Rohrbaugh TM, Raney BJr et al: Transient cortical blindness secondary to vincristine therapy in childhood
malignancies. Cancer 47:37, 1981. 28. D'Amico DJ, Kenyon KR, Ruskin JN: Amiodarone keratopathy: Drug-induced lipid storage disease. Arch Ophthalmol 99:257, 1981. 29. Volpe BT, Soave R: Formed visual hallucinations as digitalis toxicity. Ann Intern Med 91:865, 1979. 30. Aronson JF, Ford AR: The use of colour vision measurement in the diagnosis of digoxin toxicity. Q J Med 195:273, 1980. 31. Atkinson AB, McAreavey D, Trope G: Papilloedema and hepatic dysfunction apparently induced by perhexiline
maleate (Pexid). Br Heart J 43:490, 1980. 32. Svarc ED, Werner D: Isolated retinal hemorrhages associated with oral contraceptives. Am J Ophthalmol 84:50, 1977. 33. Perry HD, Mallen FJ: Cilioretinal artery occlusion associated with oral contraceptives. Am J Ophthalmol 84:56, 1977. 34. Alpert JN: Downbeat nystagmus due to anticonvulsant toxicity. Ann Neurol 4:471, 1978. 35. Gallagher B, Baumel IP, Mattson RH et al: Primidone, diphenylhydantoin and phenobarbital. Neurology 23:145, 1973. 36. Kutt H, Winters W, Kokenge R, McDowell F: Diphenylhydantoin metabolism, blood levels and toxicity. Arch Neurol 11:642, 1964. 37. Riker WK, Downes H, Olsen GD et al: Conjugate lateral gaze nystagmus and free phenytoin concentrations in plasma: Lack
of correlation. Epilepsia 19:93, 1978. 38. Sloan LL, Gilger AP: Visual effects of Tridione. Am J Ophthalmol 30:1387, 1947. 39. Skalka HW, Prchal JT: The effect of diabetes mellitus and diabetic therapy on cataract formation. Ophthalmology 88:117, 1981. 40. Albert DM, Bullock JD, Lahav M, Caine R: Flecked retina secondary to oxalate crystals from methoxyflurane anesthesia: Clinical
and experimental studies. Trans Am Acad Ophthalmol Otolaryngol 79:817, 197. 41. Muirhead JF, Scheie HG: Transient myopia after acetazolamide. Arch Ophthalmol 63:315, 1960. 42. Beasley FJ: Transient myopia and retinal edema during ethoxzolamide (Cardrase) therapy. Arch Ophthalmol 68:490, 1962. 43. Wright P: Untoward effects associated with practolol administration: Oculomucocutaneous
syndrome. Br Med J 1:595, 1975. 44. Rahi AHS, Chapman CM, Garner A et al: Pathology of practolol-induced ocular toxicity. Br J Ophthalmol 60:312, 1976. 45. Skegg DGG, Doll R: Frequency of eye complaints and rashes among patients receiving practolol
and propranolol. Lancet 2:475, 1977. 46. Harley RD, Huang NN, Macri CH et al: Optic neuritis and optic atrophy following chloramphenicol in cystic fibrosis
patients. Trans Am Acad Ophthalmol Otolaryngol 74:1011, 1970. 47. Bird AC, Sheikh HE, Anderson J et al: Changes in visual function and in the posterior segment of the eye during
treatment of onchocerciasis with diethylcarbamazine citrate. Br J Ophthalmol 64:191, 1980. 48. Barron CJ, Tepper L, Iovine G: Ocular toxicity from ethambutol. Am J Ophthalmol 77:256, 1974. 49. Derakhshan I, Forough M: Progressive visual loss after eight years on choquinol. Lancet 1:715, 1978. 50. Cohen DN: Intracranial hypertension and papilledema associated with naladixic acid
therapy. Am J Ophthalmol 76:680, 1973. 51. Katahira K: SMON reported in 1935 in Argentina. JAMA 239:2757, 1978. 52. Thylefors B, Rolland A: The risk of optic atrophy following suramin treatment of ocular onchocerciosis. Bull WHO 57:479, 1979. 53. Jay WM, Jay S: Benign intracranial hypertension with tetracycline therapy. J Pediatr 93:901, 1978. 54. Walker AR, Adamckiewicz JJ: Pseudotumor cerebri associated with prolonged corticosteroid therapy. JAMA 188:779, 1964. 55. Sundmark E: The cataract-inducing effect of systemic corticosteroid therapy. Acta Ophthalmol 44:291, 1966. 56. Williamson J, Paterson RWW, McGavin DDM et al: Posterior subcapsular cataracts and glaucoma associated with long-term
