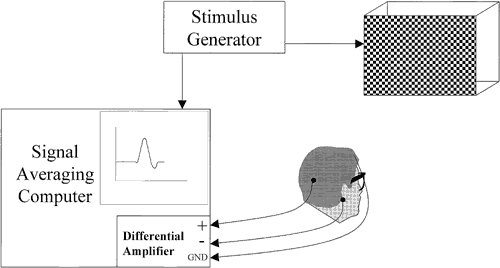
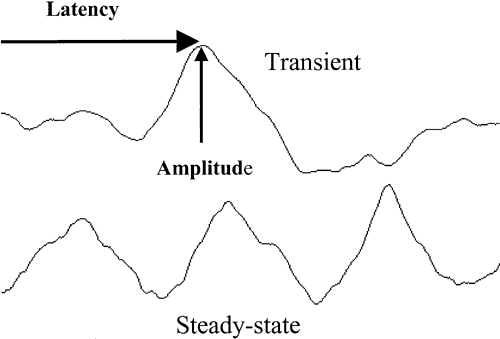
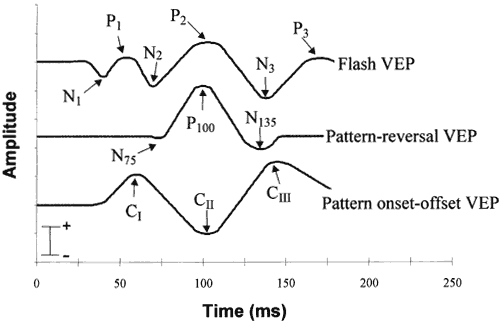
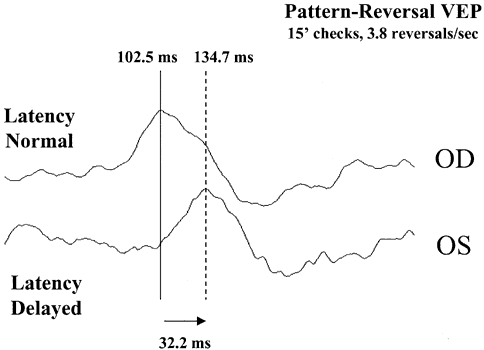
1. Adrian ED, Mathews BHC: The Berger rhythm: Potential changes from the occipital lobes in man. Brain 57:365–385, 1934. 2. Harding GFA, Odom JV, Spileers W, Spekreijse H: Standard for visual evoked potentials. Vision Res 36:3567–3572, 1996. 3. Regan D: Human Brain Electrophysiology. Evoked Potentials and Evoked Magnetic
Fields in Science and Medicine. New York, Elsevier, 1989. 4. Regan D: Evoked Potentials in Psychology, Sensory Physiology and Clinical
Medicine. London, Chapman & Hall, 1972. 5. Sokol S: Visually evoked potentials: Theory, techniques and clinical applications. Surv Ophthalmol 21:18–44, 1976. 6. Brigell MG: The Visual Evoked Potential In: Fishman GA, Birch DG, Holder
GE, and Brigell MG (eds): Electrophysiologic Testing in Disorders of
the Retina, Optic Nerve and Visual Pathway, (2nd edition). The Foundation
of the American Academy of Ophthalmology, San Francisco, 2001 pp 408–415. 7. Jeffreys DA: Cortical source locations of pattern-related visual evoked potentials recorded
from the human scalp. Nature 229:502–504, 1971. 8. Parker DM, Salzen EA, Lishman JR: The early wave of the visual evoked potential to sinusoidal gratings: Responses
to quadrant stimulation as a function of spatial frequency. Electroencephalogr Clin Neurophysiol 53:427–435, 1982. 9. Diamond LA: Latency of the steady state visual evoked potential. Electroencephalogr Clin Neurophysiol 42:125–131, 1977. 10. Cohn R, Hurley WC: Differential visual evoked cortical responses to direct and peripheral
stimulation in man. Electroencephalogr Clin Neurophysiol 61:157–160, 1985. 11. Pratt H, Bleich N, Berliner E: Short latency visual evoked potentials in man. Electroencephalogr Clin Neurophysiol 54:55–62, 1982. 12. Baseler HA, Sutter EE, Klein SA, Carney T: The topography of visual evoked response properties across the visual field. Electroencephalogr Clin Neurophysil 90:65–81, 1994. 13. Klistorner AI, Graham SL: Multifocal pattern VEP perimetry: Analysis of sectoral waveforms. Doc Ophthalmol 98:183–196, 1999. 14. Regan D, Richards W: Brightness contrast and evoked potentials. Optical Soc Am 63:606–611, 1973. 15. Harter RM, White CT: Effects of contour sharpness and check-size on visually evoked cortical
potentials. Vision Res 8:701–711, 1968. 16. Spekreijse H, Van Der Twell LH, Zuidema T: Contrast evoked responses in man. Vision Res 13:243–248, 1973. 17. Meredith TJ, Celesia GG: Pattern-reversal visual evoked potentials and retinal eccentricity. Electroencephalogr Clin Neurophysiol 53:243, 1982. 18. Harter MR: Evoked cortical responses to checkerboard patterns: Effect of check size
as a function of retinal eccentricity. Vision Res 10:1365–1376, 1970. 19. DeValois KK, DeValois RL, Yund EW: Responses of striate cortex cells to grating and checkerboard patterns.J Physiol (Lond) 291:483–505, 1979. 20. Maffei L, Fiorentini A: Electroencephalographic responses to alternating gratings in the cat. Exp Brain Res 48:327–334, 1982. 21. Hubel DH, Weisel TN: Sequence regularity and geometry of orientation columns in the monkey striate
