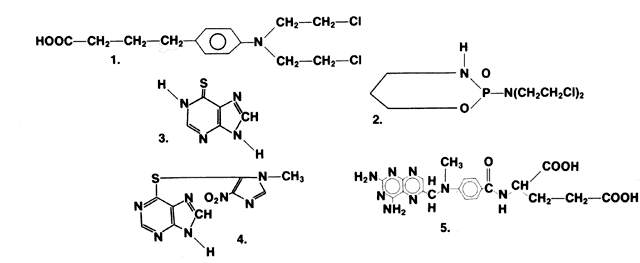
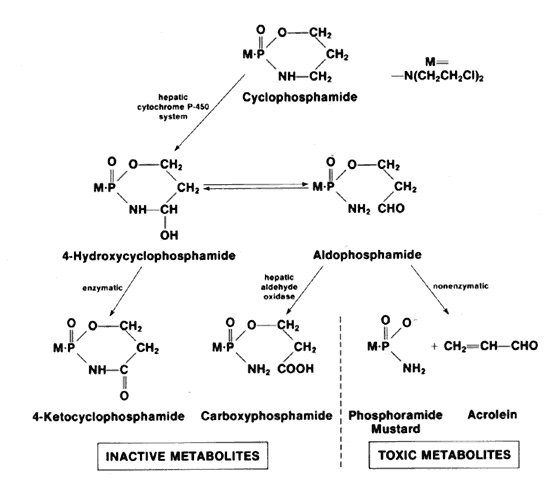
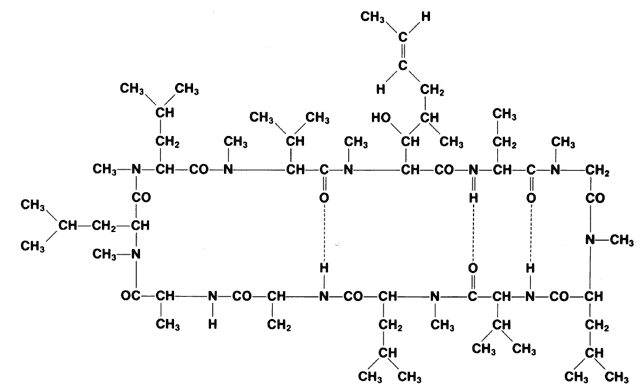
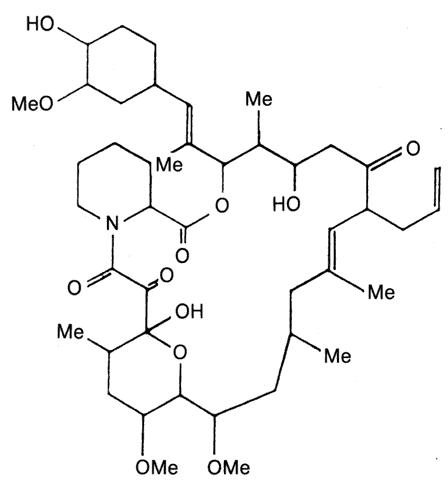
1. Hench PS, Kendall EC, Slocumb CH, Polley HF: The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocortisone: compound
E) and of pituitary adrenocorticotropic hormone on rheumatoid
arthritis. Proc Staff Meet Mayo Clinic 24:181, 1949 2. Gordon DM, McLean JM: Effects of pituitary adrenocorticotropic hormone (ACTH) therapy in ophthalmologic
conditions. JAMA 142:1271, 1950 3. Olson JA, Steffensen EH, Margulis RR et al: Effect of ACTH on certain inflammatory diseases of the eye. JAMA 142:1276, 1950 4. Whitcup SM, Salvo EC Jr, Nussenblatt RB: Combined cyclosporine and corticosteroid therapy for sight-threatening
uveitis in Behçet's disease. Am J Ophthalmol 118:39, 1994 5. Cogan DG: Immunosuppression and eye disease. Am J Ophthalmol 83:777, 1977 6. Grunwald HW, Rosner F: Acute leukemia and immunosuppressive drug use: A review of patients undergoing
immunosuppressive therapy for non-neoplastic diseases. Arch Intern Med 139:461, 1979 7. Schein PS, Winokur SH: Immunosuppressive and cytotoxic chemotherapy: Long-term complications. Ann Intern Med 82:84, 1975 8. Krumbhaar EB, Krumbhaar HD: The blood and bone marrow in yellow cross gas (mustard gas) poisoning: Changes
produced in the bone marrow of fatal cases. J Med Res 40:497, 1919 9. Calabresi P, Parks RE Jr: Antiproliferative agents and drugs used for immunosuppression. In
Gilman AG, Goodman LS, Gilman A (eds): The Pharmacological
Basics of Therapeutics, 6th ed, pp 1263–1268. New York, Macmillan, 1980 10. Dorr RT, Fritz WL: Cancer Chemotherapy Handbook. New York, Elsevier, 1980 11. Everett JL, Roberts JJ, Ross WCB: Aryl-2-halogenoalkylamines. Part XII. Some carboxylic derivatives of NNdi-2-chlorethylaniline. J Chem Soc 3:2386, 1953 12. McLean A: Pharmacokinetics and metabolism of chlorambucil in patients with malignant
disease. Cancer Treat Rev 6 (suppl):33, 1979 13. Arnold H, Bourseaux F: Synthese und Abbau cytostatisch wirksamer cyclischer N-Phosphamidester
des Bis-(ß-chlorathyl)-amins. Angew Chem 70:539, 1958 14. Brock N, Hohorst HJ: Uber die Aktivierung von Cyclophosphamid in vivo und in vitro. Arzneimittelforschung 13:1021, 1963 15. Cohen JL, Jao JY: Enzymatic basis of cyclophosphamide activation by hepatic microsomes of
the rat. J Pharmacol Exp Ther 174:206, 1970 16. Chabner BA, Myers CE, Oliverio VT: Clinical pharmacology of anticancer drugs. Semin Oncol 4:165, 1977 17. Bach JF: The Mode of Action of Immunosuppressive Agents, pp 209–210. Amsterdam, North-Holland, 1975 18. Mackiewicz U, Brelinska-Peczalska R, Konys J: The influence of cyclophosphamide on lymph node and peripheral blood lymphocytes
in mice. Arch Immunol Ther Exp 26:375, 1978 19. Starzl TE, Groth CG, Putnam CW et al: Cyclophosphamide for clinical renal and hepatic transplantation. Transplant Proc 5:511, 1973 20. Paterson PY, Hanson MA. Cyclophosphamide inhibition of experimental allergic
encephalomyelitis and cellular transfer of the disease on Lewis
rats. J Immunol 103:1311, 1969 21. Thomson JD, Austin RW, Streetman RP: The influence of chlorambucil on experimental allergic encephalomyelitis. Neurology 13:505, 1963 22. Gordon RO, Wade ME, Mitchell MS: The production of tolerance to human erythrocytes in the rat with cytosine
arabinoside or cyclophosphamide. J Immunol 103:233, 1969 23. Hall JM, Ohno S, Pribnow JF: The effect of cyclophosphamide on an ocular immune response. I: Primary
