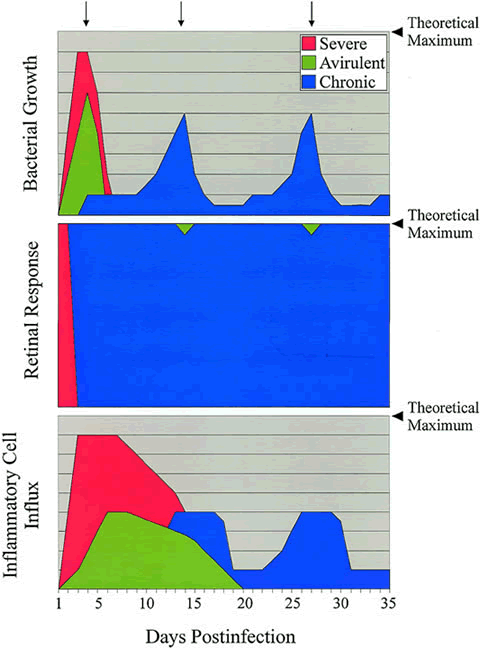
Endophthalmitis includes a relatively painless and easily treated intraocular inflammation caused by avirulent organisms such as coagulase-negative staphylococci, recurrent indolent infections caused by P. acnes, and an explosive and refractory panophthalmitis caused by Bacillus cereus.1,8,50 Certain organisms are typically associated with a predictable range of coincident inflammation. Pseudomonas aeruginosa, for example, is typically associated with more inflammation than S. epidermidis. There are always strain-specific exceptions, however. The offending microbe is not always predictive of the degree of inflammation engendered by the infection. A number of clinical reports detail severe endophthalmitis by relatively avirulent organisms, such as coagulase-negative staphylococci or Bacillus species other than B. cereus.51–55 Schematic comparisons of the infection courses of severe, avirulent, and chronic bacterial endophthalmitis are depicted in Figure 1.
Experimentally, equal numbers of different strains injected into the vitreous produce different clinical responses. However, the presence of microorganisms in the human eye does not always lead to tissue destruction. Therefore, different bacteria must possess different virulence factors. Current analysis of bacterial mutant strains deficient in individual and multiple toxins are defining specific roles for these proposed virulence factors in the pathogenesis of endophthalmitis. Additional putative virulence factors that are of interest are bacterial cell surfaces and their individual components, such as peptidoglycan, teichoic acid, polysaccharide capsules, lipopolysaccharide, and adhesive proteins. Because the bacterial cell surface is the foremost component of an organism to dialog with the host, its contribution to virulence must also be taken into account. The behavior of the organism in the eye during infection may also contribute to intraocular virulence. These aspects of endophthalmitis pathogenesis are discussed later.
STAPHYLOCCUS EPIDERMIDIS
Coagulase-negative staphylococci are a leading cause of posttraumatic and postoperative endophthalmitis, are found as normal flora of the ocular region, and are considered relatively avirulent.56 However, S. epidermidis and other coagulase-negative staphylococci have been reported to cause significant vision loss in some cases of endophthalmitis.30,51,52 In general, S. epidermidis does not secrete membrane-damaging toxins but does possess a number of adhesive proteins on its surface, including fibrinogen- and vitronectin-binding proteins, polysaccharide intercellular adhesin (PIA), and capsular polysaccharide adhesin (PS/A).57–60 The direct contribution of these adhesins in the pathogenicity of endophthalmitis must be determined.
S. epidermidis has emerged as one of the most important nosocomial pathogens often associated with nonophthalmic indwelling devices.61 In these types of infections, contamination of the implant likely occurs with a small inoculum from the patient's own normal flora during surgery. One likely source of S. epidermidis in postoperative endophthalmitis may, therefore, be organisms attached to implanted intraocular lenses (IOLs). In vitro adherence studies demonstrate that S. epidermidis adhere readily to most types of IOLs and that adherence depends on IOL materials, surface modifications, and extent of damage or irregularity of the IOL surface.62–66 Polypropylene haptics have been associated with an increased risk of postoperative endophthalmitis, presumably resulting from the increased bacterial adherence to this type of biomaterial and possibly from sequestration at the point of haptic attachment to the IOL.62 Polymethyl methacrylate (PMMA) lenses and haptics allowed less bacterial adherence than polypropylene haptics,62 whereas heparin-coated PMMA and Acrysoft lenses allowed even less adherence.63,64 Regarding the role of specific S. epidermidis adhesins in attachment to IOLs, a S. epidermidis ocular isolate containing the gene for PS/A and PIA (ica) adhered more readily to Acrysoft IOLs in vitro than a strain without the ica locus.65 It is not known what specific role these or other adhesins plays in S. epidermidis endophthalmitis pathogenesis.
