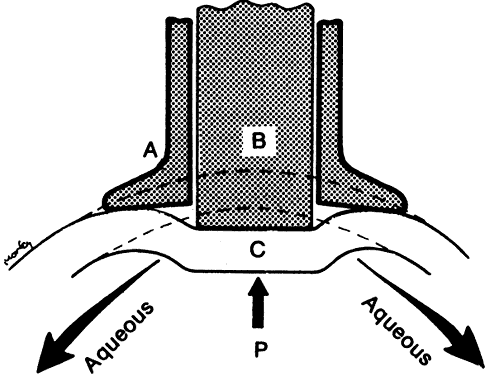
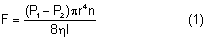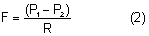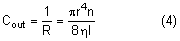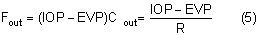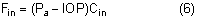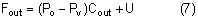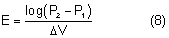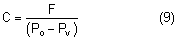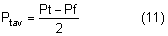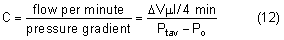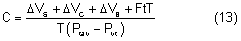After being produced by the ciliary processes in the posterior chamber, the aqueous circulates throughout the eye, coming into contact with the vitreous, lens, iris, and corneal endothelium. Aqueous drainage occurs by two pathways at the iridocorneal angle. Trabecular or conventional outflow, which predominates in humans, involves passage of aqueous through the trabecular meshwork and into Schlemm's canal, collector channels, and episcleral veins. A small portion of aqueous exits the eye by the uveoscleral or unconventional pathway across the anterior ciliary muscle and iris root, into the suprachoroidal space, and out through the emissarial channels of the sclera.
The episcleral veins have an internal pressure that reflects the central venous pressure. The episcleral venous pressure (EVP) is the lower limit of intraocular pressure in an intact eye if uveoscleral outflow is ignored. An increase in the EVP makes it more difficult for the aqueous to drain and leads to increased intraocular pressure.4
CHARACTERISTICS
The average intraocular pressure is thought to be about 15 mmHg.2,5 Under normal conditions, intraocular pressure is distributed evenly throughout the eye; therefore, the perfusion pressure of the retina, choroid, and ciliary body must be greater than intraocular pressure before blood can flow through these structures. The intraocular tissue pressure of about 15 mmHg is higher than elsewhere in the body, where the average is about 5 mmHg.6
The distensible uveal tissue absorbs about 2 mmHg of the intraocular pressure. The potential space between the uvea and sclera, the suprachoroidal space, therefore has a pressure about 2 mmHg lower than the intraocular pressure.7 After glaucoma-filtering surgery, the intraocular pressure may become very low and the suprachoroidal space may fill with a plasma transudate (choroidal effusion) and cause detachment of the choroid and ciliary body.
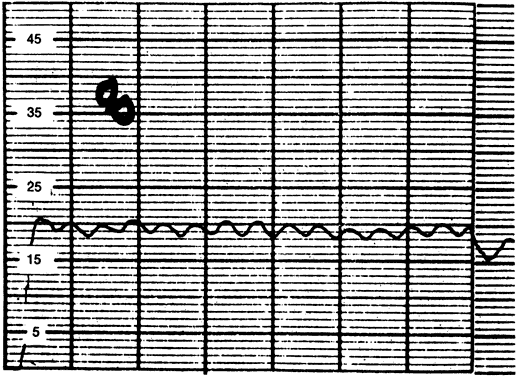
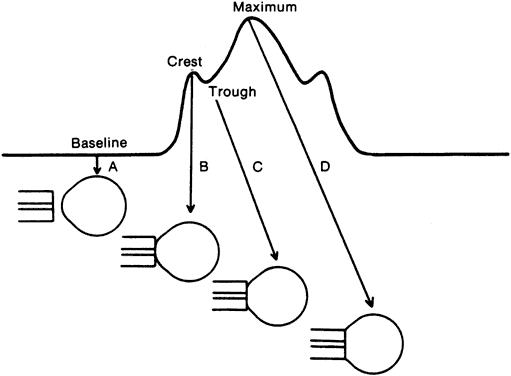
The normal intraocular pressure is pulsatile, reflecting its vascular origin (Fig. 1).8 The pulses follow the arterial pulses, and a diagnosis of cardiac arrhythmia can actually be made from a continuous measurement of the intraocular pressure.9 The amplitude of the pulse is generally 2 to 3 mmHg but may be higher if there is a large arterial pulse pressure, such as in hypertension or aortic regurgitation.8
|
The intraocular pressure is a dynamic function like heart rate and blood pressure and is influenced by many factors. A single measurement of intraocular pressure does not necessarily reflect the average pressure in that hour, day, or week.