response. Clin Exp Immunol 30:309, 1977 24. Oh JO: Effect of cyclophosphamide on primary herpes simplex uveitis in rabbits. Invest Ophthalmol Vis Sci 17:769, 1978 25. Buckley CE III, Gills JP Jr: Cyclophosphamide therapy of peripheral uveitis. Arch Intern Med 124:29, 1969 26. Mamo JG, Azzam SA: Treatment of Behçet's disease with chlorambucil. Arch Ophthalmol 84:446, 1970 27. Dinning WJ, Perkins ES: Immunosuppressive in uveitis: A preliminary report of experience with chlorambucil. Br J Ophthalmol 59:397, 1975 28. Godfrey WA, Epstein WV, O'Connor GR et al: The use of chlorambucil in intractable idiopathic uveitis. Am J Ophthalmol 78:415, 1974 29. Oniki S, Kurakazu K, Kawata K: Immunosuppressive treatment of Behçet's disease with cyclophosphamide. Jpn J Ophthalmol 20:32, 1976 30. Foster CS: Immunosuppressive therapy for external ocular inflammatory disease. Opthalmology 87:140, 1980 31. Bigos ST, Nisula BC, Daniels GH et al: Cyclophosphamide in the management of advanced Graves' ophthalmopathy: A
preliminary report. Ann Intern Med 90:921, 1979 32. Rosenbaum JT: Treatment of severe refractory uveitis with intravenous cyclophosphamide. J Rheumatol 21:123, 1994 33. Char DH: Immunology of Uveitis and Ocular Tumors, pp 65–73. New York, Grune & Stratton, 1978 34. DeFronzo RA, Braine H, Colvin OM et al: Water intoxication in man after cyclophosphamide therapy. Ann Intern Med 78:861, 1973 35. Kende G, Sirkin SR, Thomas PRM et al: Blurring of vision, a previously undescribed complication of cyclophosphamide
therapy. Cancer 44:69, 1979 36. Walpole AL: Carcinogenic action of alkylating agents. Ann NY Acad Sci 68:750, 1958 37. Hoffman GS, Kerr GS, Leavitt RY et al: Wegener's granulomatosis: an analysis of 158 patients. Ann Intern Med 116:488, 1992 38. Hitchings GH, Elion GB: Chemical suppression of the immune response. Pharmacol Rev 15:365, 1963 39. Crabtree GW: Mechanisms of action of pyrimidine and purine analogues. In
Brodsky I, Kahn SB, Conroy JR (eds): Cancer Chemotherapy, pp 35–47. New
York, Grune & Stratton, 1978 40. Lemmel E, Hurd ER, Ziff M: Differential effects of 6-mercaptopurine and cyclophosphamide on autoimmune
phenomena in NZB mice. Clin Exp Immunol 8:355, 1971 41. Dutton RW, Pearce JD: A survey of the effect of metabolic antagonists on the synthesis of antibody
in an in vitro system. Immunology 5:414, 1962 42. Borel Y, Fauconnet M, Miescher PA: Effect of 6-mercaptopurine (6-MP) on different classes of antibody. J Exp Med 122:263, 1965 43. Rollinghoff M, Schrader J, Wagner H: Effect of azathioprine and cytosine arabinoside on humoral and cellular
immunity in vitro. Clin Exp Immunol 15:261, 1973 44. Hoyer LW, Condie RM, Good RA: Prevention of experimental allergic encephalomyelitis with 6-MP. Proc Soc Exp Biol Med 103:205, 1960 45. Borel Y, Schwartz RS: Inhibition of immediate and delayed hypersensitivity in rabbits by 6-mercaptopurine. J Immunol 92:754, 1964 46. Calne RY: The rejection of renal homografts inhibition in dogs by 6-mercaptopurine. Lancet 1:417, 1960 47. Giblett ER, Anderson JE, Cohen F et al: Adenosine-deaminase deficiency in two patients with severely impaired cellular
