|
PATHOPHYSIOLOGY OF VENEREAL SYPHILIS
Transmission of Treponema pallidum subspecies pallidum results from direct contact with early syphilitic lesions; entry is thought to occur through microscopic fissures in the epidermis or mucous membranes. T. pallidum proliferates at the initial site of infection, resulting in well-circumscribed, indurated, painless lesions called chancres that often become ulcerated or encrusted. This primary stage of syphilis occurs within 10 to 90 days postinfection and typically will resolve within a few weeks. The organism disseminates during this period, and the patient enters into a period of latent infection, in which there are no signs of infection other than serologic reactivity.
Secondary syphilis occurs in nearly one third of untreated cases, appearing within 6 weeks to 6 months postinfection. Manifestations consist of multiple macular or papular skin lesions, often accompanied by mucous membrane lesions, patchy hair loss, “nickel-and-dime” lesions on the palms of the hands and soles of the feet, fever, malaise, and lymphadenopathy. Primary and secondary skin and mucosal lesions contain large numbers of T. pallidum and are highly infectious. Syphilitic patients are considered to be infectious during the first year of infection.
A period of latent, asymptomatic infection occurs in all untreated individuals and may last the lifetime of the patient. Approximately two-thirds of syphilis patients in the United States are diagnosed by serologic reactivity during the early (<1 year postinfection) or late (≥1 year) latent stages.
Tertiary (also called late) manifestations of syphilis typically occur after years to decades in approximately one third of untreated patients and consist of gummatous, cardiovascular, and neurosyphilis forms. Gummas are granuloma-like accumulations of mononuclear cells that can occur in any organ, causing damage through the displacement of normal tissue. Cardiovascular syphilis may present as aortic aneurysm or cardiac valve abnormalities. Neurosyphilis can actually occur at either early or late stages of syphilis and may result in meningovascular inflammation, spinal cord demyelination and deterioration of dorsal root ganglia (tabes dorsalis), or frank infection of the brain (paresis).
Congenital syphilis occurs following transmission of T. pallidum across the placenta during the second or third trimester of pregnancy. The range of manifestations includes stillbirth, fulminant, multiorgan infection at birth, and asymptomatic infection at birth followed by the constellation of symptoms that constitute late congenital syphilis.
OCULAR MANIFESTATIONS
Interstitial keratitis and uveitis in congenital syphilis patients were first reported by Jonathan Hutchinson in 1858.32 Since that time, ophthalmic manifestations have been recognized as common complications of both sexually transmitted (acquired) and congenital syphilis. Any region of the eye can be affected, and presentations include interstitial keratitis, granulomatous and non-granulomatous iridocyclitis, panuveitis, retinitis, retinal vasculitis, and optic atrophy.6,86 Uveitis in nearly all forms may occur at either the secondary or late stages, months to years after the initial infection. In the preantibiotic era, the majority of interstitial keratitis cases were caused by untreated late congenital syphilis, occurring at 5 to 20 years of age. Acquired syphilis also caused uveitis in up to 5% of patients.
With the wide availability of penicillin in the mid 1940s, the incidence of syphilis and its impact on ocular disease decreased dramatically in countries where health care and antibiotic therapy were readily available. Any reduction in incidence may apply only to countries and regions with health care access; occurrence of all forms of syphilis (including congenital disease) in some areas of sub-Saharan Africa and Asia often reflect preantibiotic era rates. Recent studies in the United States indicate that syphilis is still a cause of corneal scarring with ghost vessels but is rarely associated with active keratitis.86 In a referral center in New York City,11 8% of uveitis patients had reactive treponemal antibody tests, but other potential causes could be ruled out in only 4% of patients.