DIURNAL VARIATION
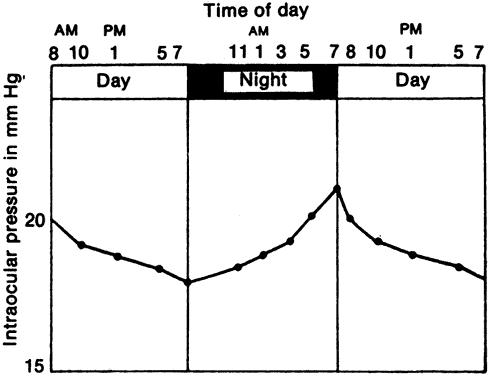
Intraocular pressure in humans follows a diurnal rhythm.4,8 Most commonly, the pressure is highest in the early morning (around 7 AM), and lowest in the evening (around 5 PM; Fig. 2) but considerable individual variation exists regarding the time of day or night when the peak and nadir occur.10–12 The intraocular pressure usually varies less than 5 mmHg from peak to nadir, although differences as large as 15 mmHg have been found. Some people with elevated intraocular pressure are found to have large diurnal peaks and troughs13 and can show variations of as much as 8 mmHg from hour to hour.14 Irregular sleep patterns alter the usual diurnal pattern of intraocular pressure.15
The diurnal pattern of intraocular pressure has been correlated with circadian rhythms of other functions, such as blood pressure, body temperature, and adrenal corticosteroid secretion. In many subjects, the intraocular pressure rises several hours after an increase in plasma adrenal corticosteroids.16,17 This pattern may be due to changes in both aqueous secretion and outflow resistance.18,19
The existence of diurnal variation implies that a single random intraocular pressure measurement does not adequately represent average pressure over a 24-hour period. Peak intraocular pressure can only be obtained by around the clock measurements or with measurements taken at different times of the day. In glaucoma patients, normal intraocular pressures recorded during office hours can give a false clinical impression of adequate pressure control.20–22 Phelps and colleagues argued that a single office pressure measurement was likely, though not certain, to be representative of the peak diurnal pressure.23 Zeimer, however, using Phelps and coworkers' data, calculated that the 95% confidence interval for such a prediction was ±6.6 mmHg, an uncertainty of more than 13 mmHg.12 A small (about 1.5 mmHg) seasonal variation in intraocular pressure has been described, with the highest pressures recorded in winter and the lowest in summer.23,24
INFLUENCING FACTORS
The intraocular pressure is subject to numerous short- and long-term influences (Tables 1 and 2). The intraocular pressure undergoes rhythmic oscillations other than those associated with the arterial pulse. It varies with the respiratory cycle, presumably through changes in venous pressure. Vasomotor variations are associated with waves having a periodicity of 3 to 8 per second (Traube-Hering waves). Although each of these variations produces changes of only 1 to 2 mmHg, the difference between the highest and lowest pressure over a 1-minute period can be as much as 8 mmHg.8
TABLE 7-1. Factors Raising Intraocular Pressure
General
Age
Systemic
Large increase in blood pressure
Increased carotid blood flow
Valsalva maneuver
Carotid-cavernous fistula
Plasma hypo-osmolarity
Hyperbaria
Local
Increased episcleral venous pressure
Blockage of ophthalmic vein
Blockage of trabecular meshwork
Co-contraction of extraocular muscles
Restricted extraocular muscle
Acute external pressure
Forced blinking
Relaxation of accommodation
Prostaglandin release (biphasic)
Hypersecretion of aqueous (?)