immunity. Lancet 2:1067, 1972 48. James GD: Immunotherapy. Trans Ophthalmol Soc UK 97:468–473, 1977 49. Foster CS, Wilson LA, Ekins MB: Immunosuppressive therapy for progressive ocular cicatricial pemphigoid. Ophthalmology 89:340, 1982 50. Andrasch RH, Pirofsky B, Burns RP: Immunosuppressive therapy for severe chronic uveitis. Arch Ophthalmol 92:247, 1978 51. Moore CE: Sympathetic ophthalmitis treated with azathioprine. Br J Ophthalmol 52:688, 1968 52. Foster CS: Immunomodulation in ophthalmology. Int Ophthalmol Clin 23:193, 1983 53. Newell FW, Krill AE, Thomson A: The treatment of uveitis with 6-mercaptopurine. Am J Ophthalmol 61:1250, 1966 54. Aoki K, Sugiura S: Immunosuppressive treatment of Behçet's disease. Mod Probl Ophthalmol 16:309, 1976 55. Yazici H, Pazarli H, Barnes CG et al: A controlled trial of azathioprine in Behçet's syndrome. N Engl J Med 322:281, 1990 56. Hooper PL, Kaplan HJ: Triple agent immunosuppression in serpiginous choroiditis. Ophthalmology 98:944, 1991 57. Hertz R: Folic acid antagonists: Effects on the cell and the patient. Clinical Staff
Conference at NIH. Ann Intern Med 59:931, 1963 58. Farber S, Diamond LK, Mercer RD et al: Temporary remission in acute leukemia in children produced by folic antagonist 4-amethopteroylglutamic acid (aminopterin). N Engl J Med 238:787, 1948 59. Skipper HT, Schabel FM Jr: Quantitative and cytokinetic studies in experimental
tumor models. In Holland JF, Frei E III (eds): Cancer Medicine, pp 629–650. Philadelphia, Lea & Febiger, 1973 60. Berenbaum MC: Prolongation of homograft survival in guinea pigs: Effect of pretreatment
with amethopterin. Transplantation 2:116, 1964 61. Levy L, Whitehouse MW: Selective stimulation of a cellular immune response by methotrexate. Agents Actions 4:113, 1974 62. Schwartz RS: Immunosuppressive drug therapy. In Rapaport FT, Dausset J (eds): Human
Transplantation, pp 440–471. New York, Grune & Stratton, 1980 63. Heppner GH, Calabresi P: Selective suppression of humoral immunity by antineoplastic drugs. Ann Rev Pharmacol Toxicol 16:367, 1976 64. Currey HLF: A comparison of immunosuppressive and anti-inflammatory agents in the rat. Clin Exp Immunol 9:879, 1971 65. Weinstein GM: Methotrexate. Ann Intern Med 86:199, 1977 66. Gerber NL, Steinberg AD: Clinical use of immunosuppressive drugs: II. Drugs 11:90, 1976 67. Ramsay NKC, Kersey JH, Robison LL et al: A randomized study of the prevention of acute graft-versus-host disease. N Engl J Med 306:392, 1982 68. Wong VG: Immunosuppressive therapy of ocular inflammatory diseases. Arch Ophthalmol 81:628, 1969 69. Foster CS, Forstot L, Wilson LA: Mortality rate in rheumatoid arthritis patients with destructive ocular
lesions: Effect of systemic immunosuppression. Ophthalmology 91:1253, 1984 70. Holz FG, Krastel H, Breitbart A et al: Low-dose methotrexate treatment in noninfectious uveitis resistant to corticosteroids. Ger J Ophthalmol 1:142, 1992 71. Shah SS, Lowder CY, Schmitt MA et al: Low-dose methotrexate therapy for ocular inflammatory disease. Ophthalmology 99:1419, 1992 72. Rootman J, Gudauskas G: Treatment of ocular leukemia with local chemotherapy. Cancer Treat Rep 69:119, 1985 73. Hargreaves MR, Mowat AG, Benson MK: Acute pneumonitis associated with low-dose methotrexate treatment for rheumatoid
arthritis: Report of five cases and review of published reports. Thorax 47:628, 1992 74. Carroll GJ, Thomas R, Phatouras CC et al: Incidence, prevalence and possible risk factors for pneumonitis in patients
with rheumatoid arthritis receiving methotrexate. J Rheumatol 21:51, 1994 75. Doroshow JH, Locker GY, Gaasterland DE et al: Ocular irritation from high-dose methotrexate therapy: Pharmacokinetics
of drug in the tear film. Cancer 48:2158, 1981 76. Wenger R: Chemistry of cyclosporin. In White DJG (ed): Cyclosporin A, pp 19–34. Amsterdam, Elsevier Biomedical Press, 1982 77. Beveridge T: Pharmacokinetics and metabolism of cyclosporin A. In White