oral corticosteroid therapy. Br J Ophthalmol 53:361, 1969. 57. Ivey KJ, Den Besten L: Pseudotumor cerebri associated with corticosteroid therapy in an adult. JAMA 208:1698, 1969 58. Ticho U, Durst A, Licht A et al: Steroid-induced glaucoma and cataract in renal transplant recipients. Isr J Med Sci 13:871, 1977. 59. Hosking GP, Elliston H: Benign intracranial hypertension in a child with eczema treated with topical
steroids. Br Med J 1:550, 1978. 60. Rooklin AR, Lampert SI, Jaeger EA et al: Posterior subcapsular cataracts in steroid-requiring asthmatic children. J Allergy Clin Immunol 63:383, 1979. 61. Skalka HW, Prchal JT: Effect of corticosteroids on cataract formation. Arch Ophthalmol 98:1773, 1980. 62. Gangtano JL, Keltner JL: Abnormalities of the pupil and visual-evoked-potential in
quinine amblyopia. Am J Ophthalmol 89:425, 1980. 63. Medina C, Kramer MD, Kurland AA: Biperiden in the treatment of phenothiazine-induced extrapyramidal
reactions. JAMA 182:1127, 1962. 64. Manor RS, Dickerman Z, Llaron Z: Myopia during bromocriptine treatment. Lancet 1:102, 1981. 65. Shimizu N, Cohen B, Bala SP et al: Ocular dyskinesias in patients with Parkinson's disease treated with
levodopa. Ann Neurol 1:167, 1977. 66. Freeling P: A trial of oxyphencyclimine. Practitioner 192:797, 1964. 67. Brothers DM, Hidayat AA: Conjunctival pigmentation associated with tetracycline medication. Ophthalmology 88:1212, 1981. 68. Laties AM: Ocular toxicology of haloperidol. In Leopold IH, Burns RP (eds): Symposium on Ocular Therapy, Vol 9. New York, John Wiley & Sons, 1976. 69. Rasmussen K, Kirk L, Faurbye A: Deposits in the lens and cornea of the eye during long-term chlorpromazine
medication. Acta Psychiatr Scand 53:1, 1976. 70. Meredith TA, Aaberg TM, Willerson D: Progressive chorioretinopathy after receiving thioridazine. Arch Ophthalmol 96:1172, 1978. 71. Kinsey VE: Cooperative study of retrolental fibroplasia and the use of oxygen. Arch Ophthalmol 56:481, 1956. 72. Miyazaki Y, Van Leeuwen L, Van Leeuwen G: Retrolental fibroplasia: A continuing dilemma for the pediatrician. Clin Pediatr 16:1091, 1977. 73. Nicholson DH, Harkness DR, Benson WE et al: Cyanate-induced cataracts in patients with sickle cell hemoglobinopathies. Arch Ophthalmol 94:927, 1976. 74. Spector RH, Carlisle J: Pseudotumor cerebri caused by a synthetic vitamin A preparation. Neurology 34:1509, 1984. 75. Fraunfelder FT, LaBraico JM, Meyer SM: Adverse ocular reactions possibly associated with isotretinoin. Am J Ophthalmol 100:534, 1985 76. Weleber RG, Denman ST, Hanifin JM, Cunningham WJ: Abnormal retinal function associated with isotretinoin therapy for acne. Arch Ophthalmol 104:831, 1986. 77. Brown RD, Grattan CEH: Visual toxicity of synthetic retinoids. Br J Ophthalmol 73:286, 1989 78. Millay RH, Klein ML, Illingworth DR: Niacin maculopathy. Ophthalmology 95:930, 1988. 79. Olivieri NL, Buncic JR, Chew E et al: Visual and auditory neurotoxicity in patients receiving subcutaneous deferoxamine
infusions. N Engl J Med 314:869, 1986. 80. Rahl AHS, Hungerford JL, Ahmed AI: Ocular toxicity of desferrioxamine: Light microscopic histochemical and
ultrastructural finding. Br J Ophthalmol 70:373, 1986. 81. Pengloan J, Dantal J, Rossazza C et al: Ocular toxicity after a single intravenous dose of desferrioxamine in 2 hemodialyzed
patients. Nephron 46:211, 1987. 82. Doroshow JH, Locker GY, Gasterland DE et al: Ocular irritation from high-dose methotrexate therapy. Pharmokinetics
