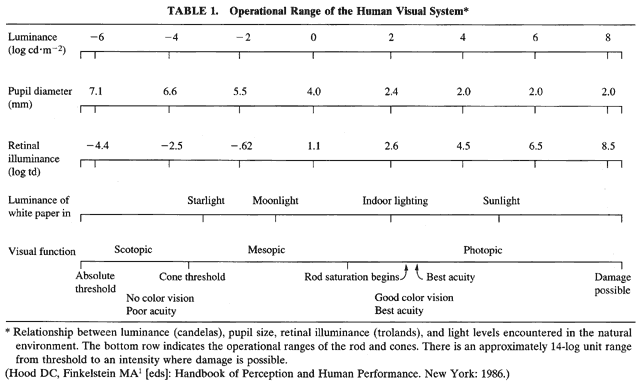
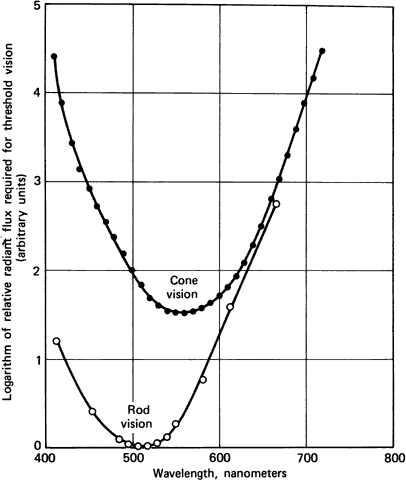
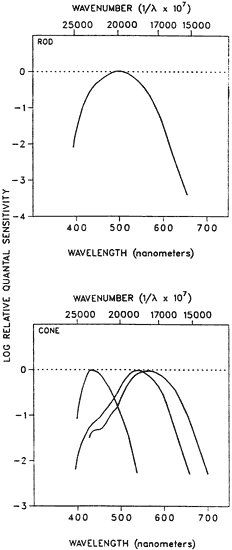
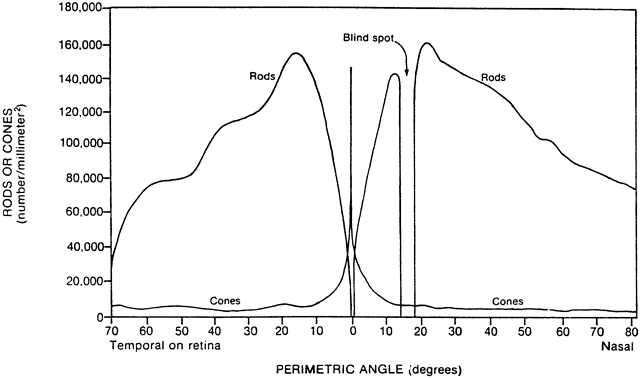
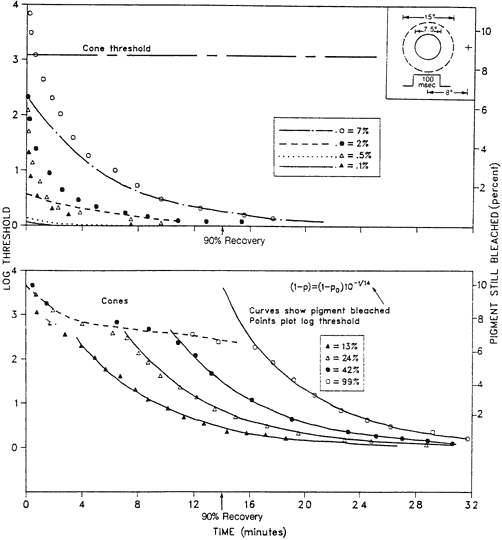
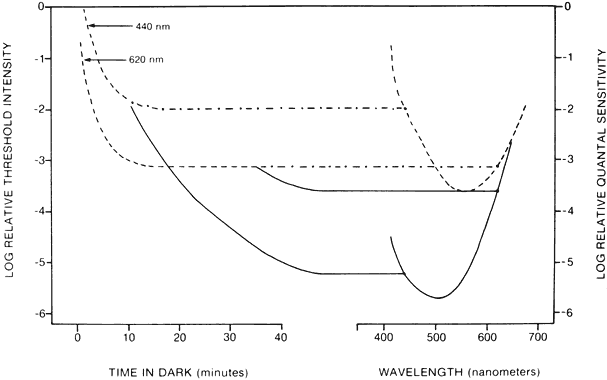
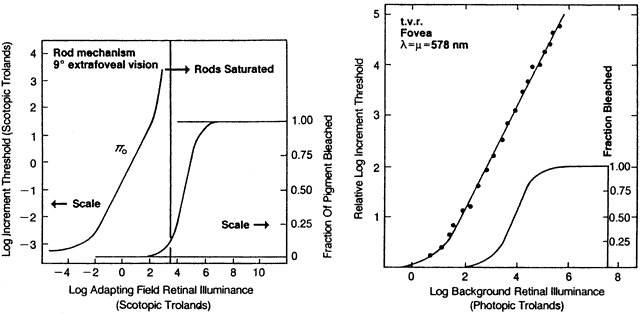
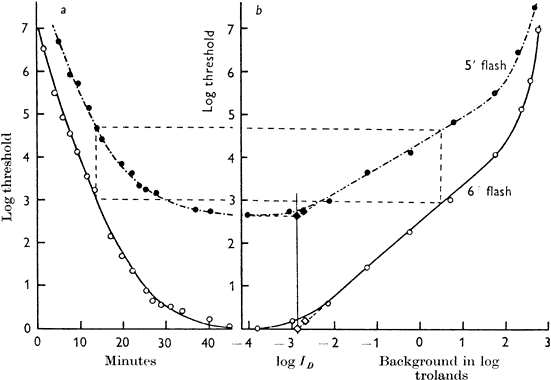
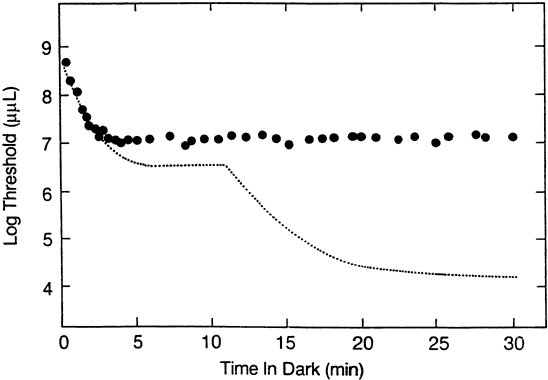
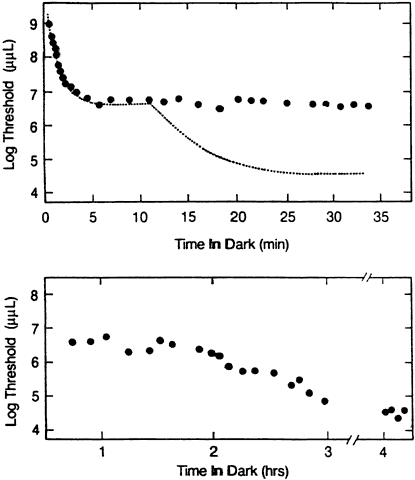
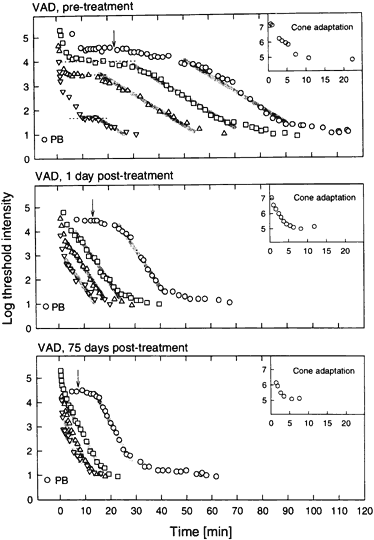
1. Hood DC, Finkelstein MA: Sensitivity to light. In Boff KR, Kaufman L, Thomas
JP (eds): Handbook of Perception and Human Performance, pp 1–66. New
York: Wiley, 1986 2. Shapley R, Enroth-Cugell C: Visual Adaptation and Retinal Gain Controls, pp 263–346. Oxford, UK: Pergamon, 1984 3. Green DG: Visual acuity, color vision, and adaptation. In Albert DM, Jacobiek
FA (eds): Principles and Practices of Ophthalmology, pp 332–349. 1st
ed. Philadelphia: WB Saunders, 1994 4. Loewenfeld IE: The Pupil. Ames, IA: Iowa State University Press, 1992 5. Crawford B: Visual adaptation in relation to brief conditioning stimuli. Proc R Soc Lond (Biol) 134:283, 1947 6. Pokorny J, Smith VC: Color vision and night vision. In Ogden TE (ed): Retina, pp 127–145. St Louis: Mosby, 1994 7. Osterberg G: Topography of the layer of rods and cones in the human retina. Acta Ophthalmol Suppl 6:1, 1935 8. McNaughton PA: Light response of the vertebrate photoreceptors. PhysiolRev 70:847, 1990 9. Stryer L: Molecular basis of visual excitation. Cold Spring Harbor Symp Quant Biol 53:283, 1988 10. Yau K-W: The Friedenwald Lecture. Phototransduction mechanism in retinal rods and
cones. Invest Ophthalmol Vis Sci 35:9, 1994 11. Rushton WAH: Bleached rhodopsin and visual adaptation. J Physiol 181:645, 1965 12. Hart WM: Visual adaptation. In Hart WM (ed): Adler's Physiology of
the Eye, pp 502–530. St Louis: Mosby, 1992 13. Tulunay-Keesey U, Saleh G, Ver Hoeve JN et al: Apparent phase reversal during stabilzied image fading. J Opt Soc Am 4:2166, 1987 14. Hahn LW, Geisler WS: Adaptation mechanisms in spatial vision: I. Bleaches and backgrounds. Vision Res 35:1585, 1995 15. Kortum PT, Geisler WS: Adaptation mechanisms in spatial vision: II. Flash thresholds and background
