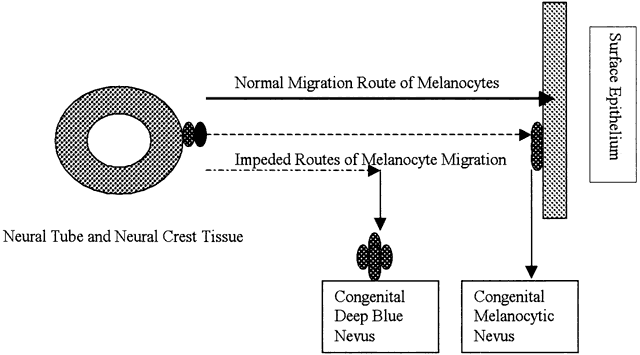
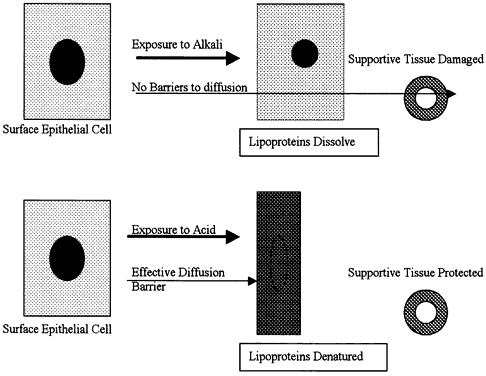
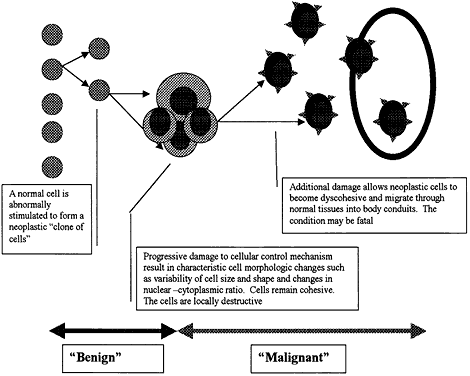
| Inflammation is one of the body's active, or reactive, methods of
protecting tissues and organs. Other and more passive protective mechanisms
include keratinization of the skin, which prevents excessive tissue
fluid loss and exposure to environmental antigens. Tears dilute and
mechanically remove surface agents; they also contain active antibacterial
agents (lysozymes).There are three levels of inflammation; each
has a different function and outcome. Acute inflammation is the most primitive level and usually results in destruction
of material or of organisms sensitive to the numerous degradative
enzymes and oxidative processes that characterize it. Enzymes are
carried in protective packets within the cytoplasm of polymorphonuclear
leukocytes; they are formed in the bone marrow and brought to the
site of tissue abnormality through vascular channels. In the region of
injury, the vascular endothelial cell membrane changes its normally
nonadhesive surface to one that attracts white blood cells. Endothelial
cells separate from each other to create spaces large enough for passage
of the white blood cells from the vascular lumen to the extracellular
matrix external to the vessel (diapedesis). White blood cells then
follow a trail of protein or other signals emanating from the site of
injury (chemotaxis). The polymorphonuclear leukocyte initially attempts
to remove noxious agents by internalizing (phagocytosis) and degrading
the material through controlled exposure to the destructive enzymes
carried by the cell. If noxious material overwhelms the cell, it simply
dissolves and externalizes the enzymes into the immediate environment, allowing
uncontrolled action of the destructive enzymes. The end
result may be destruction of extensive areas of tissue, including areas
of normal adjacent tissue (Table 3). TABLE 1-3. Characterization of Inflammatory Cells
| Cell Type | Cytologic Characteristics | Function |
| Polymorphonuclear leukocytes | Nucleus: multilobulated | Destruction of organisms and damaged tissue with proteolytic enzymes and
oxidation |
| | Cytoplasm: spherical inclusions |
| Eosinophils | Nucleus: bilobed | Influence blood vessels function |
| | Cytoplasm: spherical inclusions | |
| Basophils | Nucleus: bilobed | Produce vasoactive amines |
| | Cytoplasm: coarse basophilic granules | |
| Mast cells | Nucleus: central bland | Produce vasoactive amines |
| | Cytoplasm: featureless | |
| Lymphocytes | Nucleus: large, homogeneous | Multiple roles in recognition and destruction of protein components threatening
to cell function |
| | Cytoplasm: inconspicuous |
| Plasma cell | Nucleus: eccentric; “clock face” | Product antibodies |
| | Cytoplasm: paranuclear “halo” | |
| Epitheloid histiocyte | Nucleus: single, central location | Process antigens, macrophagic |
| | Cytoplasm: similar to epithelial cells | |
| Foreign body giant cell | Nucleus: multiple, random | Reaction to foreign materials, fungi |
| | Cytoplasm: abundant, amorphous | |
| Langerhans giant cell | Nucleus: multiple, peripheral | Associated with tuberculosis |
| | Cytoplasma: abundant, amorphous | |
| Touton giant cell | Nucleus: multiple, midperipheral | Associated with lipid abnormalities |
| | Cytoplasm: peripheral lipid | | Other cell types active in acute inflammatory processes include eosinophils, mast
cells, and basophils; they secrete vasoactive substances to
influence blood vessel function during allergic reactions. At the second level of inflammation, chronic nongranulomatous inflammation, lymphocytes
and macrophages operate in a more sophisticated and less