cortex. J Comp Neurol 158:267–293, 1974. 22. Hubel DH, Weisel TN: Receptive fields and functional architecture of monkey striate cortex. J Physiol (Lond) 195:215–243, 1968. 23. Mitchell DE, Wilkinson F: The effect of early astigmatism on the visual resolution of gratings. J Physiol 243:739–756, 1974. 24. Reed JL, Marx MS, May JG: Spatial frequency tuning in the visual evoked potential elicited by sine-wave
gratings. Vision Res 24:1057–1062, 1984. 25. Howe JW, Mitchell KW: The objective assessment of contrast sensitivity function by electrophysiological
means. Br J Ophthalmol 68:626–638, 1984. 26. Maffei L, Campbell FW: Neurophysiological localization of the vertical and horizontal visual coordinates
in man. Science 167:36–387, 1970. 27. Kupersmith MJ, Nelson JI, Seiple WH et al: The 20/20 eye in multiple sclerosis. Neurology 33:1015–1020, 1983. 28. Parker DM, Salzen EA, Lishman JR: Visual-evoked responses elicited by the onset and offset of sinusoidal
gratings. Latency, waveform, and topographic characteristics. Invest Ophthalmol Vis Sci 22:675–680, 1982. 29. Pijn JPM, Estevez O, Van Der Twell LH: Evoked potential latencies as a function of contrast: A system analytical
approach. Doc Ophthalmol 59:175–185, 1985. 30. Rouher F, Plane C, Sole P: Interet des potentiels evoques visuels dans les affections du neuf optique. Arch Ophthalmol (Paris) 29:555–564, 1969. 31. Namerow NS, Enns N: Visual evoked potentials in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 35:829–833, 1972. 32. Richey ET, Kooi KA, Tourtelotte WW. Visually evoked responses in multiple
sclerosis. J Neurol Neurosurg Psychiatry 34:275–280, 1971. 33. Halliday AM, McDonald WI, Mushin J: Delayed pattern evoked responses in optic neuritis in relation to visual
acuity. Trans Ophthalmol Soc UK 93:315–324, 1974. 34. Halliday AM, McDonald WI, Mushin J: Delayed visual evoked response in optic neuritis. Lancet 1:982–985, 1972. 35. Halliday AM, McDonald WI, Mushin J: Delayed visual evoked response in progressive spastic paraplegia. Electroencephalogr Clin Neurophysiol 37:328–335, 1974. 36. Halliday AM, McDonald WI, Mushin J: The dissociation of amplitude and latency changes in the pattern evoked
response following optic neuritis. Electroencephalogr Clin Neurophysiol 36:218–227, 1974. 37. Halliday AM, McDonald WI, Mushin J: Visual evoked response in the diagnosis of multiple sclerosis. Br Med J 4:661–664, 1973. 38. Asselman P, Chadwick DW, Marsden CD: Visual evoked responses in the diagnosis and management of patients suspected
of multiple sclerosis. Brain 98:261–282, 1975. 39. Shahrokhi F, Chiappa KH, Young RR: Pattern shift visual eoked responses. Arch Neurol 35:65–71, 1978. 40. Zeese JA: Pattern visual evoked responses in multiple sclerosis. Arch Neurol 34:314–316, 1977. 41. Perrson HE, Sachs C: Visual evoked potentials elicited by pattern reversal during provoked visual
impairment in multiple sclerosis. Brain 104:369–382, 1981. 42. Scherokman BJ, Selhorst JB, Waybright ET et al: Improved optic nerve conduction with ingestion of ice water. Ann Neurol 17:418–419, 1985. 43. Bodis-Wollner I, Hendley CD, Mylin LH, Thornton J: Visual evoked potentials and the visuogram in multiple sclerosis. Ann Neurol 5:40–47, 1979. 44. Camisa J, Mylin LH, Bodis-Wollner I: The effect of stimulus orientation on the visual evoked potential in multiple
sclerosis. Ann Neurol 10:532–539, 1981. 45. Coupland SG, Kirkham TH: Orientation-specific visual evoked potential deficits in multiple sclerosis. Can J Neurol Sci 9:331–337, 1982. 46. Neetens A, Hendrata Y, Van Rompaey J: Pattern and flash evoked responses in disseminated and selective optic