of drug in the tear film. Cancer 48:2158, 1981. 83. Hamming NA, Apple DJ, Goldberg MF: Histopathology and ultrastructure of busulfan-induced cataract. Graefes Arch Klin Exp Ophthalmol 200:139, 1976. 84. Hopen G, Mondino BJ, Johnson BL: Corneal toxicity with systemic cytarabine. Am J Ophthalmol 91:500, 1981. 85. Kaufman HE, Capella JA, Maloney ED: Corneal toxicity of cytosine arabinoside. 72:535, 1964. 86. Christophidis N, Vajda FJE, Lucas I et al: Ocular side effects with 5-fluorouracil. Aust N Z J Med 9:143, 1979. 87. Haidak DJ, Hurwitz BS, Yeung KY: Tear duct fibrosis (dacryostenosis) due to 5-fluorouracil. Ann Intern Med 88:657, 1978. 88. Straus DJ, Mausolf FA, Ellerby RA et al: Cicatricial entropion secondary to 5-fluorouracil therapy. Med Pediatr Oncol 3:15, 1977. 89. Holland EJ, Stein CA, Palestine AG et al: Suramin keratopathy. Am J Ophthalmol 106:216, 1988. 90. Muenter MD, Perry HO, Ludwig J: Chronic vitamin A intoxication in adults. Am J Med 50:129, 1971. 91. Yiannikas C, Walsh JC, McLeod JG: Visual evoked potentials in the detection of subclinical optic toxic effects
secondary to ethambutol. Arch Neurol 40:654, 1983. 92. Chatterjee VKK, Buchanan DR, Friedman AI et al: Ocular toxicity following ethambutol in standard dosage. Br J Dis Chest 80:288, 1986. 93. Casey WF, Hannon V, Cunningham A et al: Visual evoked potentials and changes in serum glycine concentration during
transurethral resection of the prostate. Br J Anaesth 60:525, 1988. 94. Wang JM, Creel DJ, Wong KC: Transurethral resection of the prostate, serum glycine levels, and ocular
evoked potentials. Anesthesiology 70:36, 1989. 95. McCormick SAD, Bartolomeo AC, Raju VK et al: Ocular chrysiasis. Ophthalmology 92:1432, 1985. 96. Weidle EG: Lenticular chrysiasis in oral chrysotherapy. Am J Ophthalmol 103:240, 1987. 97. Chuman MA, LeSage J: Color vision deficiencies in 2 cases of digoxin toxicity. Am J Ophthalmol 100:682, 1985. 98. Feiner LA, Yonger BR, Kazmier FJ et al: Optic neuropathy and amiodarone therapy. Mayo Clin Proc 62:702, 1987. 99. Gittinger JW, Asdourian GK: Papillopathy caused by amiodarone. Arch Ophthalmol 105:349, 1987. 100. Nazarian SM, Jay WM: Bilateral optic neuropathy associated with amiodarone therapy. J Clin Neurol Ophthalmol 8:25, 1988. 101. Messmer E, Font RL, Sheldon G et al: Pigmented conjunctival cysts following tetracycline/minocycline therapy: Histochemical
and electron microscopic observations. Ophthalmology 90:1462, 1983. 102. Kincaid MC, Green WR, Hoover RE et al: Iododerma of the conjunctiva and skin. Ophthalmology 88:1216, 1981. 103. Hook SR, Holladay JT, Prager TC et al: Transient myopia induced by sulfonamides. Am J Ophthalmol 101:495, 1986. 104. Oliver TRJr , Havener WH: Eye manifestations of chronic vitamin A intoxication. Arch Ophthalmol 60:19, 1958. 105. Morrice GJr , Havener WH, Kapetansky F: Vitamin A intoxication as a cause of pseudotumor cerebri. JAMA 173:1802, 1960. 106. Bickerstaff ER, Jacoby E: Oculogyric crisis with phenothiazine derivatives. Br Med J 1:647, 1960. 107. Lawden MC, Eke T, Degg C et al: Visual field defects associated with vigabatrin therapy. J Neurol Neurosurg Psychiatry 67:716, 1999. 108. Johnson MA, Krauss GL, Miller NR et al: Visual function loss from vigabatrin: Effect of stopping the drug. Neurolology 55:40, 2000. 109. Daneshvar H, Racette L, Coupland SG et al: Symptomatic and asymptomatic visual loss in patients taking vigabatrin. Ophthalmology 106:1792, 1999. 110. Arndt CF, Derambure P, De Foort-Dhellemmes S et al: Outer retinal dysfunction in patients treated with vigabatrin. Neurology 52:1201, 1999. 111. Harding GFA, Wild JM, Robertson KA et al: Separating the retinal electrophysiologic effects of vigabatrin. Neurology 55:347, 2000. 112. Malmgren K, Menachem E, Frisen L: Vigabatrin visual toxicity: Evolution and dose dependence. Epilepsia 42:609, 2001. 113. Sen HA, O'Halloran HS, Lee WB et al: Topiramate-induced acute myopia and retinal striae. Arch Ophthalmol 119:775, 2001. 114. Sankar PS, Pasquale LR, Grosskruetz CL: Uveal effusion and secondary angle-closure glaucoma associated with
