NEMATHELMINTHES: NEMATODES (ROUNDWORMS)
Toxocariasis
Toxocara canis is the round worm of dogs. The normal life cycle of Toxocara canis includes the ingestion of the larval stages of the organism by an adult dog. The eggs contain first-stage larvae, whereas second-stage larvae are found in the intestinal wall and tissues of infected animals. Third-stage larvae can be found in the lung, and fourth-stage larvae can be found in the feces of infected hosts. Second-stage larvae penetrate the intestinal wall and enter the systemic circulation of the infected dog. The larvae may reach arterioles of various organs, causing localized granuloma. During pregnancy, the encapsulated larvae in the infected bitch are released and the larvae spread, reaching the liver of the fetus via the placenta. The newborn pups may also be infected by the larvae that are passed in the milk of the bitch. The larvae migrate to the lung of the pup, reaching the third stage when they are coughed, swallowed, and then passed to the small intestines, where they become fourth-stage larvae. They mature in the intestines in 3 weeks and begin to shed eggs in large numbers in the feces for the remainder of their lifetime. Man is an incidental host for Toxocara species.
Larvae cannot come to maturity, but once they reach the circulation, they can lead to forms of parasites of infection, either (1) systemic or visceral larva migrans, or (2) ocular larva migrans.49 The systemic form of the disease occurs at age 2 to 3 years and in children with history of eating soil (pica). Children may develop cough, hepatosplenomegaly, lymphadenopathy, and eosinophilia51; ocular lesions in this form of the infection are rare.49 Ocular larvae migrans occurs at age 3 to 40 years. Patients rarely have a history of pica, and there are no systemic manifestations, such as hepatosplenomegaly or lymphadenopathy. Patients with ocular larva migrans have no fever and have mild or absent eosinophilia. The Toxocara antibody titer is usually low or negative.
The diagnosis of toxocariasis is made by clinical findings of disease and by performing serologic testing. ELISA appears to be a sensitive serologic test and can be performed on ocular fluid specimens.
The treatment of visceral larva migrans includes the use of diethylcarbamazine, thiabendazole, and mebendazole. In cases of ocular larva migrans, steroids may be used to prevent inflammatory reactions after the death of the organism. Vitrectomy is helpful in the repair of retinal detachment, which may develop in cases of ocular toxocariasis. Laser photocoagulation has been used in a few cases with varying degree of success.
Ascariasis
Ascariasis is caused by the roundworm Ascaris lumbricoides. The disease is common, but ocular complications are rare. Ectopic migration of the adult worm may occur. Kaplan and associates52 reported on an intact adult Ascaris worm that was extracted from the lacrimal duct of an 18-month-old African girl. Roche53 reported on a similar case in which an ectopic Ascaris worm was found in the lacrimal duct. Ascaris larvae do not develop in the eye, and larval granuloma do not occur.
Baylisascariasis
Baylisascariasis is caused by tissue invasion by the larvae of Baylisascaris procyonis, which are roundworms transmitted to humans by the raccoon. The adults of this young worm are long; males can measure 12 cm and females 23 cm. The larvae of this parasite are large and may reach 1000 to 2000 μm in length. They do little damage to their raccoon hosts, who become infected by eating the egg-containing larvae or a tissue of an intermediate host infected with the parasite, such as rodents and rabbits. Millions of eggs resembling Toxocara are shed daily and they can survive for many years. Humans are incidental hosts of this parasite and may acquire the disease by ingesting the eggs or drinking contaminated water. Children who keep raccoons as pets may be at risk. Ocular infection with the larvae of Baylisascaris procyonis may occur.