pathway damage. Trans Ophthalmol Soc UK 99:103–110, 1979. 47. Adachi-Usami E, Kuroda N, Yamamoto Y: Clinical analysis of non-recordable pattern VECP's in 107 patients. Fortschr Ophthalmol 85:301–303, 1988. 48. Regan D, Silver R, Murray TJ: Visual acuity and contrast sensitivity in multiple sclerosis-hidden visual
loss, an auxiliary diagnostic test. Brain 100:563–579, 1977. 49. Regan D, Whitlock JA, Murray TJ, Beverly KI: Orientation-specific losses of contrast sensitivity in multiple sclerosis. Invest Ophthalmol Vis Sci 25:324–328, 1980. 50. Plant GT: Transient visually evoked potentials to sinusoidal gratings in optic neuritis. J Neurol Neurosurg Psychiatry 46:1125–1133, 1983. 51. Kupersmith MJ, Seiple WH, Nelson JI, Carr RE: Contrast sensitivity loss in multiple sclerosis. Selectivity by eye, orientation, and
spatial frequency measured with the evoked potential. Invest Ophthalmol Vis Sci 25:632–639, 1984 52. Wildberger H: Neuropathies of the optic nerve and visual evoked potentials with special
reference to color vision and differential light threshold measured
with the computer perimeter Octopus. Doc Ophthalmol 58:147–227, 1984. 53. Berninger TA, Arden GB, Bird AC: Leber's optic atrophy. J Paediatr Genet Ophthalmol 10:211–227, 1989. 54. Carroll WM, Mastaglia FL: Leber's optic neuropathy: A clinical and visual evoked potential study
of affected and asymptomatic members of a six generation family. Brain 102:559–580, 1979. 55. Wijgaarde R, Van Lith GHM: Pattern EPS in endocrine orbitopathy. Doc Ophthalmol 48:327–332, 1979. 56. Troncoso J, Mancall EL, Schatz NJ: Visual evoked responses in pernicious anemia. Arch Neurol 36:168–169, 1979. 57. Bodis-Wollner I, Korczyn AD: Dissociated sensory loss and visual evoked potentials in a patient with
pernicious anemia. Mt Sinai J Med 47:579–582, 1980. 58. Hennerici M: Dissociated foveal and parafoveal visual evoked responses in subacute combined
degeneration. Arch Neurol 42:130–132, 1985. 59. Halliday AM: Evoked Potentials in Clinical Testing. Edinburgh, Churchill
Livingstone, 1982. 60. Brndet-Wickel CLM, Van Lith GHM, Graniewski Wijnands: Drusen of the optic disc and occipital transient pattern reversal responses. Doc Ophthalmol 50:243–248, 1981. 61. Stevens RA, Newman NM: Abnormal visual-evoked potentials from eyes with optic nerve head drusen. Am J Ophthalmol 92:857–862, 1981. 62. Wilson WB: Visual-evoked response differentiation of ischemic optic neuritis from
the optic neuritis of multiple sclerosis. Am J Ophthalmol 86:530–535, 1978. 63. Cox TA, Thompson HS, Hayreh SS, Snyder JE: Visual evoked potential and pupillary signs. Arch Ophthalmol 100:227–239, 1982. 64. Holder GE: The visual evoked potential in ischaemic optic neuropathy. Doc Ophthalmol Proc Ser 27:123–129, 1981. 65. Ikeda H, Tremain KE, Sanders MD: Neurophysiological investigaton in optic nerve disease: Combined assessment
of the visual evoked response and electroretinogram. Br J Ophthalmol 62:227–239, 1978. 66. Kupersmith MJ, Weiss PA, Carr RE: The visual-evoked potential in tobacco-alcohol and nutritional amblyopia. Am J Ophthalmol 95:307–314, 1983. 67. Cappin JM, Nissim S: Visual evoked responses in the assessment of field defects in glaucoma. Arch Ophthalmol 93:9–18, 1975. 68. Towle VL, Moskowitz A, Sokol S, Schwartz B: The visual evoked potential in glaucoma and ocular hypertension: Effects