DJG (ed): Cyclosporin A, pp 35–44. Amsterdam, Elsevier Biomedical
Press, 1982 78. Dreyfuss M, Harri E, Hofmann H et al: Cyclosporin A and C: New metabolites from Trichoderma polysporum (Link ex Pers) Rifai. Eur J Appl Microbiol 3:125, 1976 79. Nussenblatt RB: Cyclosporine: Immunology, pharmacology, and therapeutic uses. Surv Ophthalmol 31:159, 1986 80. Thommen K: Antimalarial activity of cyclosporin A. Agents Action 11:770, 1981 81. Borel JF, Feurer C, Gubler HU et al: Biological effects of cyclosporin A: A new antilymphocytic agent. Agents Actions 6:468, 1976 82. Borel JF, Feurer C, Magnee C et al: Effects of the new anti-lymphocyte peptide cyclosporin A in animals. Immunology 32:1017, 1977 83. Borel JF, Wiesinger D: Effect of cyclosporin A on murine lymphoid cells. In
Lucas DO (ed): Regulatory Mechanisms in Lymphocyte Activation, pp 716–718. New
York, Academic Press, 1976 84. Wiesinger D, Borel JF: Studies on the mechanism of action of cyclosporin A. Immunology 156:454, 1979 85. Larsson EL: Cyclosporin A and dexamethasone suppress T-cell responses by selectively
acting at distinct sites of the triggering process. J Immunol 124:2828, 1980 86. Cross SL, Halden NF, Lenardo MJ, Leonard WJ. Functionally distinct NF-kappa
B binding sites in the immunoglobulin kappa and IL-2 receptor alpha
chain genes. Science 244:466, 1989 87. Hess AD, Tutschka PJ: Effect of cyclosporin A on human lymphocyte responses in vitro. I. CsA
allows for the expression of alloantigen-activated suppressor cells while
preferentially inhibiting the induction of cytolytic effector lymphocytes
in MLR. J Immunol 124:2601, 1980 88. Horsburgh T, Wood P, Brent L: Suppression of in vitro lymphocyte reactivity by cyclosporin A: Existence
of a population of drug-resistant cytotoxic lymphocytes. Nature 286:609, 1980 89. Sweny P, Tidman N: The effect of cyclosporin A on peripheral blood T cell subpopulations in
renal allografts. Clin Exp Immunol 47:445, 1982 90. Kunkl A, Klaus GGB: Selective effects of cyclosporin A on functional B cell subsets in the
mouse. J Immunol 125:2526, 1980 91. Introna M, Allavena P, Spreafico F et al: Inhibition of human natural killer activity by cyclosporin A. Transplantation 31:113, 1981 92. Calne RY, Rolles K, White DJG et al: Cyclosporin A initially as the only immunosuppressant in 34 recipients
of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 2:1033, 1979 93. Calne RY, Rolles K, White DJG et al: Cyclosporin A in clinical organ grafting. Transplant Proc 13:349, 1981 94. Powles RL, Clink HM, Spence D et al: Cyclosporin A to prevent graft-versus-host disease in man after allogeneic
bone marrow transplantation. Lancet 1:327, 1980 95. Reitz BA, Wallwork JL, Hunt SA et al: Heart-lung transplantation: Successful therapy for patients with pulmonary
vascular disease. N Engl J Med 306:557, 1982 96. Wick G, Muller PU, Schwarz S: Effect of cyclosporin A on spontaneous autoimmune thyroiditis of obese
strain (OS) chickens. Eur J Immunol 12:879, 1982 97. Laupacis A, Stiller CR, Gardell C et al: Cyclosporin prevents diabetes in BB Wistar rats. Lancet 1:10, 1983 97a. Bolton C, Borel JF, Cuzner ML et al: Autoimmunity: Cyclosporin A therapy
in experimental allergic encephalomyelitis. In White DJG (ed): Cyclosporin