destructive manner. Although an acute inflammatory reaction ends with
destructive chaos, a chronic inflammatory reaction initiates the reparative
processes of all tissues of the body. Even though mature lymphocytes
appear with light microsopy to be similar, the functions of lymphocytes
are markedly diverse. Some cells are capable of producing antibodies; during
this process they evolve morphologically to form plasma
cells. Other cells retain their nondescript appearance but are capable
of completely destroying the cell membranes of other cells. Still
other lymphocytes are able to produce protein signals (cytokines) to
influence fibroblasts, which, in turn, may influence other cells (e.g., superficial squamous epithelial cells). These end-user cells may then
produce cytokines themselves to complete the feedback loop, resulting
in a carefully controlled, nonchaotic system. However, the reparative
tissue never regains the functional diversity or the specialization of
the original tissue. The final level of inflammation is the most specific of all. The small, so-called
granules of reactive tissue noted grossly in specific inflammatory
reactions led to the term, granulomatous inflammatory reaction. The
granules, which represent accumulations of activated mononuclear
inflammatory cells, have a generous amount of cytoplasm and resemble
a simple surface epithelial cell; they are thus called epithelioid histiocytes. Activation
usually occurs when the cells encounter large quantities
of poorly digested antigens or of organisms that can proliferate
intracellularly. In the presence of complex stimuli, epithelioid histiocytes
duplicate nuclear material without the associated division of
cell cytoplasm, resulting in the formation of multinucleate giant cells. There
are three types of multinucleate giant cells: Langhans giant
cells, which have a peripheral ring of nuclei (associated with tuberculosis); Touton
giant cells, which have a midperipheral ring of nuclei
surrounded by a peripheral ring of lipid (associated with xanthogranulomatous
disease); and foreign body giant cells, which have randomly
dispersed nuclei (associated with foreign material and fungi). The presence
of granulomatous inflammation limits disease categories to a rather
small number, such as infection with complex microorganisms (e.g., fungi) or complex foreign material (e.g., wood fragments). In many clinical settings, the inflammatory process itself may be abnormal. Sarcoidosis
is the disregulation of chronic granulomatous inflammation
expressed as noncaseating granulomas in multiple organ systems, particularly
in the hilar lymph nodes of the lung. Inappropriate phagocytosis
or inability to process ingested material may produce skin lesions (e.g., as found in Xanthelasma) composed of histiocytes (tissue macrophages) filled
with lipid material. Disruption or circumvention of intracellular
mechanisms that usually destroy ingested bacteria may lead to diseases
such as tuberculosis and lepromatous leprosy. Similar abnormalities
are seen in acquired immunodeficiency syndrome (AIDS), in which organisms
such as Mycobacterium avium-intracellulare accumulate and apparently proliferate within macrophages. Macrophages
or lymphocytes may inadvertently transport viruses or other infective
particles and cause diseases such as spongiform encephalopathy (Creutzfeldt-Jakob
disease). Cell-mediated destruction may result in such clinically
unfavorable outcomes as corneal graft rejection. Apparent shared
antigens may also cause disease in completely separate organ systems, as
occurs in Graves' disease, in which there is tissue stimulation
of both thyroid follicles and of extraocular muscle tissue. |