Ulcerative early lesions of syphilis are thought to increase the transmission rate of human immunodeficiency virus (HIV). Conversely, HIV infection may affect the manifestations of syphilis,31,50 including ocular lesions. For example, dense vitreitis was identified in three patients infected with HIV.37 In addition, coinfection with HIV, particularly in the late symptomatic phase, may result in deficient antibody responses and lack of reactivity in nontreponemal and treponemal serologic tests for syphilis. For these reasons, it is recommended that patients suspected of having syphilis also be tested for HIV antibody reactivity. Another consideration is that corticosteroid treatment may alter the ocular manifestations of syphilis or serologic reactivity.
LABORATORY DIAGNOSIS
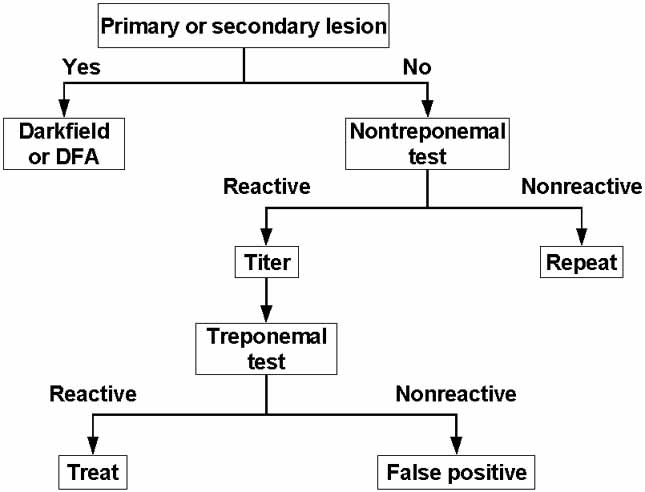
No ocular manifestations are pathognomonic for syphilis, so diagnosis is largely dependent on a combination of history, general clinical signs, detection of T. pallidum (when early lesions are present), and serologic analysis.51 A general scheme for the diagnosis of syphilis is depicted in Figure 2.
T. pallidum in patient specimens can be detected by darkfield microscopy, immunofluorescent staining, polymerase chain reaction (PCR), or animal inoculation. Characteristic primary or secondary lesions combined with the demonstration of T. pallidum in lesion exudates or biopsy samples by darkfield microscopy or immunostaining techniques is considered to be a definitive diagnosis.51 However, detection of T. pallidum by these means is not always available. In addition, spirochetes are present in exceedingly low numbers past the primary and secondary stages and are then difficult to detect by any method.
Immunofluorescent identification usually involves direct fluorescent antibody (DFA-TP) staining of lesion exudates or tissue using fluorescein-labeled anti-T. pallidum polyclonal or monoclonal antibodies.38,51 Immunohistochemical techniques have also been developed.29 T. pallidum has been detected in aqueous humor using darkfield microscopy or immunofluorescence of concentrated samples,68,87 but the numbers are exceedingly small and artifacts can yield false-positive interpretations.
PCR has been used to detect T. pallidum DNA in blood, tissue, and cerebrospinal fluid56,74 but is not routinely available. Inoculation of a rabbit was used to isolate the type strain T. pallidum subspecies pallidum Nichols from a patient's cerebrospinal fluid in 1906, and animal inoculation is still the only procedure available for the isolation of T. pallidum strains. It is conceivable that these sensitive methods could be used to detect or isolate T. pallidum from ocular fluids or biopsies.
Diagnosis of syphilis is highly dependent on serologic tests, including the so-called nontreponemal and treponemal antibody assays.8,16,51 Nontreponemal tests detect reaginic antibodies that react with cardiolipin combined with lecithin; these antibodies arise for unknown reasons during treponemal infections. The Rapid Plasma Reagin (RPR) circle card test is the most commonly used nontreponemal assay, although the Venereal Disease Research Laboratory (VDRL) test is the only assay available for testing the reactivity of cerebrospinal fluid. Treponemal assays detect anti-T. pallidum antibodies using whole T. pallidum, sonicates, or recombinant antigens. The T. pallidum particle agglutination assay (TP-PA) replaced the microhemagglutination T. pallidum (MHA-TP) test and is widely used today, but the fluorescent treponemal antibody-absorbed (FTA-ABS) test and enzyme immunoassays are also available. The nontreponemal tests are of value because they can be quantitated by titration, and typically the reciprocal titer decreases after treatment. The treponemal tests have the advantage of being specific for treponemal antigens, but reactivity is usually retained after treatment. Both types of tests may yield false-positive reactions in autoimmune disease, which should be kept in mind when attempting to distinguish autoimmune from syphilitic disease.