A, 135–142. Amsterdam, Elsevier Biomedical Press, 1982 98. Shepherd WFI, Coster DJ, Chin Fook T et al: Effect of cyclosporin A on the survival of corneal grafts in rabbits. Br J Ophthalmol 64:148, 1980 99. Salisbury JD, Gebhardt BM: Suppression of corneal allograft rejection by cyclosporin A. Arch Ophthalmol 99:1640, 1981 100. Hunter PA, Wilhelmus KR, Rice NSC et al: Cyclosporin A applied topically to the recipient eye inhibits corneal graft
rejection. Clin Exp Immunol 45:173, 1981 101. Nussenblatt RB, Rodrigues MM, Wacker WB et al: Cyclosporin A: Inhibition of experimental autoimmune uveitis in Lewis rats. J Clin Invest 67:1228, 1981 102. Nussenblatt RB, Rodrigues MM, Salinas-Carmona MC et al: Modulation of experimental autoimmune uveitis with cyclosporin A. Arch Ophthalmol 100:1146, 1982 103. Nussenblatt RB, Salinas-Carmona MS, Waksman BH et al: Cyclosporin A: Alterations of the cellular immune response in S-antigen-induced
experimental autoimmune uveitis. Int Arch Allergy Appl Immunol 70:289, 1983 104. Sumner HL, Meyers-Elliot RH: Cellular events in the initiation and progression of rhodopsin-induced
experimental autoimmune uveitis (EAU-retinopathy) in strain 13 guinea
pigs. Invest Ophthalmol Vis Sci 22 (suppl 3):263, 1982 105. Nussenblatt RB, Palestine AG, Rook AH et al: Cyclosporine therapy of intraocular
inflammatory disease. Lancet ii:235, 1983 106. Nussenblatt RB, Palestine AG, Chan CC: Cyclosporine therapy in the treatment of intraocular inflammatory disease
resistant to systemic corticosteroids or cytotoxic agents. Am J Ophthalmol 96:275, 1983 107. Towler HM, Whiting PH, Forrester JV: Combination low dose cyclosporin A and steroid therapy in chronic intraocular
inflammation. Eye 4 (pt 3):514, 1990 108. Secchi AG, De Rosa C, Pivetti-Pezzi P et al: Open noncontrolled multicenter long-term trial with cyclosporin in endogenous
non-infectious uveitis. Ophthalmologica 202: 217, 1991 109. Leznoff A, Shea M, Binkley KE et al: Cyclosporine in the treatment of nonmicrobial inflammatory ophthalmic disease. Can J Ophthalmol 27:302, 1992 110. de Smet MD, Rubin BI, Whitcup SM et al: Combined use of cyclosporine and ketoconazole in the treatment of endogenous
uveitis. Am J Ophthalmol 113:687, 1992 111. Shulman H, Striker G, Deeg HJ et al: Nephrotoxicity of cyclosporin A after allogeneic marrow transplantation. N Engl J Med 305:1392, 1981 112. Hedley D, Rowles RL, Morgenstein GR: Toxicity of cyclosporin A in patients
following bone marrow transplantation. In White DJG (ed): Cyclosporin
A, pp 545–552. Amsterdam, Elsevier Biomedical Press, 1982 113. Palestine AG, Austin HA, Balow JE et al: Renal histopathologic alterations in patients treated with cyclosporine
for uveitis. N Engl J Med 314:1293, 1986 114. Isenberg DA, Snaith ML, Al-Khader AA et al: Cyclosporine relieves arthralgia, causes angioedema. N Engl J Med 303:754, 1980 115. Kino T, Hatanabe H, Hashimoto M et al: FK506, a novel immunosuppressant isolated from streptomyces. I. Fermentation, isolation, and
physiochemical and biological characteristics. J Antibiot 40:1249, 1987 116. Kino T, Hatanaka H, Miyato S et al: FK506, a novel immunosuppressant isolated from a streptomyces. II. Immunosuppressive
effect of FK506 in vitro. J Antibiot 40:1256, 1987 117. Mochizuki M, Masuda K, Sakane T et al: A clinical trial of FK506 in refractory uveitis. Am J Ophthalmol 115:763, 1993 118. Mochizuki M: Japanese FK506 Study Group on Refractory Uveitis in Behçet's
Disease. In Wechsler B, Godeau P (eds): Behçet's
Disease: Proceedings of the 6th International Conference on Behçet's