Onchocerciasis
Onchocerciasis is a disease caused by the nematode Onchocerca volvulus. The disease is known to afflict approximately 30 million people living in Africa and Central and South America. Humans are the only definitive host for O. volvulus. Other related species of Onchocerca may produce a similar disease in domesticated animals. Onchocerca cervicalis produces a disease in the horse similar to that found in man.54 Furthermore, onchocerciasis has been produced in experimental animals. Onchocerca lienalis may produce onchocerciasis in the guinea pig under experimental conditions.55 In humans, the adult worm of O. volvulus is found in the subcutaneous tissues, where it becomes coiled and gets encapsulated in fibrous tissues. Such tumors may contain adult worms and microfilariae. Microfilariae are present in skin nodules, subcutaneous tissue, and the skin. The adult worm gets encapsulated and enmeshed in a protective coating of the host's fibrous tissue, and the manner by which it derives its nutrition is not known. Most of the signs and symptoms of onchocerciasis are due to the presence of microfilariae in the tissues. The microfilariae may have a tendency to spread systemically and, in untreated patients, are found in the bloodstream. The main route into the eye, however, is from the skin of the face through the conjunctiva or cornea or by way of the bloodstream.56 The microfilariae possess a certain enzyme activity that allows it to penetrate through collagenous connective tissue, such as the cornea.
The principal intermediate hosts of O. volvulus are many species of the black fly Simulium. Microfilariae are picked up from the skin by the black fly; the metamorphosis to an infective larva takes place in 6 to 10 days in the thoracic muscles. The mature larva migrates to the proboscis of the black fly, and when the infected black fly bites, the larva escapes to the skin of a new host and penetrates the wound. The larva grows in the host to become an adult worm in approximately 1 year. A heavily infected mother with microfilaria parasitemia may pass the disease to her fetus through direct transplacental microfilarial transmission. The possibility, however, has not been confirmed. It has been estimated that heavily infected patients may harbor between 50 and 200 million microfilariae in the skin at any one time. In Africa, the infection is common in regions of rapidly flowing small streams, where Simulium black flies breed. The prevalence of the disease decreases markedly beyond a distance of 5 miles from the stream. This is due to the fact that the distribution of the insect vector rarely goes beyond 2 to 3 miles from the water sources. In their daily biting cycle, female flies bite from dawn to dusk, usually outdoors and on the lower extremities.
Onchocerciasis is an unusual parasitic disease in humans. Because the most significant pathologic changes are due to the microfilarial stages of the parasite, the severe manifestations of the disease may take many years to develop. In many other parasitic infections due to nematodes, the parasite does not multiply in the body. Each microfilaria measures approximately 270 to 320 μm in length. The organism can be identified in tissue sections. Skin biopsy specimens of suspected areas help in establishing the diagnosis. The snip biopsy specimens of the skin can be examined for living microfilariae, either in water or in saline. The number of microfilariae can be expressed quantitatively.
In onchocerciasis, blinding lesions may affect the anterior and posterior segments, causing sclerosing keratitis, anterior uveitis, optic atrophy, and chorioretinopathy. The pathogenesis of chorioretinopathy remains to be elucidated. Several possible mechanisms have been suggested, including inflammatory reactions to dead microfilariae, eosinophil-derived toxic proteins, immune complex disease, secretory products from the microfilariae, and autoimmunity.57–62 Many authors have supported the hypothesis that autoimmune reactions may play a role in the pathogenesis of chorioretinopathy in onchocerciasis. Recently, a recombinant antigen (designated Ov39) was isolated from an adult female O. volvulus cDNA library that demonstrated immunologic cross-reactivity with a 44-kilodalton component of the retinal pigment epithelium and other ocular tissues.63,64 Cooper and associates64a studied patients with onchocercal chorioretinopathy and determined whether they had enhanced lymphoproliferative response to Ov39 compared with those without chorioretinal disease. The lymphoproliferative responses to Ov39 were not found to be enhanced in patients with onchocercal chorioretinopathy. The role of Ov39-specific cellular autoreactivity in the pathogenesis of onchocercal chorioretinopathy could not be demonstrated, and the theory of molecular mimicry as a cause in the induction of autoimmunity could not be proved.
There are several laboratory tests to determine the diagnosis of onchocerciasis. The immunologic tests that are available include skin tests, gel diffusion tests, passive hemagglutination tests, complement fixation tests, immunofluorescent antibody tests, and ELISAs. The Mazzoti test is a useful aid in the diagnosis of onchocerciasis. This test consists of giving a small dose (50 mg) of diethylcarbamazine to an adult patient and then observing the development of the clinical reaction in the skin. The reaction consists of the development of discrete papular eruptions within 15 minutes after the ingestion of diethylcarbamazine.65–67 The test is usually read at 3 hours and again at 24 hours.
