VASCULATURE
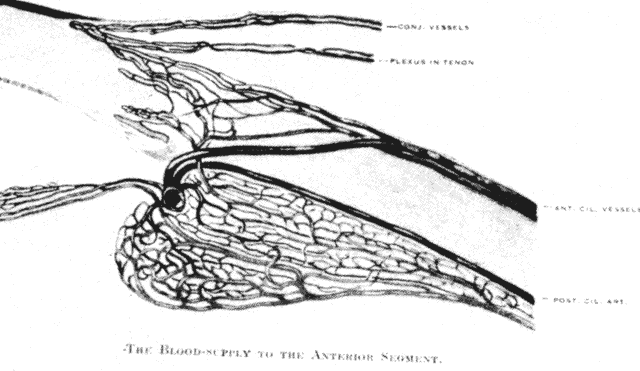
The lacrimal artery, the superior and inferior medial palpebral arteries, and the branches of the ophthalmic artery that supply the extraocular muscles and ciliary body form the rich blood supply of the conjunctiva, eyelids, and lacrimal gland (Fig. 1).1 This blood supply can be thought of as a natural immunologic defense. When the eyes are inflamed, the blood vessels dilate, fluid leaks into the extravascular spaces, and leukocytes migrate into these spaces from the bloodstream. More specifically, macrophages, polymorphonuclear leukocytes, lymphocytes, C-reactive protein, immunoglobulins, and so forth are the elements of the defense system that are brought to these ocular tissues during inflammation.2
|
Since many blood vessels are grossly visible in such tissues as the conjunctiva, immunologic inflammatory disease, which produces only subtle changes in buried organs such as the kidney, may cause conjunctival inflammation that is readily apparent early in its course.
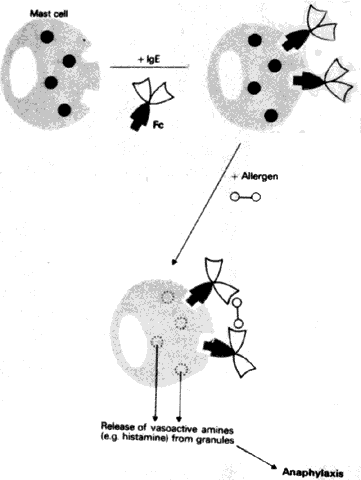
There are neurogenic and nonneurogenic causes of vasomotor activity. The capillaries, precapillaries, and small arterioles are activated by chemical materials; the large vessels near the terminal arterioles are activated by the nervous system. The nonneurogenic causes are complicated but are basically (1) the vasoactive amines, including histamine and 5-hydroxytryptamine, (2) the serum proteases and polypeptides known as kinins, and (3) the prostaglandins. Most inflammatory reactions, in which the vessels dilate and their permeability increases, are biphasic: there is an immediate response (medicated by histamine-like substances) that reaches its maximum 8 to 10 minutes after application of the noxious stimulus, and there is a late response (mediated by kinins and prostaglandins) that occurs after an intervening normal period.
Since the normal cornea is devoid of blood vessels, the immunologic mechanisms are less effective and the tissue does not respond readily to antigenic insults (i.e., microorganisms, corneal grafts, and so forth). Necrosis and the subsequent accumulation of polymorphonuclear leukocytes vascularize the cornea and alter its privileged immune status.3
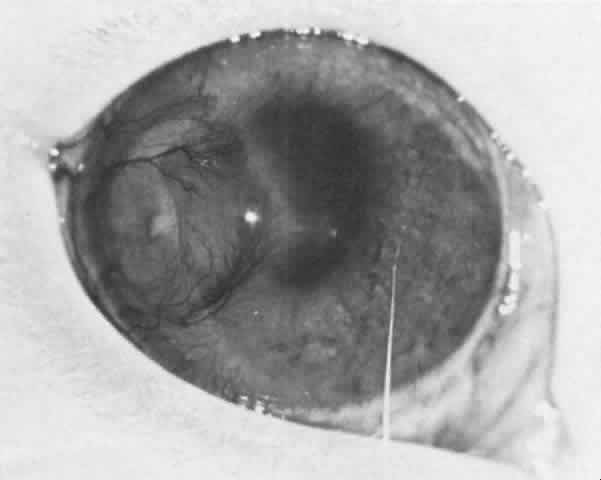
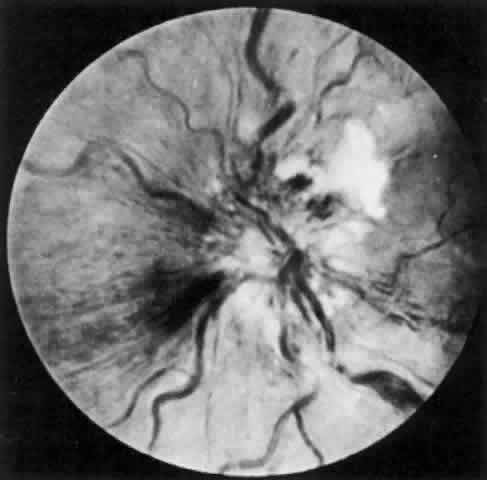
The behavior of corneal allografts is illustrative. When the results of one series were averaged, hosts with avascular corneal beds had rejected 3.5% of the grafts in 10 months, hosts with mildly vascularized corneal beds had rejected 13.3% in 4 months, and hosts with moderately vascularized corneal beds had rejected 65% in 2 months.4 It has also been shown that in experimental animals, 95% of lamellar corneal grafts and 75% of penetrating grafts placed in avascular beds survived for long periods of time. This was true even in animals sensitized by permitting them to reject large skin grafts from the same donors that provided the corneal buttons. These amply sensitized animals could not convey the sensitized lymphocytes to their avascular corneas.5 It is also significant that the graft rejection line usually starts at the site of maximum vascularization (Fig. 2).