Disease, pp 655–660. Amsterdam, Excerpta Medica, 1993 119. Stendahl O, Molin L, Dahlgren C: The inhibition of polymorphonuclear leukocyte cytotoxicity by dapsone. J Clin Invest 62:214, 1978 120. Barranco VP: Inhibition of lysosomal enzymes by dapsone. Arch Dermatol 110:563, 1974 121. Millikan LE, Conway FR: Effect of drugs on the Pillemer pathway--dapsone. J Invest Dermatol 62:541, 1974 122. Thompson DM, Souhami R: Suppression of the Arthus reaction in the guinea pig by dapsone. Proc R Soc Med 68:273, 1975 123. Griffin JD, Garnick MB: Eye toxicity of cancer chemotherapy: A review of the literature. Cancer 48:1539, 1981 124. Williams K, Capstick RB, Lewis DA et al: Anti-inflammatory actions of dapsone and its related biochemistry. J Pharm Pharmacol 28:555, 1976 125. Katz SI: Dermatitis herpetiformis. In Lichtenstein LM, Fauci AS (eds): Current
Therapy in Allergy and Immunology, 1983-1984, pp 154–156. Philadelphia, BC
Decker, 1983 126. Cupps TR, Fauci AS: Cutaneous vasculitis. In Lichtenstein LM, Fauci AS (eds): Current
Therapy in Allergy and Immunology, 1983-1984, pp 136–139. Philadelphia, BC
Decker, 1983 127. Rogers RS, Seehafer JR, Perry HO: Treatment of cicatricial (benign mucous membrane) pemphigoid with dapsone. J Am Acad Dermatol 6:215, 1982 128. Diaz LA, Provost TT: Bullous pemphigoid. In Lichtenstein ML, Fauci AS (eds): Current
Therapy in Allergy and Immunology, 1983-1984, pp 171–172. Philadelphia, BC
Decker, 1983 129. DeGowin RL: A review of therapeutic and hemolytic effects of dapsone. Arch Intern Med 120:242, 1967 130. Malawista SE: The action of colchicine in acute gouty arthritis. Arthritis Rheum 19 (suppl):835, 1975 131. Mizushima Y, Matsumura N, Mori M et al: Colchicine in Behçet's disease. Lancet 1:1037, 1977 132. Baer JC, Foster CS, Raizman MB: Ocular Behçet's syndrome: Clinical presentation and visual
outcome in 26 patients. Ophthalmology 96 (suppl):128, 1989 133. Zurier RB: Prostaglandins, immune responses and inflammation. In Helmsen
RJ, Suran AA, Gery I et al (eds): Immunology of the Eye Workshop II: Autoimmune
Phenomena and Ocular Disorders. Immunology Abstracts 273, 1981 134. Williams TJ, Peck MJ: Role of prostaglandin-mediated vasodilation in inflammation. Nature 270:530, 1977 135. Kuehl FA Jr, Humes JL, Egan RW et al: Role of prostaglandin endoperoxide PGG2 in inflammatory processes. Nature 265:170, 1979 136. Moncada S, Ferreira SH, Vane JR: Prostaglandins, aspirin-like drugs and the oedema of inflammation. Nature 246:217, 1973 137. Turner SR, Campbell JA, Lynn WS: Polymorphonuclear chemotaxis toward oxidized lipid components of cell membranes. J Exp Med 141:1437, 1975 138. Leung KH, Mihich E: Prostaglandin modulation of development cell-mediated immunity in culture. Nature 288:597, 1980 139. Zurier RB, Ballas M: Prostaglandin E1 (PGE1) suppression of adjuvant arthritis. Arthritis Rheum 16:251, 1973 140. Kunkel SL, Thrall RS, McCormick JR et al: Suppression of immune complex vasculitis in rats by prostaglandins. J Clin Invest 64:1525, 1979 141. Vane JR: Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like
drugs. Nature 231:232, 1971 142. Ferrerra SH, Moncada S, Vane JR: Indomethacin and aspirin abolish prostaglandin release from the spleen. Nature 231:237, 1971 143. Smith JB, Willis AL: Aspirin selectively inhibits prostaglandin production in human platelets. Nature 231:235, 1971 144. Flower RJ, Moncada S, Vane JR: Analgesic-antipyretics and anti-inflammatory
agents; drugs employed in the treatment of gout. In Gilman AG, Goodman
LS, Gilman A (eds): The Pharmacological Basics of Therapeutics, 6th