of check size, field size, and stimulation rate. Invest Ophthalmol Vis Sci 24:175–183, 1983. 69. Atkin A, Bodis-Wollner I, Podos SM et al: Flicker threshold and pattern VEP latency in ocular hypertension and glaucoma. Invest Ophthalmol Vis Sci 24:1524–1528, 1983. 70. Wanger P, Persson HE: Pattern electroretinograms in unilateral glaucoma. Invest Ophthalmol Vis Sci 24:749–753, 1983. 71. Bobak P, Bodis-Wollner I, Harnois C, Maffei L, Mylin L, Podos S, Thornton J: Pattern electroretinograms and visual-evoked potentials in glaucoma and
multiple sclerosis. Am J Ophthalmol 96:72–78, 1983. 72. Klistorner A, Crewther DP, Crewther SG: Separate magnocellular and parvocellular contributions from temporal analysis
of the multifocal VEP. Vision Res 37:2161–2169, 1997. 73. Klistorner AI, Graham SL, Grigg, JR, Billson FA: Multifocal topographic visual evoked potential: Improving objective detection
of local visual field defects. Invest Ophthalmol Vis Sci 39:937–950, 1998. 74. Klistorner AI, Graham SL: Early magnocellular loss in glaucoma demonstrated using the pseudorandomly
stimulated flash visual evoked potential. J Glaucoma 8:140–148, 1999. 75. Adachi-Usami E, Kuroda N, Yamamoto Y: Clinical analysis of non-recordable pattern VECPs in 107 patients. Fortschr Ophthalmol 85:301–303, 1988. 76. Graham SL, Klistorner AI, Grigg JR, Billson FA: Objective VEP perimetry in glaucoma: Asymmetry analysis to idenify early
deficits. Journal of Glaucoma 9:10–19, 2000. 77. Halliday AM, Halliday E, Kriss A et al: The pattern evoked potential in compression of the anterior visual pathways. Brain 99:357–374, 1976. 78. Holder GE: Chiasmal and retrochiasmal lesions. In: Heckenlively JR and
Arden GB (eds): Principles and Practice of Clinical Electrophysiology
of Vision. Mosby, St. Louis, 1991, pp 557–564. 79. Kooi KA, Guvener AM, Bagchi BK: Visual evoked responses in lesions of the higher optic pathways. Neurology 15:857–862, 1965. 80. Vaughan HG, Katzman R, Taylor J: Alterations of visual evoked response in the presence of homonymous visual
defects. Electroencephalogr Clin Neurophysiol 15:737–751, 1963. 81. Wilderberger GH, Van Lith GHM, Wigngaarde R, Mak GTM: Visually evoked cortical potentials in the evaluation of homonymous and
bitemporal visual field defects. Br J Ophthalmol 60:273–278, 1976. 82. Kuroiwa Y, Celesia GG: Visual evoked potentials with hemifield pattern stimulation. Arch Neurol 38:86–90, 1981. 83. Blumhardt L, Barrett G, Halliday A: The asymmetrical visual evoked potential to pattern reversal in one half
field and its significance for the analysis of visual field defects. Br J Ophthalmol 60:454–461, 1977. 84. Maitland CG, Aminoff MJ, Kennard C, Hoyt WF: Evoked potentials in the evaluation of visual field defects due to chiasmal
or retrochiasmal lesions. Neurology 32:986–991, 1982 85. Barrett G, Blumhardt LD, Halliday AM et al: Paradoxical reversal of lateralization of the half-field trode montages. J Physiol (Lond) 258:63–64, 1976. 86. Crevits L, Van Lith G, Viifvinkel-Brulnenga S: On and off contribution to the combined occipital on-off response to a
pattern stimulus. Ophthalmologica 84:169–173, 1982. 87. Stensaas SS, Eddington DK, Bodelle WH: The topography and variability of the primary visual cortex in man. J Neurosurg 40:747–755, 1974. 88. Brecelj J, Cunningham K: Occipital distribution offovea half-field responses. Doc Ophthalmol 59: 157–165, 1985. 89. Kirkham TH, Coupland SG: Abnormal electroretinograms and visual evoked potentials in chronic papilledema