Trauma and non-graft-related inflammation (both of which dilate the vessels) and interrupted sutures left in place too long (causing neovascularization) can increase graft rejection by affecting the vasculature.
LYMPHATIC VESSELS AND REGIONAL LYMPH NODES
The lymphatics are arranged in pretarsal and posttarsal plexuses and are connected by cross channels. The posttarsal channels drain the conjunctiva and tarsal glands, and the pretarsal channels drain the skin and skin structures. Both groups drain from the lateral side into the preauricular and parotid lymph nodes, and from the medial side into the submandibular lymph nodes.
Although cell-lined lymphatic vessels do not occur in nonvascularized corneas, their presence in vascularized corneas seems to have been well established by the experimental work of Collin, Busacca, Mann, Aoki, and Smolin and their colleagues.6–10
Lymphatics and regional lymph nodes play a significant role in immunologic reactions (e.g., in transplantation). Certain tissues, such as hamster cheek-pouch, testes, brain, anterior chamber, lens, and avascular cornea, are devoid of conventional lymphatic drainage. Privileged in this respect, they support the continued growth of grafted tissue.11,12 In the conjunctiva and vascularized cornea, however, the lymphatics increase the host's ability to recognize nonself corneal antigen.
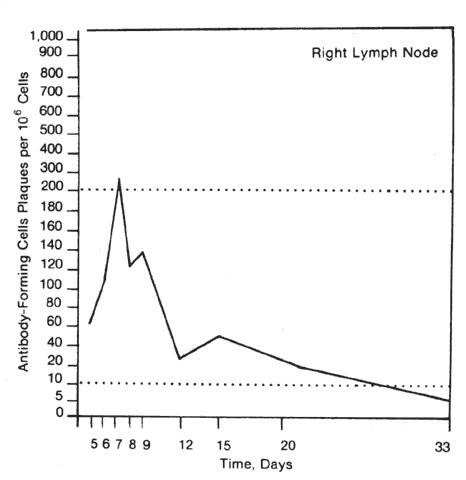
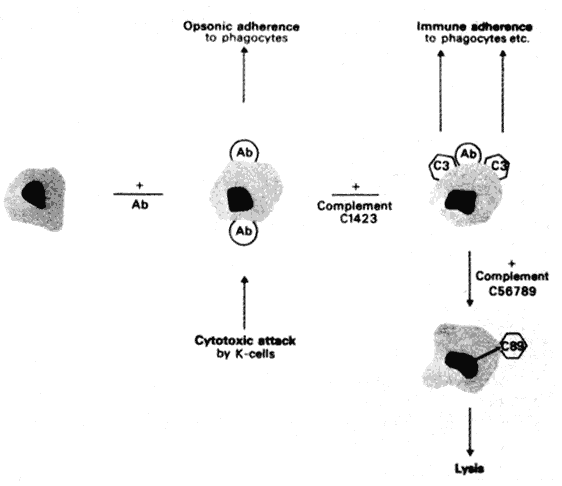
Langerhans' cells, which are more abundant peripherally in the cornea than centrally, may process the antigen, thus enhancing immune responsiveness. Depletion of these cells may prolong nonrecognition and graft survival. After the unilateral intracorneal injection of rabbit eyes with bovine gamma globulin, many antibody-forming cells are found in the homolateral draining lymph nodes, corneal limbus, and uveal tract. The first sites at which these antibody-forming cells can be detected are the draining nodes (Fig. 3).13
|
TEAR FILM AND LACRIMAL APPARATUS
In addition to the blood vessels and lymphatic channels, there are other features of the external ocular tissue that affect the immunologic reaction. The intact epithelial surface, the low surface temperature, the low pH of the tear film, the presence of macrophages, and lid blinking are important as natural immunologic defense mechanisms. The tears themselves are also a natural immune defense. They not only wash away antigen and potentially pathogenic bacteria but contain bacteriostatic and bactericidal substances as well.