topiramate use. Arch Ophthalmol 119:1210, 2001. 115. Vobig M, Klotz T, Staak M et al: Retinal side-effects of sildenafil. Lancet: 353:375, 1999. 116. Luu JK, Chappelow AV, McCulley TJ et al: Acute effects of sildenafil on the electroretinogram and multifocal electroretronogram. Am J Ophthalmol 132:388, 2001. 117. Gabrieli CB, Regine F, Vingold EM et al: Subjective visual halos after sildenafil (Viagra) administration. Ophthalmology 108:877, 2001. 118. Fraunfelder FT, Fraunfelder FW, Edwards R: Ocular side effects possibly associated with isotretinoin usage. Am J Ophthalmol 132:299, 2001 119. Hoerauf H, Schmidt-Erfurth U, Asiyo-Vogel M et al: Combined choroidal and retinal ischemia during interferon therapy: Indocyanine
green angiographic and microperimetric findings. Arch Ophthalmol 118:580, 2000. 120. Hejny C, Sternberg P Jr , Lanson DH et al: Retinopathy associated with high-dose interferon alpha-2b
therapy. Am J Ophthalmol 131:782, 2001. 121. Esmaeli B, Koller C, Papadopoulos N et al: Interferon-induced retinopathy in asymptomatic cancer patients. Ophthalmology 108:858, 2001. 122. Tokai R, Ikeda T, Miyaura T et al: Interferon-associated retinopathy and cystoid macular edema. Arch Ophthalmol 119:1077, 2001. 123. Flash AJ, Dolan BJ: Progression of amiodarone induced cataracts. Doc Ophthalmol 83:323, 1993 124. Mantyjarvi M, Tuppurainen K, Ikaheimo K: Ocular side effects of amiodarone. Survey Ophthalmol 42:360, 1998. 125. Grierson DJ: Hydroxychloroquine and visual screening in a rheumatology outpatient clinic. Ann Rheumat Dis 56:188, 1997. 126. Block JA: Hydroxychloroquine and retinal safety. Lancet 351:771, 1998. 127. Chiu AM, Chuenkongkaew WL, Cornblath WT: Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol 126:116, 1998. 128. Wolin MJ: Digoxin visual toxicity with therapeutic blood levels of digoxin. Am J Ophthalmol 125:406, 1998. 129. Madreperla SA, Johnson MA, Nakatani K: Electrophysiologic and electroretinographic evidence for photoreceptor
dysfunction as a toxic effect of digoxin. Arch Ophthalmol 112:807, 1994. 130. Alhalel A, Ziu I, Versano D et al: Ocular effects of hyocine in double dose transdermal administration and
its reversal by low dose pyridostigmine. Aviat Space Environ Med 66:11, 1995. 131. Eltze M, Mutschler E, Lambrecht G: Affinity profiles of pizotifen, ketotifen and other tricyclic antimuscarinics
at muscarinic receptor subtypes M1, M2, and M3. Eur J Pharmacol 211:283, 1992. 132. Kubo N, Shirakawa O, Kuno T et al: Antimuscarinic effects of antihistaminics: Quantitative evaluation by receptor
binding assay. Jap J Pharmacol 43:277, 1987. 133. Harnois C., Samson J, Malenfant M et al: Canthaxanthin retinopathy anatomic and functional reversibility. Arch Ophthalmol 107:538, 1989. 134. Espaillat A, Aiello LP, Arrigg PG et al: Canthaxanthin retinopathy. Arch Ophthalmol 117:412, 1999. 135. Moorthy RS, Valluri S, Jampol LM: Drug-induced uveitis. Survey Ophthalmol 42:557, 1998. 136. Jewelewicz DA, Schiff WM, Brown S: Rifabutin-associated uveitis in an immunosuppressed pediatric patient
without acquired immunodeficiency syndrome. Am J Ophthalmol 125:872, 1998. 137. Bhagat N, Read RW, Rao NA: Rifabutin-associated hypopyon uveitis in human immunodeficiency
virus–negative immunocompetent individuals. Ophthalmol 108:750, 2001. 138. Siris ES: Biphosphonates and uveitis. Lancet 341:436, 1993. 139. Mbekeani JC, Slamovits TL, Schwartz BH et al: Ocular inflammation associated with alendronate therapy. Arch Ophthalmol 117:837, 1999. 140. Sabroe RA, Archer CB, Harlow D et al: Minocycline-induced discolouration of the sclerae. Brit J Dermatol 135:314, 1996. 141. Fraunfelder FT, Randall JA: Minocycline-induced scleral pigmentation. Ophthalmol 104:936, 1997. | 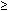 250 mg/d
for 4 mo
250 mg/d
for 4 mo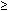 200,000
U/d for 2 mo
200,000
U/d for 2 mo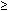 3.2 g
3.2 g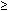 8.1 mg/kg/d
for 2 mo
8.1 mg/kg/d
for 2 mo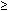 48 mg
IV in 14 mo
48 mg
IV in 14 mo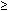 3 g/d
3 g/d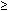 6
mo
6
mo