using time-difference analysis. J Can Sci Neurol 8:243–248, 1981. 90. Kooi KA, Sharbrough FW: Electrophysiological findings in cortical blindness. Report of a case. Electroencephalogr Clin Neurophysiol 20:260–263, 1966. 91. Barnet AB, Manson JI, Wilner E: Acute cerebral blindness in childhood. Neurology 20:1147–1156, 1970. 92. Duchowny MS, Weiss IR, Majlessi H, Barnet AB: Visual evoked responses in childhood cortical blindness after head trauma
and meningitis. Neurology 24:933–940, 1974. 93. Hess CW, Meienberg O, Ludin HP: Visual evoked potentials in acute occipital blindness. J Neurol 227:193–200, 1982. 94. Frank Y, Torres F: Visual evoked potentials in the evaluation of ‘cortical blindness’ in
children. Ann Neurol 6:126–129, 1979. 95. Howe JW, Harcourt RB, Mitchell KW: Prognostic value of the visual evoked response. Trans Ophthalmol Soc UK 99:251–256, 1979. 96. Regan D, Regal DM, Tibbles JAR: Evoked potentials during recovery from blindness recorded serially from
an infant and his normally sighted twin. Electroencephalogr Clin Neurophysiol 54:465–468, 1982. 97. Kupersmith MJ, Nelson JI: Preserved visual evoked potential in infancy
cortical blindness. Neuro-ophthalmology. 98. Perris C, Gotffries CG, Von Knorring L: Visual averaged evoked responses in psychiatric patients. Relationship
to levels of 5-hydroxy-indoleacetic acid, homovanillic acid and tryptophan
in cerebrospinal fluid. J Psychiatr Res 15:175–181, 1979. 99. Ellenberger C, Petro DJ, Ziegler SB: The visually evoked potential in Huntington disease. Neurology 28:95–97, 1978. 100. Oepen G, Doerr M, Thoden U: Visual (VEP) and somatosensory (SSEP) evoked potentials in Huntington's
chorea. Electroencephalogr Clin Neurophysiol 51:666–670, 1981. 101. Hammond EJ, Wilder BJ: Efect of gamma-vinyl GABA on human pattern evoked visual potentials. Neurology 35:1801–1803, 1985. 102. Declerck AC, Arnoldussen OW, te Dorsthorst M: Alterations in transient visual-evoked potentials induced byclonazepam
and sodium valproate. Neuropsychobiology 14:39–41, 1985. 103. Kupersmith MJ, Shakin E, Siegel IM, Lieberman A: Visual system abnormalities in patients with Parkinson's disease. Arch Neurol 39:284–286, 1982. 104. Bodis-Wollner I, Yahr MD: Measurements of visual evoked potentials in Parkinson's disease. Brain 101:661–671, 1978. 105. Bodis-Wollner I, Yahr MD, Mylin L, Thornton J: Dopami-nergic deficiency and delayed visual evoked potentials in humans. Ann Neurol 11:478–483, 1982. 106. Yasui M: Electrophysiological study on Wilson's disease-changes of visual evoked
potential and EEG by D-penicillamine treatment. Wakayama Med Rep 21:49–55, 1978. 107. Hamois C, Di Paolo T, Marcotte G, Daigle M: Retinal dopamine content in parkinsonian patients. Invest Ophthalmol Vis Sci 29:200, 1988. 108. Paranhos FRL, Katsumi O, Arai M, Nehemy MB, Hirose T: Pattern reversal visual evoked response in retinitis pigmentosa. Doc Ophthalmol 96:321–331, 1999. 109. Bass SJ, Sherman HJ, Bodis-Wollner et al: Visual evoked potentials in macular disease. Invest Ophthalmol Vis Sci 26:1071–1074, 1985. 110. Celesia GG, Kaufman D: Pattern ERGs and visual evoked potentials in maculopathies and optic nerve
diseases. Invest Ophthalmol Vis Sci 26:726–735, 1985. 111. Lennerstrand G: Delayed visual evoked cortical potentials in retinal disease. Acta Ophthalmol 60:497–504, 1982. 112. Harding GFA: The use of the visual evoked potential to flash stimuli in