adaptation. Vision Res 35:1595, 1995 16. Barlow HB: Optic nerve impulses and Weber's Law. Cold Spring Harbor Symp Quant Biol 30:539, 1965 17. Dowling JE: The site of visual adaptation. Science 155: 273, 1967 18. Lankheet MJ, Rowe MH, van Wezel RJ et al: Horizontal cell sensitivity in the cat retina during prolonged dark adaptation. Vis Neurosci 13:885, 1996 19. Dizhoor A, Ray S, Kumar S et al: Recoverin: A calcium sensitive activator of retinal rod guanylate cyclase. Science 251:915, 1991 20. Barlow HB: Retinal noise and absolute threshold. J Opt Soc Am 46:634, 1956 21. Barlow HB: Increment threshold at low intensities considered as signal noise discriminations. J Physiol 136:469, 1957 22. Rose A: The sensitivity performance of the human eye on an absolute scale. J Opt Soc Am 38:196, 1948 23. Lamb TD: The role of photoreceptors in light-adaptation and dark-adaptation
of the visual system. In Blakemore C (ed): Vision: Coding and efficiency, pp 161–168. Cambridge, UK: Cambridge University Press, 1990 24. Aulhorn E: Über die Bezeihung zwischen Lichtsinn und Sehasharfe. Graef's Arch Ophthalmol 167:4, 1964 25. Van Nes FL, Bouman MA: Spatial modulation transfer in the human eye. J Opt Soc Am 57:401, 1967 26. Blakemore C, Campbell FW: On the existence of neurones in the human visual system, selectively sensitive
to the orientation and size of retinal images. J Physiol 203:237, 1969 27. Ohzawa I, Sclar G, Freeman RD: Contrast gain control in the cat's visual system. J Neurophysiol 54:651, 1985 28. Carandini M, Ferster D: A tonic hyperpolarization underlying contrast adaptation in cat visual
cortex. Science 276: 949, 1997 29. Kelly DH: Adaptation effects on spatio-temporal sine-wave threshold. Vision Res 12:89, 1972 30. Ratliff F, Riggs LA: Involuntary motions of the eye during monocular fixation. J Exp Psychol 40:687, 1950 31. Ditchburn RW, Ginsborg BL: Vision with a stabilized image. Nature 170:36, 1952 32. Riggs LA, Ratliff F, Cornsweet JC et al: The disappearance of steadily fixated visual test objects. J Opt Soc Am 43: 495, 1953 33. Barlow HB, Sparrock JMB: The role of after-images in dark adaptation. Science 144:1309, 1964 34. Jacobson DM, Thompson HS, Bartley JA: X-linked cone dystrophy: Clinical characteristics of affected male and
female carriers. Ophthalmology 96:895, 1989 35. Kemp CM, Jacobson SG, Faulkner DJ et al: Visual function and rhodopsin levels in humans with vitamin A deficiency. Invest Ophthalmol Vis Sci 46:188, 1988 36. Hecht S, Haig C, Wald C: The dark adaptation of retinal fields of different size and location. J Gen Physiol 19:321, 1935 37. Rushton WAH, Powell DS. The rhodopsin content and the visual threshold