Biofilm formation is considered to be an important virulence factor of coagulase-negative staphylococci and, in particular, S. epidermidis. Nosocomial infections are commonly associated with bacterial biofilm formation on implanted medical devices. Biofilm formation occurs as organisms replicate collectively on the surface of a biomaterial, where they become embedded in a multilayered mass of bacteria and extracellular polysaccharide.52,67 Organisms residing within the biofilm are usually sessile, and the biofilm as a whole is often resistant to antibiotic treatment and the host immune response. Because of the propensity for S. epidermidis to adhere to and form biofilms on several types of medical implant devices, it is reasonable to hypothesize that a similar situation could arise for contamination of IOLs and subsequent postoperative endophthalmitis.
As stated earlier, although relatively avirulent, S. epidermidis and other coagulase-negative staphylococci have been reported to cause fulminant endophthalmitis.30,51,52 Experimental models of S. epidermidis endophthalmitis have been initiated with a large number of bacteria (104 to 108),68–72 but virulent experimental infections have been achieved with as few as 50 organisms.72 In the absence of toxin production, precisely what causes such a robust inflammatory response remains in question. In experimental rat and rabbit models of S. epidermidis endophthalmitis, bacteria were readily cleared from the eye within 3 days of infection.37,69 This spontaneous sterilization coincided with intravitreal infiltration of neutrophils, plasma cells, B cells, and monocytes and measurable antibody to teichoic acid. No pathologic changes were noted in the retina.37 The complement system is also an integral participant in normal host defenses against experimental S. epidermidis endophthalmitis. Impaired host defenses against experimental staphylococcal endophthalmitis were observed in decomplemented guinea pigs.70 These data together suggest that antibodies and complement may assist the cellular immune response in bacterial clearing, leading to spontaneous sterilization in S. epidermidis endophthalmitis. The paradox of the significant number of microbe-positive anterior chamber taps recovered at the conclusion of human cataract surgery73–75 and the infrequency of endophthalmitis argues for the potency of the ocular immune response to control invasion by a limited number of avirulent microorganisms. Although these findings provide a hypothesis for relatively mild forms of S. epidermidis endophthalmitis, what triggers pathogenesis in more severe cases remains an open question.
STAPHYLOCOCCUS AUREUS
In contrast to S. epidermidis, S. aureus is a virulent intraocular pathogen. S. aureus endophthalmitis has been reported to result in final visual acuities of 20/400 or worse in more than half of the reported cases.76–79 Increased multiple antibiotic resistance in staphylococcal clinical isolates may also ultimately lead to an increased number of treatment failures.
S. aureus produces a number of putative virulence factors, namely adhesins, cytolytic toxins, and proteolytic enzymes, that are controlled by a system of global transcriptional regulators called staphylococcal accessory regulator (sar) and accessory gene regulator (agr).80 This quorum-sensing system recognizes the increase in bacterial numbers accompanying active infection and coordinately regulates the secretion of adhesins and other cell wall-associated proteins during the exponential phase of growth and the production of extracellular toxins and enzymes during the postexponential phase of growth. Mutants of S. aureus deficient in global regulation, therefore, produce cell wall-associated proteins and adhesins throughout the entire growth cycle but do not produce toxins. Such mutants have been analyzed in experimental models of S. aureus endophthalmitis in the rabbit and rat.81,82 Eyes infected with wild type S. aureus demonstrate focal retinal destruction with a rapid decline in electroretinogram (ERG) b-wave amplitude and a more intense intraocular inflammatory response than in eyes infected with a defective agr system.81,82 The intraocular virulence of S. aureus with mutations in both agr and sar global regulatory systems was almost completely attenuated.83 These studies suggested an important role for global regulation of extracellular toxins in S. aureus endophthalmitis pathogenesis.