the diagnosis of visual defects. In Desmedt JT (ed): Visual Evoked Potentials
in Man: New Developments. Oxford, Clarendon, 1977, pp 500–508. 113. Folk JC, Thompson HS, Han DP, Brown CK: Visual function abnormalities in central serous retinopathy. Arch Ophthalmol 102:1299–1302, 1984. 114. Papakostopoulos D, Dean Hat C, Cooper C, Natsikos V: Combined electrophysiological assessment of the visual system in central
serous retinopathy. Electroencephalogr Clin Neurophysiol 59:77–82, 1984. 115. Sherman J, Bass J, Noble KG et al: Visual evoked potential (VEP) delays in central serous choroidopathy. Invest Ophthalmol Vis Sci 27:214–221, 1986. 116. Puvanendran K, Devathasan G, Wong PK: Visual evoked response in diabetes. J Neurol Neurosurg Psychiatry 46:643–649, 1983. 117. Babel J, Stangos N, Korol S et al: Ocular Electrophysiology: A Clinical
and Experimental Study of Electroretinogram, Electro-oculogram and Visual
Evoked Response. Stuttgart, Georg Thieme, 1977. 118. Bird TD, Griep E: Pattern reversal visual evoked potentials. Studies in Charcot-Marie-Tooth
hereditary neuropathy. Arch Neurol 38:739–741, 1981. 119. Rossini PM, Prichin M, Treviso M et al: Checkerboard reversal pattern and flash VEPs in dialysed and non-dialysed
subjects. Electroencephalogr Clin Neurophysiol 52:435–444, 1985. 120. Cohen SN, Syndulko K, Rever B et al: Visual evoked potentials and long latency event-related potentials in chronic
renal failure. Neurology 33:1219–1222, 1983. 121. Fenwick PBC, Stone SA, Bushman J, Enderby D: Changes in the pattern reversal visual evoked potential as a function of
inspired nitrous oxide concentration. Electroencephalogr Clin Neurophysiol 57:178–183, 1984. 122. Yaar I, Ron E, Modan M et al: Long-term cerebral effects of small doses of X-irradiation in childhood
as manifested in adult visual evoked responses. Ann Neurol 8:261–268, 1980. 123. Carroll WM, Kriss A, Baraitser M et al. The incidence and nature of visual pathway involvement in Friedrich's
ataxia: A clinical and visual evoked potential study of 22 patients. Brain 103:413–434, 1980. 124. Comi G, Medaglini S, Locatelli T et al: Multimodal evoked potentials in
acquired immunodeficiency syndrome. In: Barber C, Blum T (eds): Evoked
Potentials III. Boston, Butterworth, 1987, pp 408–412. 125. Kirkham TH, Coupand SG: An ERG and VEP study in Friedreich's ataxia. Can J Neurol Sci 8:289–293, 1981. 126. Livingstone IR, Mastaglia FL, Edis R, Howe JW: Visual involvement in Friedreich's ataxia and hereditary spastic ataxia. Arch Neurol 38:75–84, 1981. 127. Lowitzsch K, Westoff M: Optic nerve involvement in neurosyphilis: Diagnostic evaluation by pattern
reversal visual evoked potentials (VEP). EEG-EMG. 11:77–87, 1980. 128. Oepen G, Doerr M, Thoden U: Visual (VEP) and somatosensory (SSEP) evoked potentials in Huntington's
chorea. Electroencephalogr Clin Neurophysiol 51:666–675, 1981. 129. Hennerici M, Wenzel D, Freund HJ: The comparison of small-size rectangle and checkerboard stimulation for
the evaluation of delayed visual evoked responses in patients suspected
of multiple sclerosis. Brain 100:119–136, 1977. 130. Regan D, Whitlock JA, Murray TJ, Beverly KI: Orientation-specific losses of contrast sensitivity in multiple sclerosis. Invest Ophthalmol Vis Sci 25:324–328, 1980. 131. Livingston IR, Mastaglia FL, Edis R, Howe JW: Visual involvement in Friedreich's ataxia and hereditary spastic ataxia. Arch Neurol 38:75–79, 1981. 132. Bird TD, Crill WE: Pattern-reversal visual evoked potentials in the hereditary ataxias and