Clinical studies have demonstrated the efficacy of ivermectin in onchocerciasis.67,68 Ivermectin is given as a single oral dose of 150 μg/kg of body weight once or twice a year. It should not be given to children younger than 5 years of age or to those weighing less than 15 kg; to pregnant women; or to nursing mothers in the infant's first week of life.
Trichinosis
Trichinosis is a disease caused by Trichinella spiralis. The disease is common, though generally subclinical, and the infection affects certain areas of Central and North America and central Europe. The disease is perpetuated by the habit of eating undercooked pork. The larvae of Trichinella are produced by the adult worm in the gut wall, where they burrow in gaining access to the lymphatics and reaching the circulation. The larvae are carried to all parts of the body; they penetrate striated muscle with their spear-shaped apparatus and eventually become encysted and calcified. The orbicularis muscle is frequently affected, causing periorbital edema and conjunctivitis. In rare cases, papilledema and retinal hemorrhages may occur.
Loiasis
Loiasis is a parasitic infection in humans caused by the roundworm Loa loa. The adult worm is a threadlike, cylindrical parasite that has the ability to inhabit the ocular adnexa and the subconjunctival tissue. It may live in humans for up to 17 years, and the intermediate hosts and vectors are members of the Chrysops genus. The disease is restricted to Africa. Loa loa is called the eye worm, but it is primarily a cutaneous pathogen. The microfilariae are blood borne and may in rare cases invade the uveal tissue and the optic nerve, as well as many other organs in the body.
The parasite is known for its occasional presentation as an adult worm under the bulbar conjunctiva. Patients may complain of irritation and foreign-body sensation as the worm creeps in the periocular tissues. Temporary inflammatory reactions may cause swelling of the eyelid (Calabar swelling), which is a characteristic finding in patients with Loa loa infections. Loiasis causes vague symptoms of low-grade fever, myalgia, and paresthesia. The adult worm can migrate under the conjunctiva, and involvement of the eye causes irritation, hyperemia, periorbital swelling, congestion, pain, photophobia, and decreased vision.
Loiasis is diagnosed by detecting eosinophilia, and by demonstrating microfilariae in peripheral blood films taken during the day; microfilariae may be found in 20% to 30% of patients.
Treatment is accomplished by surgical removal of the adult filarial worms wherever accessible. The best time to remove the worms is when they appear under the conjunctiva. The use of cocaine or other local anesthetics may cause immobilization of the worm and allow surgical removal. Cryoprobe extraction may be successful in removing a subconjunctival Loa loa. Chemotherapy with diethylcarbamazine may also be effective.
The concomitant use of antihistamines or corticosteroids may be indicated in patients who have allergic reactions after the use of diethylcarbamazine. Prednisone may be given simultaneously with oral diethylcarbamazine. This proves to be efficacious in moderating the adverse reaction to diethylcarbamazine.
Wuchereriasis
Wuchereria bancrofti is another cause of systemic filariasis. The parasite rarely leads to ocular complications. Retinal involvement with hemorrhages have been reported.
