CLASSIFICATION
The earliest classification of poxviruses was based on disease symptoms and gross pathological manifestations. Later criteria included morphological characterization of virions and staining patterns of cytoplasmic inclusion bodies in infected cells. The poxvirus family is divided into two subfamilies: Entomopoxvirinae (poxviruses of insects) and Chordopoxvirinae (poxviruses of vertebrates). The Chordopoxvirinae are further classified into genera through studies of cross-protection in animals and cross-neutralization in tissue culture. Species of the same genera cross-protect or cross-neutralize each other and share similar biological properties. Current taxonomic classification is based on viral genomic DNA sequences. Vaccinia virus is the prototypic member of the orthopoxvirus genus along with closely related cowpox, ectromelia, monkeypox, and variola (smallpox) viruses.
VIRION MORPHOLOGY
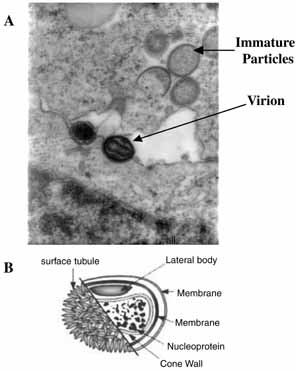
Poxviruses are the largest of all animal viruses and can just be visualized by light microscopy. High-resolution electron microscopy shows virions to be oval or brick-shaped structures 200 to 400 nm in length, with axial ratios of 1.2 to 1.7 (Fig. 1A). Images of electron microscopic thin sections show the surface membrane to enclose a biconcave or cylindrical core that contains genomic DNA.
The vaccinial genome comprises a linear molecule of double-stranded DNA with covalently closed termini. Terminal hairpins constitute two isomeric, imperfectly paired, “flip-flop” DNA forms consisting of inverted complementary sequences. Variably sized, tandem repeat sequence arrays are present near the ends. Each virion contains one DNA molecule associated with proteins and organized in a nucleoprotein core. One or two lateral bodies are present in the concave region between the core wall and a single membrane, which contains a number of virus-encoded proteins. This virion form is known as the intracellular mature virus (Fig. 1B).
REPLICATION CYCLE
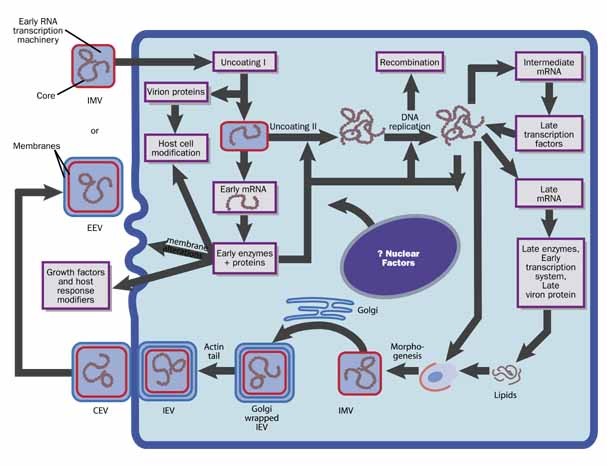
The intracellular replication cycle of vaccinia virus involves a sequence of events (Fig. 2).5 The vaccinia virion containing early RNA transcription machinery attaches to and fuses with the plasma membrane. Within 15 minutes the virion transcription machinery is activated (uncoating I). Early genes are expressed that code for a variety of functions that modify the host cell for optimal virus replication, attenuate the host response to infection, and mediate viral synthetic processes. After further uncoating (uncoating II), between 1.5 to 6 hours postinfection, the viral genome is replicated via concatamers.
|
From progeny DNA templates, late transcription factors are expressed from intermediate genes, and late gene RNA is synthesized. Late genes encode the early transcription system, enzymes, and structural proteins necessary for virion assembly. By 4 hours postinfection, virion morphogenesis commences with the formation of membrane structures in the intermediate compartment and the packaging of resolved unit-length genomic DNA. The intracellular mature virus (IMV) has one membrane derived from the intermediate compartment. Some IMVs acquire an additional double layer of intracellular membrane derived from the trans Golgi network that contains unique virus proteins, the intracellular enveloped virus (IEV).
IEVs are transported to the periphery of the cell where fusion with the plasma membrane ultimately results in release of extracellular enveloped virus (EEV) or, if attached to the exterior surface of the plasma membrane, as cell-associated enveloped virus (CEV). While IMV and CEV/EEV are infectious, the external antigens on the two virion forms are different. Thus, each virion type probably binds to different cellular receptors and are likely uncoated by different mechanisms. EEVs are thought to be the most important form involved in cell-to-cell spread and systemic disease.