spinal degenerations. Ann Neurol 9:243–250, 1981. 133. Harden A, Pampiglione G: Neurophysiological studies (EEG/ERG/VEP/SEP) in 88 children
with so-called neuronal ceroid lipofuscinosis. In: Armstrong
A, Kopang N, Rider JA (eds): Ceroid-Lipofuscinosis (Batten's
Disease). New York, Elsevier Biomedical Press, 1982, pp 61–69. 134. Gottlob I: Neuronal ceroid lipofuscinosis. In: Heckenlively JR, Arden GB (eds): Principles
and Practice of Clinical Electrophysiology of Vision. Mosby, St. Louis, 1991, pp 786–792. 135. Byngartner J, Epstein CM: Voluntary alteration of visual evoked potentials. Ann Neurol 12:475–478, 1982. 136. Lovasik JV, Spafford M, Szymkiw M: Modification of pattern reversal VERs
by ocular accommodation. Vision Res 25:599–608, 1985. Üc 137. Ellington RJ: Variability of visual evoked responses in the human newborn. Electroencephalogr Clin Neurophysiol 29:10–14, 1970. 138. Barnet AB, Friedman SL, Weiss LP et al: VEP development in infancy and early childhood. A longitudinal study. Electroencephalogr Clin Neurophysiol 49:476–489, 1980. 139. Blom JL, Barth PG, Visser SL: The visual evoked potential in the first six years of life. Electroencephalogr Clin Neurophysiol 48:395–405, 1980. 140. Moskowitz A, Sokol S: Developmental changes in the human visual system as reflected by the latency
of the pattern reversal VEP. Electroencephalogr Clin Neurophysiol 56:1–15, 1983. 141. Fenwick PBC, Brown D, Hennesey J: The visual evoked response to pattern reversal in ‘normal’ 6–11 year
old children. Electroencephalogr Clin Neurophysiol 51:49–62, 1981. 142. Sokol S: Measurement of infant visual acuity from pattern reversal evoked potentials. Vision Res 18:33–39, 1978. 143. Spekreijse H: Maturation of contrast EPs and development of visual resolution. Arch Ital Biol 116:358–369, 1979. 144. Tyler CW, Apkarian P, Levi DM, Nakayama K: Rapid assessment of visual function: An electronic sweep technique for
the pattern VEP. Invest Ophthalmol Vis Sci 18:703–713, 1979. 145. Norcia AM, Tyler CW: Spatial frequency sweep VEP: Visual acuity in the first year of life. Vision Res 25:1399–1408, 1985. 146. Norcia AM, Tyler CW, Hamer RD: High contrast sensitivity in the young human infant. Vision Res 29:44–49, 1988. 147. Perry NW, Childers DG, McCoy JG: Binocular addition of the visual evoked response at different cortical
locations. Vision Res 8:567–573, 1968. 148. Ellenberger C, Shufflesworth DE: Electrical correlates of normal binocular vision. Arch Neurol 35:834–837, 1978. 149. Jakobson P, Lennerstrand G: Binocular interaction in the VEP to grating stimulation II. Spatial frequency
effects. Acta Ophthalmol (Copenh) 63:290–296, 1985. 150. Amigo G, Fiorentini A, Prichio M, Spinelli D: Binocular visin tested with visual evoked potentials in children and infants. Invest Ophthalmol Vis Res 17:910–915, 1978. 151. Srebro R: The visually evoked response. Arch Ophthalmol 96:839–844, 1978. 152. Harter MR, Seiple WH, Salmon L: Binocular summation of visual evoked responses to pattern stimuli in humans. Vision Res 13:1433–1446, 1973. 153. Spekreijse H, Van Der Twell LH, Regan D: Interocular sustained suppression: Correlations with evoked potential amplitude