Thelaziasis
Thelaziasis is a zoonotic eye infection that occurs in dogs, cattle, sheep, horses, deer, and other animals; it is caused by a nematode of the genus Thelazia. The eye worm has been reported in western North America and the Far East. The adult worms live and lay their eggs in the lacrimal ducts and conjunctival sacs of the host animals. The worms and/or hatched first-stage larvae are ingested by flies of the genera Musca and, less commonly, Fannia, as they feed on the host animal's eye secretions. The first-stage larvae migrate from the intestine of the fly to its ovarian follicles, where they molt twice and develop into the infective third-stage larvae, eventually working their way into the proboscis of the fly. When the fly feeds on the eye secretions of an uninfected animal, the infective larvae are released into the lacrimal fluid, where they molt into fourth-stage larvae. They then migrate to the lacrimal ducts, where they develop to adulthood. Humans rarely serve as incidental hosts. Human thelaziasis is caused by Thelazia californiensis in the United States69–71; Thelazia callipaeda (also known as Filaria lacrimalis) in India,72 Bangladesh,73 Korea,74 Indonesia,75 Japan,76 Thailand,77 China,78,79 and Russia80; and Habronema in Australia.81 The threadlike eye worms have a creamy-white cuticular covering with closely packed transverse striations.74 Females measure up to 1.7 cm in length; males are somewhat smaller. In reported cases of thelaziasis in humans, the worms are usually located in the conjunctival cul-de-sac, but rarely in the anterior chamber.73
Diagnosis of thelaziasis is based on observation of the worms, and their identification after removal. Treatment consists of removing the surface worms with forceps under topical anesthesia. Worms located more deeply are removed surgically.81 Small worms may be missed initially, and may cause recurrence of symptoms as they grow. Ivermectin has been used successfully in the treatment of thelaziasis in cattle.82
Dirofilariasis
Dirofilariasis is a zoonotic disease among raccoons, dogs, and cats, which is accidentally transmitted to humans presumably by the same vector arthropods that infect their usual animal hosts, namely Culex and Aedes mosquitoes. Filariid worms of the genus Dirofilaria are the causative agents of this disease, of which three species have been identified in humans: Dirofilaria tennius, parasitic in raccoons, which is the most common species acquired in North America; Dirofilaria immitis, the heartworm of dogs; and Dirofilaria repens, which is normally found in the subcutaneous tissues of cats and dogs in Asia, Europe, and South America.
Dirofilaria conjunctivae, so named because of the tendency of the parasite to locate near the eye, does not show any significant taxonomic differences from Dirofilaria repens.83,84 Taxonomic identification of worms belonging to the genus Dirofilaria is based on cuticular morphology,85 anatomic location, and geographic considerations.
Humans are a suboptimal host: that is, the parasite dies before producing microfilariae. Immature worms, unmated adult worms, and sexually mature worms containing microfilariae have been detected in humans, but microfilariae have never been found in human blood. The female worm is approximately 155 mm long and the male approximately 85 mm long. Most reports show a well-encapsulated, nonviable parasite in subcutaneous nodules or in cardiopulmonary “coin” lesions and infarcts. For reasons that are unclear, infection in children is rare. The ocular form of dirofilariasis in man is cosmopolitan, with cases reported sporadically from Australia,86 Asia,87–91 Europe,92–94 Africa,95 and the Americas.96–98 In reviewing the cases of dirofilarial ophthalmic infections in various parts of the world, it is apparent that common sites of involvement, listed from most to least common, are the subcutaneous tissues of the eyelids and periorbital region,94,96,97,99–109 conjunctiva,87,93,95,102–117 orbit,88,93,94,106,118–120 vitreous,121–125 anterior chamber,86,98,126 and sclera. Presenting symptoms include eye irritation, ocular pain, itching and redness, and diplopia. Detection of the viable parasite itself is perhaps the clearest indication of dirofilariasis, although it is more common to find a well-encapsulated, nonviable parasite causing orbital proptosis and symptoms of an orbital tumor. Diagnosis is based on serologic testing. A fluorescent antibody test is used that can differentiate Dirofilaria from Toxocara and Ascaris infections, and a recently developed diagnostic ELISA technique is highly specific.127
Local excision of the mass of encapsulated parasites or surgical removal of the viable worms is adequate treatment. If secondary infection of the orbit is suspected, treatment with intravenous penicillin and/or corticosteroids is advisable.
Dracunculiasis
Dracunculiasis, also known as dracunculosis or dracontiasis, is caused by the presence of the guinea worm Dracunculus medinensis in the deep connective and subcutaneous tissues of humans. The adult worms are elongated nematodes. The females can measure up to 120 cm in length and approximately 2 mm in diameter; however, the male is less than 4 cm in length. The gravid female, whose body is almost completely filled by its larvae-distended uterus, approaches the human skin surface, usually in the distal portions of the lower extremities. The adult worms provoke the formation of burning blisters that finally ulcerate. If the affected part comes in contact with water, a loop of the worm's uterus prolapses through its body wall and ruptures, liberating large numbers of first-stage larvae. These are ingested by copepods of the genera Cyclops, Mesocyclops, Tropocyclops, and Thermocyclops, in which they undergo two molts to reach the infective third-stage larvae. If these copepods are swallowed in drinking water, the contained infective larvae are released and migrate to subcutaneous tissue, where they undergo two further molts before maturation. After mating, the male worms die and become encysted, while the fertilized females move to the extremities and emerge through the skin about a year after infection.
