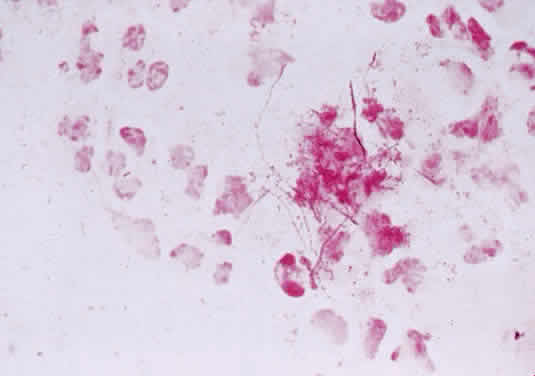
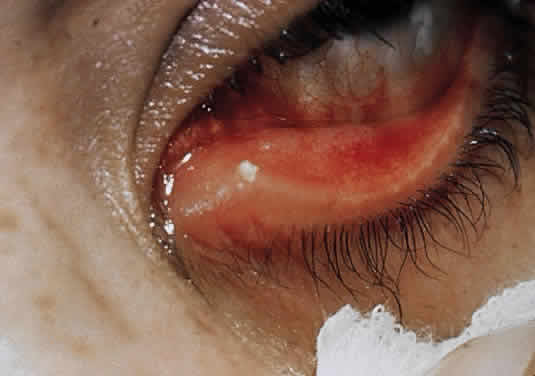
1. Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH: Manual of Clinical
Microbiology. 6th ed. Washington DC, ASM Press, 1995 2. Lever W, Schaumburg-Lever G: Actinomycosis. In Histopathology of the Skin, p 385. 7th
ed. Philadelphia, WB Saunders, 1990 3. Finegold SM: General aspects of anaerobic infection. In Finegold SM, George
WL (eds): Anaerobic Infections in Humans, p 135. San Diego, Academic
Press, 1989 4. Yeager B, Hoxie J, Weisman R, Greenburg M, Bilaniuk L: Actinomycosis in the acquired immunodeficiency syndrome related complex. Arch Otolaryngol Head Neck Surg 112: 1293, 1986 5. Rippon J. Actinomycosis. In Medical Mycology: The Pathogenic Fungi and
the Pathogenic Actinomycetes, p 30. 3d ed. Philadelphia, WB Saunders, 1988 6. Moore LVH, Moore WEC, Cato EP, Smibert RM, Burmeister JA, Best AM: Bacteriology of human gingivitis. J Dent Res 66:989, 1987 7. Slack JM, Gerencser MA: Actinomyces, Filamentous Bacteria, Biology and
Pathogenicity. Minneapolis, Burgess Publishing Co., 1975 8. Milder B: Actinomycosis. In Fraunfelder F, Roy F, Meyer S (eds): Current
Ocular Therapy 2, p 36. Philadelphia, WB Saunders, 1985 9. Bennhoff D: Actinomycosis: Diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope 94: 1198, 1984 10. Kuijper E, Wiggerts H, Jonker G, Schaal K, DE Gans J: Disseminated actinomycosis due to Actinomyces meyeri and Actinobacillus actinomycetem comitans. Scand J Infect Dis 24:667, 1992 11. Jani A, Casibang V, Mufarri J: Disseminated actinomycosis presenting as a testicular mass: A case report. J Urol 143:1012, 1990 12. Moschella S, Hurley H: Systemic bacterial and nonvenereal spirochetal infections. In
Dermatology, p 760. 3d ed. Philadelphia, WB Saunders, 1992 13. Feder H: Actinomycosis manifesting as an acute painless lump of the jaw. Pediatrics 85:858, 1990 14. Tyrrell J, Noone P, Prichard J: Thoracic actinomycosis complicated by Actinobacillus actinomycetem comitans. Respir Med 86:341, 1992 15. Miyamoto M, Fang F: Pyogenic liver abscess involving Actinomycoses. Clin Infect Dis 16:303, 1993 16. Shauly Y, Nachum Z, Gdal OM et al: Adjunctive hyperbaric oxygen therapy for actinomycotic lacrimal canaliculitis. Graefes Arch Clin Exp Ophthalmol 231:429, 1993 17. Pine L, Hardin H, Turner L, Robert S: Actinomycotic lacrimal canaliculitis. Am J Ophthalmol 49:1278, 1960 18. Fedukowicz H: Bacteria. In External Infections of the Eye, p 41. Philadelphia, Appleton-Century-Crofts, 1985 19. Roussel T, Olson E, Rice T, Meisler D, Hall G, Miller D: Chronic postoperative endophthalmitis associated with Actinomyces species. Arch Ophthalmol 109:60, 1991 20. Blanksma L, Slijper J: Actinomycotic dacryocystitis. Ophthalmologica 176:145, 1977 21. Scheie HG, Albert DM: Textbook of Ophthalmology, p 376. 9th ed. Philadelphia, WB
Saunders, 1977 |