and distribution. Vision Res 12:521–524, 1972. 154. Tyler CW, Apkarian PA: Effects of contrast, orientation, and binocularity in the pattern evoked
potential. Vision Res 25:755–766, 1985. 155. Apkarian A, Nakayama K, Tyler CW: Binocularity in the human visual evoked potential: Facilitation, summation
and suppression. Electroencephalogr Clin Neurophysiol 51:32–48, 1981. 156. Lennerstrand G, Jakobsson P: Visual evoked potentials to binocular stimulation with disparate patterns. Acta Ophthalmol (Copenh) 60:373–385, 1982. 157. Bodis-Wollner I, Barris MC, Mylin LH et al: Binocular stimulation reveals cortical components of the human visual evoked
potential. Electroencephalogr Clin Neurophysiol 52:298–305, 1981. 158. Braddick O, Atkinson J, Julesz B et al: Cortical binocularity in infants. Nature 288:363–365, 1980. 159. Salier JP, Gnust JM, Pandey JP, Fudenberg HH: Development of stereopsis and cortical binocularity in human infants: Electrophysiological
evidence. Science 213:213–215, 1981. 160. Lehmann D, Skrandies W, Lindenmaier C: Sustained cortical potentials evoked in humans by binocularly correlated, uncorrelated
and disparate dynamic random-dot stimuli. Neurosci Lett 10:129–130, 1978. 161. Glass JD, Crowder JV, Kennerdell JS, Merikangas JR: Visually evoked potentials from occipital and precentral cortex in visually
deprived humans. Electroencephalogr Clin Neurophysiol 43:207–217, 1977. 162. Arden GB, Barnard WM: Effects of occlusion on the visual evoked response in amblyopia. Trans OphthalmolSoc UK 99:419–426, 1979. 163. Sokol S: Abnormal evoked potential latencies in amblyopia. Br J Ophthalmol 67:310–314, 1983. 164. Fiorentini A, Maffei L, Prichio M, Spinelli D: An electrophysiological correlate of perceptual suppression in anisometropia. Vision Res 18:1617–1621, 1978. 165. Wanger P, Persson HE: Visual evoked responses to pattern-reversal stimulation in childhood amblyopia. Acta Ophthalmol (Copenh) 58:697–706, 1980. 166. Gittinger JW, Sokol S: The visual evoked potential in the diagnosis of congenital ocular motor
apraxia. Am J Ophthalmol 93:700–703, 1982. 167. Wright CE, Williams DE, Drasdo N, Harding GFA: The influence of age on the electroretinogram and visual evoked potential. Doc Ophthalmol 59:365–384, 1985. 168. Shearer DE, Dustman RE: The pattern reversal evoked potential: The need for laboratory norms. Am J Electroencephalogr Technol 20:185–200, 1980. 169. Celesia GG, Daly RF: Effects of aging on visual evoked responses. Arch Neurol 34:403–407, 1977. 170. Shaw NA, Cant BR: Age-dependent changes in the amplitude of the pattern visual evoked potential. Electroencephalogr Clin Neurophysiol 51:671–673, 1981. 171. Adachi-Usami E: Aging and pattern visual evoked cortical potential. In: Heckenlively
JR, Arden GB (eds): Principles and Practice of Clinical
Electrophysiology of Vision. Mosby, St. Louis, 1991, pp 417–424. 172. Harding GFA: Technical issues in visual evoked potential recording. In: Heckenlively
JR, Arden GB (eds): Principles and Practice of Clinical
Electrophysiology of Vision. Mosby, St. Louis, 1991, pp 435–441. 173. Apkarian P: Methodology of testing for albinism with visual evoked cortical
potentials. In: Heckenlively JR, Arden GB (eds): Principles and Practice
of Clinical Electrophysiology of Vision. Mosby, St. Louis, 1991, pp 425–434. 174. Apkarian P: Albinism. In: Heckenlively JR, Arden GB (eds): Principles and
Practice of Clinical Electrophysiology of Vision. Mosby, St. Louis, 1991, pp 773–782. |