Dracunculiasis is highly endemic in poverty-stricken areas, particularly rural communities lacking any form of public drinking water supply. It tends to be seen in India, western and central Africa, and southwestern Asia.
A few cases128–130 of ocular dracunculiasis have been reported in the literature over the past five decades. In these cases, the worms were found behind the orbital septum128 in the subconjunctival space,129 in an intraocular abscess, and in a cystic mass of the upper lid.130 Retrobulbar invasion results in proptosis and loss of the eye, whereas more superficial involvement causes intense edema of the soft tissue of the eyelid and conjunctiva. Diagnosis is based on demonstration of the Dracunculus female in surgical specimens.
Treatment consists of surgical removal of the worms. Supportive drug treatment includes oral administration of niridazole, thiabendazole, metronidazole,131 and mebendazole.132
PLATYHELMINTHES: CESTODES (TAPEWORMS)
Echinococcosis
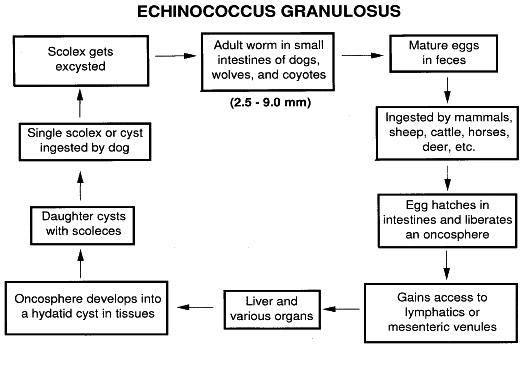
Echinococcosis is caused by Echinococcus granulosus. The adult worm lives in the intestines of dogs and humans, who are intermediate hosts for the parasite (Fig. 1). The adult worm is small, measuring 2.5 to 9 mm in length. It does not harm the canine host. When eggs are ingested by humans, they liberate larvae that may reach the tissues and undergo central vesiculation, forming a cyst. Intraocular hydatid cysts are rare but have been reported. More commonly, orbital hydatid cysts may cause proptosis and restriction of ocular movements.133,134
Cysticercosis
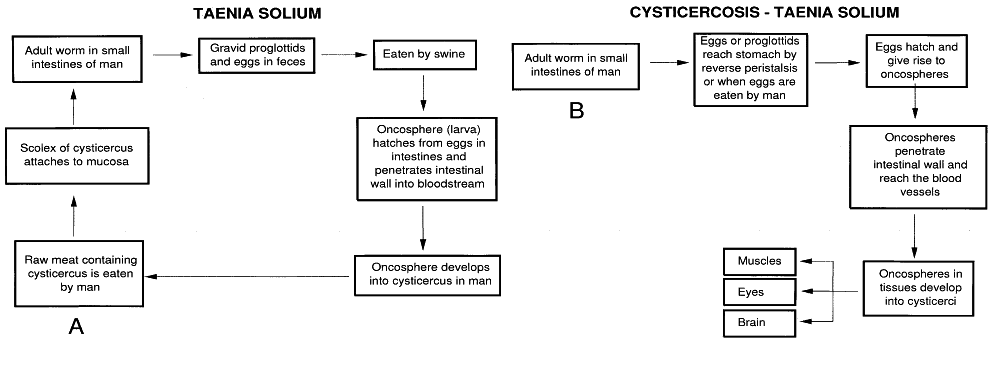
Cysticercosis is a common cause of serious morbidity in Mexico and Central American countries. It is caused by the eggs of the adult pork tapeworm Taenia solium, which are either ingested or released by reverse peristalsis in cases of intestinal obstruction (Fig. 2A and B). Eggs mature, and the larvae penetrate the intestinal mucosa, reaching the retinal circulation. The larva of Taenia solium (Cysticercus cellulosae) is the most common tapeworm that invades the human eye. The larvae (cysticerci) may cause localized abscesses of the eyelids135–137 and conjunctiva.138 A subconjunctival abscess may form secondary to the larva of Taenia solium (Cysticercus).139
|
The Cysticercus larvae may reach the eye by way of the choroidal circulation and gain access to the subretinal space.140 The larvae may migrate under the retina and can live in the eye for years. Persistence of the larvae within the ocular tissue may stimulate chronic inflammatory reactions and fibrosis.141 In rare instances, the larvae may be seen in the anterior chamber. In the subretinal location, the parasite may stay in the eye for a while, but with increasing fluid accumulation in the bladder of the Cysticercus, the retina may become detached. The Cysticercus may gain free access to the vitreous cavity and occasionally may cross through the pupillary opening and appear in the anterior chamber. Death of the larvae within the ocular tissues can stimulate a severe inflammatory reaction.