of human rods. Vision Res 12:1073, 1972 38. Hecht S, Schlaer S, Pierenne MH: Energy, quanta, and vision. J Gen Physiol 25:819, 1942 39. Dowling JE: Chemistry of visual adaptation in the rat. Science 188:114, 1960 40. Rushton WAH: Rhodopsin measurement and dark adaptation in a subject deficient in cone
vision. J Physiol 156: 193, 1961 41. Enoch JM: The two-color threshold technique of Stiles and derived component
color mechanisms. In: Jameson D, Hurvich L (eds): Visual Psychophysics, Handbook
of Sensory Physiology. Vol II/4. Berlin: Springer-Verlag, 1972 42. Rushton WAH. The Ferrier lecture. Visual adaptation. Proc R Soc Lond B 162:20, 1965 43. Rushton WAH: The sensitivity of rods under illumination. J Physiol 178:141, 1965 44. Crawford BH: The effect of field size and pattern with the change of visual sensitivity
with time. Proc R Soc Lond B 123:94, 1940 45. Blakemore C, Rushton WAH: Dark adaptation and increment thresholds in a rod monochromat. J Physiol (Lond) 181:612, 1965 46. Krill AE, Martin D: Photopic abnormalities in congenital stationary night-blindness. Invest Ophthalmol Vis Sci 10: 625, 1971 47. Heckenlively JR, Weleber RG: X-linked recessive cone dystrophy with tapetal-like sheen. Arch Ophthalmol 104: 1328, 1986 48. Carr RE, Gouras P: Oguchi's disease. Arch Ophthalmol 73:646, 1965 49. Dryja TP, Berson EL, Rao VR et al: Heterozygous missense mutation in the rhodopsin gene as a cause of congenital
stationary night blindness. Nat Genet 4:280, 1993 50. Dryja TP, Hahn LB, Reboul T et al: Missense mutation in the gene encoding the alpha subunit of rod transducin
in the Nougaret form of congenital stationary night blindness. Nat Genet 13:358, 1996 51. Sandberg G, Pawlyk BS, Jeffery D et al: Rod and cone function in the Nougaret form of stationary nightblindness. Arch Ophthalmol 116:867, 1998 52. Cideciyan AV, Zha XY, Nielsen L et al: Null mutation in the rhodopsin kinase gene slows recovery kinetics of rod
and cone phototransduction. Proc Natl Acad Sci USA 95:328, 1998 53. Yamamoto S, Khani SC, Berson EL et al: Evaluation of the rhodopsin kinase gene in patients with retinitis pigmentosa. Exp Eye Res 65:249, 1997 54. Khani SC, Nielsen L, Vogt TM: Biochemical evidence for pathogenicity of rhodopsin kinase mutations correlated