Sapse and co-workers identified the following seven proteins in human tears: specific tear prealbumin, serum albumin, ceruloplasmin, transferrin or lactoferrin, immunoglobulin A (IgA), immunoglobulin G (IgG), and lysozyme.14 The lysozyme comes from the type A cell in the lacrimal gland. (The type B cell supplies a neutral glycoprotein and sulfosialomucin.15) The tear prealbumin, IgA, lysozyme, and lactoferrin are the major tear proteins.16 The bacteriostatic substances in the tears are lactoferrin, lysozyme, and a nonlysozyme antibacterial agent (beta lysin?) that often appears to act in concert with other immunologic mechanisms.17,18 Lactoferrin has a concentration of about 2 mg/mL in tears and represents one of the major tear proteins.19 Lactoferrin, as well as other tear proteins (tear-specific prealbumin, lysozyme, secretory IgA), is synthesized and excreted by the lacrimal gland.20 Lactoferrin appears to act directly on certain strains of bacteria21 and it may interfere with the complement system and regulate granulocyte and macrophage colony-stimulating factors.22
The tears contain appreciable amounts of IgA, IgG, and complement, all of which are probably derived from the normal plasma cells lying beneath the conjunctival epithelium and in the stroma of the lacrimal gland.23,24 Although most of the plasma cells in and around the eye are located in the lacrimal gland, there are also significant numbers in the conjunctiva, fewer in the accessory gland, and insignificant numbers in the limbal tissue.25 Staining patterns indicate the presence of IgG, IgD, IgA, and IgE in the lacrimal gland. IgM is absent.
The conjunctiva may contain localized areas (similar to Peyer's patches) for processing antigen. The mucous membrane-associated tissue (MALT) may be part of an integrated system of MALT present in other tissue and allows for the “homing” of antibody to the ocular tissue, especially to the lacrimal gland. In the conjunctiva, these lymphoid collections are sometimes referred to as CALT.26
Although tear IgA undoubtedly comes from the plasma cells, the increase in tear IgG during acute inflammation, associated with increased vascular permeability, indicates that some of these immunoglobulins are derived from the circulating blood. Most of the tear IgA has an attached secretory piece (formed by the lacrimal gland epithelium) and is relatively resistant to proteolytic enzymes.27 In an environment rich in proteolytic enzymes, this allows it to act as antibody and to play a role in the regulation of the normal bacterial and viral flora of the mucous membranes.28
IMMUNOGLOBULIN DISTRIBUTION
The conjunctiva has a rich supply of immunoglobulins. Extracellular IgG, IgA, and IgM, all of which have been found in the conjunctiva's substantia propria, may come from the rich vascular supply, the abundant plasma cells, the tear film, the lacrimal gland, or more likely, from all four sources.29
McMaster and colleagues feel that most of the proliferation of lymphatic tissue in the conjunctiva is a response to infection by organisms in the environment.30
IgG and IgA are found in the cornea, at the same levels centrally and peripherally, and at one-half and one-fifth the serum levels, respectively; IgMis found less often and only rarely in the central cornea. Albumin is present in the cornea, with less of it centrally than peripherally.31 The immunoglobulins have been found almost exclusively in the stroma but sometimes also in the epithelium and endothelium.
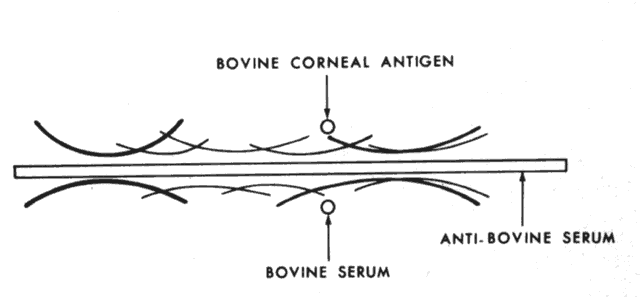
Other serum proteins (alpha-globulin, beta-globulin) are found in the corneal stroma, and nonserum proteins are found in the corneal epithelium.32 In serologic studies some investigators have shown organ specificity for some corneal antigens and species specificity for others (Fig. 4).33