Cysticercus larvae may be removed from the subretinal location by surgical intervention without permanent damage to the eye. Once the Cysticercus reaches the vitreous, the prognosis is worse and its removal more difficult. If the parasite dies within the vitreous cavity, its toxic cyst fluid is released, leading to a severe inflammatory reaction. Subretinal Cysticercus can be treated with photocoagulation in an attempt to kill the organism and provide a firm scar. Pretreating such patients with systemic corticosteroids is mandatory to minimize the inflammatory reaction. The ocular adnexa, including the conjunctiva and extraocular muscles, may be involved by the Cysticercus.
The most serious form of the disease is cysticercosis of the brain, which leads to seizures and sometimes paralysis and death. The central nervous system lesions seem to resolve spontaneously and may relentlessly cause hydrocephalus and death. Death of larvae within the brain can lead to localized inflammation, fibrosis, and calcification. If the larvae are in the Aqueduct of Sylvius, it can cause hydrocephalus. The severity of the disease depends strictly on the number of eggs ingested. Radiography on the subcutaneous tissue may reveal calcification at the sites of the Cysticercus larvae.
Coenuriasis
Coenuriasis, a tapeworm disease also known as coenurosis, is caused by Coenurus cerebralis, the larval cystic form of the taeniid worms Multiceps multiceps and Taenia bremneri. The parasite affects primarily the central nervous system of sheep, causing the syndrome of “blind staggers.” In humans, who are an unusual incidental host, Coenurus bladder worms have been reported in the central nervous system and occasionally in the eye and subcutaneous tissues, particularly in Africa.
In intraocular cases, it is commonly found in the subretinal space and vitreous,142 and rarely in the anterior chamber. In superficial infestations, however, it is mostly located in the subconjunctival space and less commonly elsewhere.49 It is believed that subconjunctival and subcutaneous infestation, which occurs mostly in children, results from inoculation of the larva directly into the skin or through the conjunctiva during early toddler life, when the skin and eyes are frequently close to ground that may be contaminated.
Sparganosis
Sparganosis is infection with spargana (plerocercoid larvae) of various species of the diphyllobothriid tapeworm Spirometra, which cannot grow to adult worms in humans, but can reach maturity in certain animals, such as cats and dogs.49
It has been shown that eggs of Spirometra hatch in water, and the emerging coracidia are ingested by copepods of the genus Cyclops, where they develop into procercoid larvae.49 When infected copepods are swallowed by cats or dogs, the procercoids transform into plerocercoid larvae and develop into adult worms. Humans, frogs, snakes, and some birds are acceptable paratenic hosts, although infection remains at the plerocercoid level.
Sparganosis may be transmitted in one of the following ways: (1) by drinking water contaminated with copepods harboring the infective procercoids; (2) by ingesting the raw flesh of frogs, snakes, or birds containing the infective plerocercoids; or (3) by poulticing open wounds with the flesh of infected frogs or snakes, a recognized practice in the Far East.
Presenting symptoms of ocular sparganosis are nodule formation, lacrimation, edema, redness and pain, and in the case of retrobulbar invasion, a bulging orbit with corneal ulceration. A presumptive preoperative diagnosis may be made on demonstration of periocular nodules.