ed pp 1263–1268. New York, Macmillan, 1980 145. Pinals RS, Frank S: Relative efficacy of indomethacin and acetylsalicylic acid in rheumatoid
arthritis. N Engl J Med 276:512, 1967 146. Godfrey RG, Calabro JJ, Mills D et al: A double blind crossover trial of aspirin, indomethacin, and phenylbutazone
in ankylosing spondylitis. Arthritis Rheum 15:110, 1972 147. Zuckerman H, Reiss U, Rubenstein I: Inhibition of human premature labor by indomethacin. Obstet Gynecol 44:787, 1974 148. Heymann MA, Rudolph AM, Silverman NH: Closure of the ductus arteriosus in premature infants by inhibition of
prostaglandin synthesis. N Engl J Med 295:530, 1976 149. Klein RM, Katzin HM, Yannuzzi LA: The effect of indomethacin pretreatment on aphakic cystoid macular edema. Am J Ophthalmol 87:487, 1979 150. Miyake K: Prevention of cystoid macular edema after lens extraction by topical indomethacin. I. A
preliminary report. Albrect Von Graefes Arch Klin Exp Ophthalmol 203:81, 1977 151. Yannuzzi LA, Landau AU, Turtz AI: Incidence of aphakic cystoid macular edema with the use of topical indomethacin. Ophthalmology 88:947, 1981 152. Miyake K, Sugiyama S, Norimatsu I et al: Prevention of cystoid macular edema after lens extraction by topical indomethacin. III. Radioimmunoassay measurement of prostaglandins in the
aqueous during and after lens extraction procedures. Albrect Von Graefes Arch Klin Exp Ophthalmol 209:83, 1978 153. Kraff MC, Sanders DR, Jampol LM et al: Prophylaxis of pseudophakic cystoid macular edema with topical indomethacin. Ophthalmology 89:885–890, 1982 154. Miyake K, Miyake Y, Maekubo K et al: Incidence of cystoid macular edema after retinal detachment surgery and
the use of topical indomethacin. Am J Ophthalmol 95:451, 1983 155. Jampol LM: Pharmacologic therapy of aphakic cystoid macula edema. Ophthalmology 89:891, 1982 156. Perkins ES, MacFaul PA: Indomethacin in the treatment of uveitis: A double-blind trial. Trans Ophthalmol Soc UK 85:53, 1965 157. Abelson MB, Butrus SI, Weston JH: Aspirin therapy in vernal conjunctivitis. Am J Ophthalmol 95:501, 1983 158. Abel JJ, Rowntree LG, Turner BB: Plasma removal with return of corpuscles. J Pharm Exp Ther 5:625, 1914 159. Skoog WA, Adams WS: Plasmapheresis in a case of Waldenström's macroglobulinemia. Clin Res 7:96, 1959 160. Branda RF, Moldow CF, McCullough JJ et al: Plasma exchange in the treatment of immune disease. Transfusion 15:570, 1975 161. Jones JV: Plasmapheresis in SLE. Clin Rheum Dis 8:243, 1982 162. Schur PH, Sandson J: Immunologic factors and clinical activity in systemic lupus erythematosus. N Engl J Med 278:533, 1968 163. Frank MM, Hamburger MI, Lawley TJ et al: Defective reticuloendothelial system Fc-receptor function in systemic lupus
erythematosus. N Engl J Med 300:518, 1979 164. Dandona P, Marshall NJ, Bidey SP et al: Successful treatment of exophthalmos and pretibial myxoedema with plasmapheresis. Br Med J 10:374, 1979 165. Lewis RA, Slater N, Croft DN: Exophthalmos and pretibial myxoedema not responding to plasmapheresis. Br J Med 11:390, 1979 166. Saraux H, Le Hoang P, Audebert AA et al: Intérêt de la plasmapherese dans le traitement du syndrome
de Behçet. Bull Soc Ophtalmol Fr 82:41, 1982 167. Wing EJ, Bruns FJ, Fraley DS et al: Infectious complications with plasmapheresis in rapidly progressive glomerulonephritis. JAMA 244:2423, 1980 168. Cardella CL: Plasma exchange--a new approach to immune modulation in rheumatic diseases. J Rheumatol 6:606, 1979 169. Inderbitzen T: The relationship of lymphocytes, delayed cutaneous allergic reactions and