The contribution of individual extracellular S. aureus toxins to endophthalmitis pathogenesis has also been analyzed using strains genetically engineered to be deficient in the specific toxins of interest. Analyses of S. aureus mutants defective in alpha-toxin (a membrane-damaging cytolysin), beta-toxin (a sphingomyelinase), and gamma-hemolysin (a bicomponent cytolysin) were analyzed in an experimental rabbit model of endophthalmitis.84 The absence of gamma-hemolysin did not alter the course or severity of endophthalmitis compared with that of infection caused by wild type S. aureus. However, declines in ERG b-wave amplitudes and overall retinal destruction on postinfection day 2 in untreated eyes infected with alpha-toxin-deficient or beta-toxin-deficient mutants were significantly less than that of eyes infected with wild-type S. aureus. There was a cumulative positive effect on the preservation of retinal function on postinfection day 2 in eyes infected with a triple mutant defective in alpha-toxin, beta-toxin, and gamma-hemolysin production. Differences in endophthalmitis severity were less clear after day 2, because significant progressive declines in ERG b-wave amplitudes occurred regardless of the S. aureus strain analyzed.84
Genomic analysis of S. aureus clinical ocular isolates demonstrated that all isolates tested possessed the gene encoding alpha-toxin.85 These data together suggest that globally regulated toxins, particularly alpha-toxin, are key virulence factors in S. aureus endophthalmitis. Arresting S. aureus toxin production by inactivation of global regulatory systems during the early stages of infection may, therefore, be a more viable therapeutic option than the targeting of individual toxins.
In addition to having detrimental effects on retinal function, experimental S. aureus endophthalmitis also incites significant intraocular inflammation. In experimental S. aureus endophthalmitis models in the rabbit and the rat, antibody levels to staphylococcal ribitol teichoic acid were detected, and neutrophils, lymphocytes, plasma cells, and monocytes infiltrated into the vitreous during the early stages of infection.35,36 Neutrophil adhesins E-selectin and intercellular adhesion molecule 1 (ICAM-1) were also expressed before leukocyte migration into the vitreous.86 As with experimental S. epidermidis endophthalmitis, this cellular response correlated with clearance of staphylococci from the vitreous and spontaneous sterilization.36 Although metabolically inactive S. aureus and purified cell wall sacculi have been shown to elicit significant intraocular inflammation in an experimental sterile endophthalmitis model,87 the specific cell wall components eliciting such are response have yet to be identified.
ENTEROCCUS FAECALIS
Enterococcus faecalis is a gram-positive facultative anaerobe commonly associated with antibiotic-resistant nosocomial infections. E. faecalis is most often isolated from infected filtering blebs following glaucoma surgery and is the causative agent in 4% to 8% of postoperative endophthalmitis.5,6,30,51,88 The visual outcomes of E. faecalis endophthalmitis are commonly poor, with as many as 80% of cases resulting in a final visual acuity of 20/200 or worse.30,51,88 The development of multidrug-resistant strains over the past two decades compounds the problem of treating E. faecalis infections. Fortunately, clinical reports of enterococci resistant to all antibiotics, including vancomycin, have yet to include ocular isolates.33,89
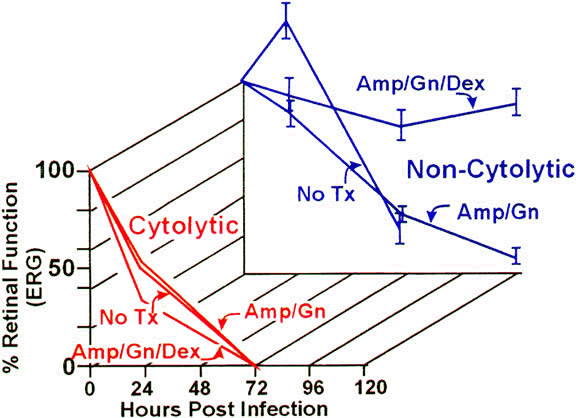
Approximately 50% of E. faecalis ocular isolates produce a cytolysin that disrupts mammalian and bacterial cell membranes.90 In an experimental rabbit endophthalmitis model, cytolysin-producing E. faecalis caused more fulminant and destructive changes in retinal architecture and a rapid decline in ERG b-wave amplitude than did noncytolytic E. faecalis.39,91 Transmission electron microscopy demonstrated retinal tissue damage occurring several hours before light microscopic detection or changes in retinal function in eyes infected with cytolytic E. faecalis. Experimental endophthalmitis with cytolytic E. faecalis was also refractory to intravitreal antibiotic and anti-inflammatory treatment, whereas noncytolytic E. faecalis endophthalmitis was resolved with a similar therapeutic regimen39 (Fig. 2). The results suggested that the presence of the cytolysin likely contributes to treatment failures and that treatment success may necessitate targeting of the cytolysin as an adjunct to therapy.
