EPIDEMIOLOGY
Explosive epidemics of AHC were initially reported in West Africa and Indonesia in 1969 with subsequent pandemic spread.3 A causative agent of AHC was first recovered by Kono and coworkers4 from conjunctival scrapings of patients with AHC. The isolate was identified as a new type of enterovirus when the first outbreak of AHC in Japan occurred, during the worldwide pandemic of 1969 to 1972. The first pandemic of AHC was caused by EV70 in Africa, Southeast Asia (including Singapore and Hong Kong), Japan, and India in 1969 to 1971, with several million cases. In 1970, a year before EV70 was found, Yin-Murphy5 reported a large outbreak of epidemic conjunctivitis in Singapore, where she isolated a new enterovirus from the conjunctival scrapings of patients. The virus was identified as the causative agent of the disease, which was called Singapore epidemic conjunctivitis by Lin and Yin-Murphy.6 The virus was later renamed CA24v.7
Epidemics of this disease recurred during the last quarter of the 20th century. Both viruses cause the same disease, according to viral isolation, serologic investigation, and the reverse transcription-polymerase chain reaction (RT-PCR) method. AHC spread throughout Southeast Asia and first occurred outside Asia in American Samoa in 1986, where nearly half the population was affected.8 After a sporadic outbreak in French Polynesia in 1982, EV70 was introduced into the Western hemisphere, including the United States.9 CA24v was responsible for the epidemics of 197510 and 1985.11,12
There is a noticeable difference in the geographic distribution of these two viruses. EV70 has widely spread to various parts of the world from its original focus in West Africa, and it has so far caused two major pandemics in 1969 to 1972 and 1980 to 1982. AHC caused by CA24v first appeared in Singapore and Malaysia in 1970, and since then, AHC epidemics have been intermittently observed only in Southeast and East Asia and India.
CLINICAL CHARACTERISTICS
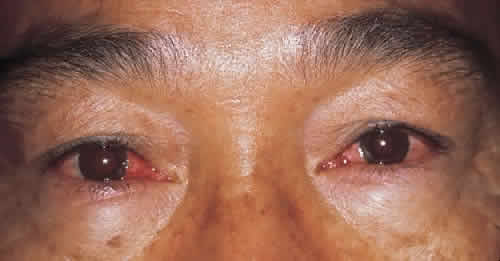
The onset is sudden and alarming. After beginning with one eye, the other often becomes inflamed in a matter of hours. Most patients develop signs in the second eye within 1 to 3 days (Fig. 1). Pain accompanies the other symptoms.
|
The most common symptoms are a profuse and predominantly watery discharge, a foreign body sensation, burning, photophobia, swelling of the eyelids, and pain in the upper eyelid that is aggravated on bending over. Although patients often complained of coryza, fewer than 5% showed systemic manifestations, such as fever or malaise. Ocular symptoms are often usually severe enough, however, to disrupt routine activities for several days.
Signs of AHC caused by EV70 and CA24v are similar, and it is impossible to determine the causative virus from clinical characteristics. Edematous swelling of the lid occurs in 72% to 100% of patients, although severity varies. Swelling usually subsides within 3 or 4 days. Preauricular lymph nodes become enlarged and palpable in 65% of patients, and approximately 80% are tender.13 The upper conjunctivae become inflamed from a mild to a severe degree, with injection, infiltration, and follicle formation. In moderately inflamed eyes, the fornix is reddened and cloudy, obscuring blood vessels.
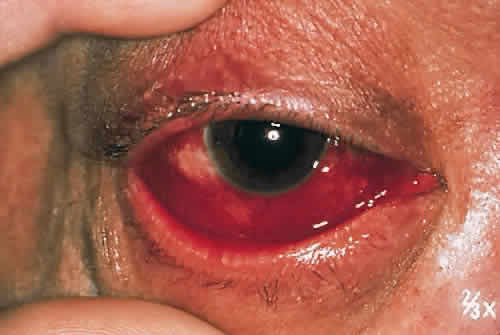
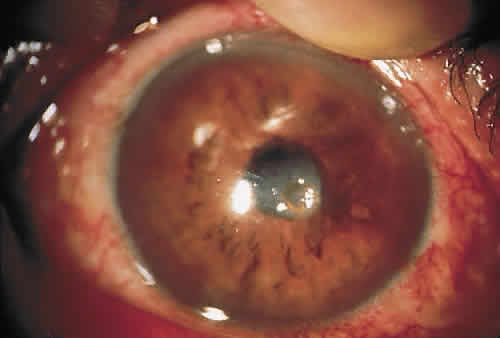
The most characteristic finding is marked hemorrhagic involvement of the bulbar conjunctival and subconjunctival layers. Punctate hemorrhages often appear within a few hours of onset of symptoms and rapidly coalesce to form larger hemorrhagic patches (Fig. 2). These patches tend to form ridges concentric with the corneoscleral limbus and occasionally become confluent (Fig. 3). Although 40% of patients have with unilateral involvement, more than 90% have bilateral disease when examined the next day.
|
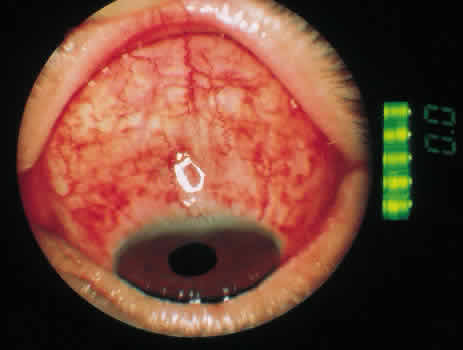
Corneal complications are generally rare. The incidence of epithelial keratitis ranges from 0% to 30% (Fig. 4). Extraocular symptoms and signs are usually mild and resolve without sequelae.
|
Most symptoms resolve by the fourth day of illness, as do most physical findings. The most persistent signs are conjunctival hemorrhages and follicles, which may aid physicians in making the diagnosis of AHC when patients present during the convalescent phase of disease.