histamine. Int Arch Allergy 8:150, 1956 170. Taub RN, Deutch V: Antilymphocytic serum. Pharmacol Ther 2:89, 1977 171. Waksman BH, Arbouys S, Arnason BG: The use of specific “lymphocyte” antisera to inhibit hypersensitive
reactions of the “delayed” type. J Exp Med 114:997, 1961 172. Mandel MA, Asofsky R: The effects of heterologous anti-thymocyte sera in mice. I. The use of
a graft-versus-host assay as a measure of homograft reactivity. J Immunol 100:1319, 1968 173. Monaco AP, Wood ML, Gray JG et al: Studies on heterologous anti-lymphocyte serum in mice. II. Effect on the
immune response. J Immunol 96:229, 1966 174. Kerbel RS, Eidinger D: Variable effects of antilymphocyte serum on humoral antibody formation: Role
of thymus dependency of antigen. J Immunol 106:917, 1971 175. Touraine JL, Malik MC, Traeger J: Antilymphocyte globulin and thoracic
duct drainage in renal transplantation. In Salaman JR (ed): Immunosuppressive
Therapy, pp 55–73. Philadelphia, JB Lippincott, 1981 176. Cosimi AB, Burton RC, Kung PC et al: Evaluation in primate renal allograft of monoclonal antibody to human T
cells subclasses. Transplant Proc 13:499, 1981 177. Prentice HG, Blacklock HA, Janossy G et al: Use of anti-T-cell monoclonal antibody OKT3 to prevent acute graft-versus-host
disease in allogeneic bone marrow transplantation for acute leukemia. Lancet 1:700, 1982 178. Ring J, Dechant W, Seifert J et al: Immunosuppression with antilymphocyte globulin (AL6) in the treatment of
ophthalmic disorders. Ophthalmol Res 10:82, 1978 179. Brown PS, Parenteau GL, Dirbas FM et al: Anti-Tac-H, a humanized antibody to the interleukin 2 receptor, prolongs
primate cardiac allograft survival. Proc Natl Acad Sci USA 88:2663, 1991 180. Soulilou JP, Peyronnet P, Le Mauff B et al: Randomized controlled trial of a monoclonal antibody against the interleukin-2 receptor (33B3. 1) as compared with rabbit antithymocyte globulin
for prophylaxis against rejection of renal allografts. N Engl J Med 322:1175, 1990 181. Blaise D, Olive D, Hirn M et al: Prevention of acute GVHD by in vivo use of anti-interleukin-2 receptor
monoclonal antibody (33B3.1): a feasibility trial in 15 patients. Bone Marrow Transplant 8:105, 1991 182. Whitcup SM, Chan CC, Li Q, Nussenblatt RB: Expression of cell adhesion molecules in posterior uveitis. Arch Ophthalmol 110:662, 1992 183. Whitcup SM, DeBarge LR, Rosen H et al: Monoclonal antibody against CD11b/CD18 inhibits endotoxin-induced uveitis. Invest Ophthalmol Vis Sci 34:673, 1993 184. Whitcup SM, DeBarge LR, Caspi RR et al: Monoclonal antibodies against ICAM-1 (CD54) and LFA-1 (CD11a/CD18) inhibit
experimental autoimmune uveitis. Clin Immunol Immunopathol 67:143, 1993 185. Wells H: Studies on the chemistry of anaphylaxis. III. Experiments with isolated
proteins, especially those of hen's egg. J Infect Dis 9:147, 1911 186. Bitar DM, Whitacre CC: Suppression of experimental autoimmune encephalomyelitis by the oral administration
of myelin basic protein. Cell Immunol 112:364, 1988 187. Nussenblatt RB, Caspi RR, Mahdi R et al: Inhibition of S-antigen induced experimental autoimmune uveoretinitis by
oral induction of tolerance with S-antigen. J Immunol 144:1689, 1990 188. Weiner HL, Mackin GA, Matsui M et al: Double-blind pilot trial or oral tolerization with myelin antigens in multiple
sclerosis. Science 259 (5099):1321, 1993 189. Trentham DE, Dynesius-Trentham RA, Orav EJ et al: Effects of oral administration of type II collagen on rheumatoid arthritis. Science 261 (5129):1727, 1993 |