PLATYHELMINTHES: TREMATODES (FLUKES)
Schistosomiasis
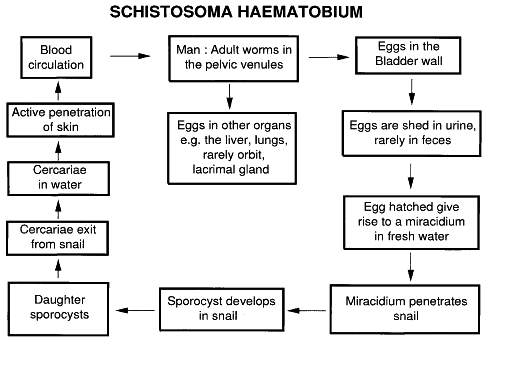
Schistosomiasis is a disease caused by Schistosoma haematobium, Schistosoma mansoni, and Schistosoma japonicum. The life cycle of S. haematobium is shown in Figure 3. Although the adult worms of Schistosoma live in the intravascular space, the eggs of S. haematobium are found in urine of humans, and the eggs of S. mansoni and S. japonicum are found in feces of humans. The eggs of Schistosoma are liberated from the tissues into the lumen of the bladder or the intestine, and the miracidium is liberated from the eggs. The miracidium escapes when a person urinates or defecates in water; the miracidium then swims in the water searching for the appropriate snail to penetrate; it then undergoes a cycle of development, giving rise to cercariae, which are infective to humans. Humans acquire the infection by exposure to water into which the snails have liberated cercariae. The cercariae have certain organs that allow them to penetrate the skin of humans and invade the circulatory system.
Intraocular infection by Schistosoma is extremely rare. Occlusion of the central retinal artery has been reported to be caused by the egg of S. mansoni. Occasionally, eggs may be discharged into the circulation, and embolization of the eggs may lead to development of a granuloma in the ocular adnexa. The conjunctiva may be the site of the localized granuloma owing to embolization of the eggs of S. haematobium.143,144 In rare instances, the granuloma may be found in the lacrimal gland.145
Paragonimiasis
Paragonimiasis is primarily a lung infection and is often referred to as pulmonary distomiasis. It is caused by trematodes of the genus Paragonimus, particularly the Oriental lung fluke Paragonimus westermani in humans. The adult worms are reddish brown, plump, ovoid flukes measuring up to 16 mm in length and 8 mm in width. They encyst in the lungs, and the eggs discharged in the bronchioles are either expectorated with sputum or swallowed and subsequently passed in feces.1 Eggs hatch in water after 3 to 4 weeks, and the miracidia invade snails belonging to several families, mainly Thiaridae, Pleuroceridae and Hydrobilidae.1,2 In 3 to 5 months, successive generations of mother sporocysts, rediae, daughter rediae, and finally cercariae are produced in snails. The cercariae then leave the snails and penetrate freshwater crabs and crayfish, where they encyst in the gills, muscles, legs, and viscera, developing into the infective metacercariae in 6 to 8 weeks. Humans acquire the infection by eating raw or undercooked crab or crayfish containing the infective metacercariae.
Paragonimiasis in humans is largely restricted to the Far East, where dietary habits (consumption of raw fish) favor infection by Paragonimus westermani. Furthermore, most of the flukes migrate to the lungs. Consequently, extrapulmonary paragonimiasis is rarely seen in humans, especially outside the Far East.
Ocular symptoms of the disease result from cerebral infection, or, much less commonly, relate to an existing worm invasion of the subconjunctival space, eyelid, or orbital tissue.146–148 In cases of cerebral paragonimiasis, the main ophthalmologic signs include homonymous hemianopia, papilledema, impaired visual acuity, and optic atrophy. The clinical features of intraocular paragonimiasis include repeated attacks of severe intraocular pain that is not alleviated by analgesics. Anterior uveitis and spontaneous hyphema develop, along with profound visual loss and secondary glaucoma.
In most cases, a diagnosis of paragonimiasis can be made after demonstration of the characteristic eggs in aspirated pleural effusion, or in the sputum or feces. Pulmonary involvement can be demonstrated by x-ray of the chest. Serologic tests are used for diagnosis of extrapulmonary paragonimiasis. Complement fixation test, countercurrent electrophoresis, and ELISA are found to be highly sensitive and specific.149 An intradermal test is available for screening in epidemiologic surveys.
Ocular paragonimiasis is treated by surgical removal of the worm. When the parasite invades the vitreous, vitrectomy should be performed. The use of systemic praziquantel or niclofolan is recommended.