BACILLUS CEREUS
B. cereus is a ubiquitous gram-positive aerobic saprophyte found primarily in the soil and most commonly recognized as an agent of acute food poisoning. B. cereus, however, causes one of the most explosive and devastating forms of endophthalmitis, often resulting in loss of useful vision, and sometimes the eye, within a few days following inoculation. Bacilli are most commonly introduced into the anterior or posterior segment on contaminated projectiles following a penetrating injury or from the posterior segment vasculature as a consequence of bacteremia. Bacilli replicate and migrate rampantly throughout all parts of the eye, and an intense intraocular inflammatory response occurs in parallel with deteriorating retinal architecture.84,87 In the worst-case scenario, the infection spreads unhindered into periocular tissues, ocular architecture collapses, and the eye is lost. Although B. cereus is exquisitely sensitive to most antibiotics, this devastating consequence often occurs regardless of prompt and proper therapeutic and surgical intervention following clinical detection of infection.
Clinical reports have historically credited the unique virulence of B. cereus to toxins produced during infection.19,20,50,92,93 B. cereus produces a number of membrane-damaging cytolysins, enterotoxins, and proteases that are putative ocular toxin candidates.94 However, to date, three of these toxins (hemolysin BL, phosphatidylinositol-specific phospholipase C, and phosphatidylcholine-specific phospholipase C) have been shown individually to contribute little to the overall pathogenesis of experimental Bacillus endophthalmitis.95,96 It is likely, therefore, that if toxins are involved in the virulence of Bacillus endophthalmitis, they do so in a multifactorial fashion.
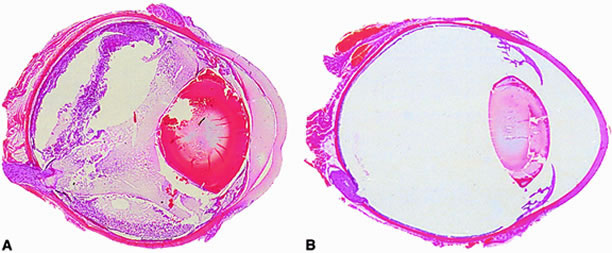
The Bacillus cell wall may also contribute to intraocular inflammation during endophthalmitis. Metabolically inactive B. cereus and purified B. cereus cell walls do not affect retinal function. However, these preparations are highly inflammogenic in experimental models of sterile endophthalmitis.87 Another significant virulence factor related to bacterial structure may be that of bacterial motility. The unusual motile capability of B. cereus in the eye appears to contribute significantly to experimental endophthalmitis pathogenesis. Bacillus strains genetically engineered to be deficient in motility could not migrate throughout the eye, did not grow as well in the vitreous, and were significantly less virulent than wild type motile bacilli97 (Fig. 3). These results highlight bacterial motility as a possible therapeutic target for Bacillus and perhaps other motile organisms in endophthalmitis.
PROPIONIBACTERIUM ACNES
P. acnes is a ubiquitous gram-positive anaerobe found as normal flora of the eyelid and conjunctiva. It is also an opportunistic pathogen that has been associated with nosocomial prosthetic device infections and chronic inflammatory diseases such as acne vulgaris, arthritis, and osteomyelitis. P. acnes can cause an acute and mild form of endophthalmitis that is readily responsive to treatment but can also cause an unusual form of chronic and recurrent endophthalmitis following extracapsular cataract extraction and IOL implantation.7–9,98,99 P. acnes from the surrounding ocular flora can adhere to PMMA IOLs100 and, therefore, are a likely source of lens contamination. Despite adequate therapeutic and surgical intervention, late-onset treatment failures and persistent infections are common with P. acnes intraocular infection.