with the Oguchi form of congenital stationary night blindness. Proc Natl Acad Sci USA 17:2824, 1998 55. Yamamoto H, Simon A, Eriksson U et al: Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed
dark adaptation in fundus albipunctatus. Nat Genet 22:188, 1999 56. Jay M: On the heredity of retinitis pigmentosa. Br J Ophthalmol 66:405, 1982 57. Molday RS: The Friedenwald lecture. Photoreceptor membrane proteins, phototransduction, and
retinal diseases. Invest Ophthalmol Vis Sci 39:2493, 2000 58. Dryja TP: Molecular genetics of retinitis pigmentosa. Hum Molec Genet 4:1739, 1995 59. Krill AE: Hereditary retinal and choroidal diseases. New York: Harper & Row, 1972. 60. Carr RE: Vitamin A deficiency. In Heckenlively JR, Arden GB (ed): Principles
and Practice of Clinical Electrophysiology of Vision, pp 737–740. St
Louis: Mosby, 1991. 61. Hussaini SH, Henderson T, Morrell AJ et al: Dark adaptation in early primary biliary cirrhosis. Eye 12:419, 1998 62. Rayner RJ, Tyrrell JC, Hiller EJ et al: Night blindness and conjunctival xerosis caused by vitamin A deficiency
in patients with cystic fibrosis. Arch Dis Childhood 64:1151, 1989 63. Cideciyan AV, Pugh EN, Lamb T et al: Rod plateaux during dark adaptation in Sorby's fundus dystrophy and
vitamin A deficiency. Invest Ophthalmol Vis Sci 38:1786, 1997 64. Ernest JT, Krill AE: The effect of hypoxia in visual function. Invest Ophthalmol Vis Sci 10:323, 1971 65. Havelius U, Bergquist D, Falke P et al: I. Impaired dark adaptation in symptomatic carotid artery disease. Neurology 49:1353, 1997 66. Havelius U, Bergquist D, Hindfelt B et al: II. Improved dark adaptation after carotid endartectomy. Evidence of a
long-term ischemic penumbra? Neurology 49:1360, 1997 67. Dannemiller JL: The early phase of dark adaptation in human infants. Vision Res 25:207, 1985 68. Hansen RM, Fulton AB: Electroretinographic assessment of background adaptation in 10-week-old
infants. Vision Res 31:1501, 1991 69. McFarland RA, Fisher MB: Alterations in dark adaptation as a function of age. J Gerontol 10:424, 1956 70. Coile DC, Baker HD: Foveal dark adaptation, photopigment regeneration, and aging. Vis Neurosci 8:27, 1992 71. Jackson GR, Owsley CR, Cordle EP et al: Aging and scotopic sensitivity. Vision Res 38:3655, 1998 72. Pulos E: Changes in rod sensitivity through adulthood. Invest Ophthalmol Vis Sci 30:1738, 1989 |