TREATMENT
Treatment should be consistent with the stage of disease, HIV status, and other factors.2 A single dose of benzathine penicillin (2.4 million units intramuscularly [IM]) is currently recommended for primary, secondary, and early latent syphilis, whereas three equivalent dosages at weekly intervals are recommended for late latent, latent of unknown duration, or tertiary syphilis.2 Aqueous crystalline penicillin G is recommended for neurosyphilis (3–4 million units intravenous [IV] every 4 hours for 10–14 days) and for congenital syphilis in infants (100,000–150,000 units/kg per day for 10 days). Re-covery of viable T. pallidum from CSF has been reported in neurosyphilis patients treated with benzathine penicillin, consistent with its poor penetration of blood–brain barrier; recurrence of manifestations in HIV-positive individuals also occurred.44 For this reason, treatment of patients with ocular syphilis with a regimen of aqueous penicillin G consistent with current neurosyphilis treatment guidelines should be considered.
ANIMAL MODELS
The earliest report of an animal model of ocular syphilis occurred before the discovery of T. pallidum. In 1881, Haensell30 described the occurrence of keratitis and iritis after inoculation of a syphilitic lesion exudate into the anterior chamber of a rabbit eye. In 1906, 1 year after T. pallidum was first identified by Schaudinn and Hoffman,65 Bertarelli14 also demonstrated the occurrence of corneal lesions in rabbits after T. pallidum inoculation. As part of their extensive characterization of experimental syphilis in the rabbit, Brown and Pearce17 described the eye manifestations observed after infection. In the 1960s, Wells and Smith84 and Elsas et al.26 were able to demonstrate ocular manifestations after inoculation of owl monkeys or squirrel monkeys with T. pallidum via the cornea, vitreous, cisterna magna, carotid artery, skin, and intravenous routes. The pathologic findings varied but included pupillary inequality, non-granulomatous iridocyclitis, retinal hemorrhage, multiple lens opacities, and flare. In addition, T. pallidum could be found in the aqueous humor using either darkfield microscopy or immunofluorescent staining up to 1 year after inoculation. Although five of the six monkeys studied developed serologic reactivity in the VDRL, FTA-ABS, and T. pallidum immobilization (TPI) tests, the reactivity was often weak (e.g., VDRL titers never exceeded 1:4). It is unclear whether this low reactivity was caused by poor antibody responses in these animals or by the inadequacy of the serologic tests for serum samples obtained from monkey species.
Concerns have long existed that corneal transplants could serve as a potential source of T. pallidum infection. Corneas from donors with reactive RPR tests were excluded from the transplant pool, resulting in a substantial reduction in the number of corneas available for transplant. In an extensive study by Dr. Elliot Randolph published in 1949,59 the presence of infectious organisms in corneas from rabbits infected with T. pallidum for 3 or more months was analyzed to provide information on the potential for transmission by corneal transplantation. None of the recipient rabbits was infected after injection of corneal extracts from 40 infected ``donor'' rabbits. In more recent studies,45 rabbits were infected by intratesticular inoculation, and 10 days later the corneas were removed, minced, and extracted. When injected into naïve rabbits, these extracts produced lesions if not washed with saline before extraction, indicating the presence of T. pallidum. However, with saline rinsing, no lesions were obtained, consistent with the presence of viable treponemes in blood or ocular fluids but not in the corneal tissue itself. In addition, incubation of T. pallidum in OptiSol® cornea storage medium was found to render the organisms noninfectious within 24 hours. These data were used in combination with other information to remove RPR reactivity as a criterion for the exclusion of corneas for transplantation.