Chronic postoperative P. acnes endophthalmitis is characterized by a recurrent inflammation with granulomatous keratic precipitates and white plaques on the posterior capsule or IOL that may be observed weeks to months following surgery.99 These intracapsular plaques are thought to represent P. acnes sequestered inside the peripheral capsular bag.101–103 These sequestered plaques likely contribute to the host's inability to clear the infection and the failure of antibiotics to reach the sequestered bacterial plaques. Although there is little evidence that P. acnes exists as a biofilm in these infections, the resistance to killing by host inflammatory cells, the refractory nature of the infection to therapy, and plaque formation are consistent with the presence of a biofilm. The inherent slow replication rate of P. acnes is also thought to contribute to the indolent nature of the disease.104P. acnes does not produce membrane-damaging toxins but does produce proteases that may contribute to virulence during infection.105,106 Adherence factors of P. acnes have not been well studied, but P. acnes, like S. aureus and S. epidermidis, produce a fibronectin-binding protein that may aid in adhesion of P. acnes to IOLs, other types of ocular biomaterials, or intraocular structures.107 The role of P. acnes proteases and adhesion molecules in the pathogenesis of experimental endophthalmitis has yet to be analyzed.
GRAM-NEGATIVE PATHOGENS AND CANDIDA
Although clinical series vary in the frequency of isolation of gram-negative organisms, gram-positive isolates greatly outnumber gram-negative isolates in endophthalmitis. The paucity of experimental data on the pathogenic mechanisms of gram-negative bacterial endophthalmitis reflects the overall predominance of gram-positive organisms isolated from these cases. Gram-negative organisms accounted for only 6% of cases in the EVS study2 but up to 20% of posttraumatic cases of endophthalmitis.15 Common gram-negative endophthalmitis isolates include Klebsiella, Pseudomonas, and Hemophilus. The ocular pathogenesis of Pseudomonas is the best understood of all gram-negative pathogens because of the abundance of experimental data that exists for P. aeruginosa keratitis. It is unknown, however, whether the findings in experimental P. aeruginosa corneal infections extend to P. aeruginosa endophthalmitis. Many gram-negative organisms, including Pseudomonas, are avid biofilm producers, which may be a source of infection via adherence to IOLs in postoperative endophthalmitis. In endogenous gram-negative endophthalmitis, a number of cases reported were secondary to sepsis and had a moderately poor prognosis.108,109 Increasing reports of gram-negative endogenous endophthalmitis in diabetics highlight the danger of an immunocompromised state and metastatic infections with these opportunistic pathogens.110–112
Fungi are one of the most common causes of endogenous endophthalmitis. The prolonged use of intravenous devices, long-term antibiotic therapy, and the use of immunosuppressive agents have contributed greatly to the rise in frequency of fungemia and endogenous fungal endophthalmitis. Candida is the most common cause of endogenous endophthalmitis in immunocompromised patients, patients on long-term broad-spectrum antibiotics, and intravenous drug abusers.113–116 Incidence of Candida endophthalmitis in patients with disseminated candidiasis have been reported to range from 3% to as high as 40%, depending on the definition criteria of the study and the patient population included in the survey.117–120Candida has a particular predilection for the eye in cases of disseminated candidiasis. Endogenous Candida endophthalmitis generally results from metastatic spread to the choroidal and retinal vasculature with generation of fungal microabscesses, followed by extension of organisms into the vitreous.121,122 Although candidemia and subsequent Candida chorioretinitis and endophthalmitis are associated with immunocompromised and hospitalized patients, it is unclear why the eye is such a frequent site for deep tissue invasion. It is also unclear why patients with acquired immunodeficiency syndrome (AIDS) from human immunodeficiency virus (HIV) infection are often colonized with Candida but have a relatively low risk of Candida endophthalmitis. Candida chorioretinitis and endophthalmitis are also seen in nonimmunocompromised patients who experience multiple episodes of transient candidemia, as in the setting of intravenous drug abuse.