Pharmacologic
Intravenous ketamine
Succinylcholine (co-contraction)
Cycloplegic agents
Corticosteroids (in some)
TABLE 7-2. Factors Lowering Intraocular Pressure
General
Exercise
Systemic
Large decrease blood pressure
Decreased carotid blood flow
Decreased central or jugular venous pressure
Sympathetic stimulation
Parasympathetic stimulation
Hypothermia
Acidosis
Plasma hyperosmolarity
Adrenalectomy
General anesthesia
Local
Decreased episcleral venous pressure
Decreased ophthalmic artery blood flow
Prolonged external pressure
Retrobulbar anesthesia
Ocular trauma
Intraocular surgery
Retinal detachment
Choroidal detachment
Inflammation
Accommodation
Increased aqueous outflow
Pharmacologic
Parasympathomimetics
Epinephrine
α-Agonists
α-Antagonists
Carbonic anhydrase inhibitors
Prostaglandin derivatives
Cardiac glycosides
Guanethidine
9-Tetrahydrocannabinol
β-Adrenergic antagonists
Muscle relaxants
Dopamine agonists
The intraocular pressure is relatively immune to physiologic changes in arterial blood pressure. Large swings in blood pressure cause the intraocular pressure to vary in the same direction, however.26,27 Ligating one carotid artery causes a drop in intraocular pressure on that side, presumably due to a decrease in aqueous secretion, although the effect diminishes over several days.28 External carotid compression has a similar result, and measurements of the magnitude of the pressure drop and the rate of recovery have been used to estimate carotid blood flow.29 Interestingly, when one carotid artery is ligated or compressed, the intraocular pressure in the contralateral eye rises, possibly due to the increased blood flow and pressure in the contralateral carotid artery.28
Obstruction of the venous return from the eye or head raises the venous pressure in the episcleral system and thus raises the intraocular pressure. The intraocular pressure rises about 0.8 mmHg for each 1 mmHg rise in EVP.30 Conditions raising EVP include external pressure on the jugular vein, compression of the superior vena cava by a tumor, cavernous sinus thrombosis, arteriovenous fistula in the cavernous sinus (transmitting arterial pressure directly to the venous system) and any change in the orbital apex (e.g., neoplastic, inflammatory, dysthyroid) that compromises the venous drainage of the eye. A tight collar or necktie may also compress the jugular vein and raise the intraocular pressure.
The Valsalva maneuver, in which expiration is forced against a closed glottis producing no air flow, is a related condition that increases venous pressure and hence intraocular pressure.31 Increased intrathoracic pressure collapses the superior vena cava, obstructing venous inflow from the head. Coughing and straining with defecation produce similar changes.
Changes in position affect the intraocular pressure. There is a 2- to 4-mmHg increase in intraocular pressure when changing from the sitting or upright position to the supine position.32–34 Placing the head below the level of the heart in Trendelenburg position may cause a considerable temporary increase in the intraocular pressure.34 Inverted posture may rapidly lead to intraocular pressures of more than 30 mmHg.36,37 These changes parallel an increase in the venous pressure in the head but whether this is the mechanism for the increased intraocular pressure is not known. Some patients with glaucoma may have a much larger change in intraocular pressure with position than normal individuals.38
External pressure on the eye increases the intraocular pressure, at least initially. The eye cannot expand significantly to accommodate the fluid displaced by indenting the globe and the intraocular pressure rises. The external pressure, however, accelerates the rate of aqueous outflow and the displaced volume eventually leaves the eye.1,4 If the external pressure is released, the decreased intraocular volume yields an intraocular pressure that is lower than the initial intraocular pressure before applying external pressure. This sequence of events forms the basis by which repeated tonometry decreases intraocular pressure and is why some surgeons massage the eye before intraocular surgery.39,40 Measurement of outflow facility in an eye during tonography is also based on the decrease in intraocular pressure after external pressure.
Forced eyelid closure is a form of external pressure on the eye. Blinking causes a pressure rise of 5 to 10 mmHg, and forced blinking has resulted in recorded intraocular pressures as high as 90 mmHg.39 Voluntary lid fissure widening, as is common during tonometry, is associated with an increased intraocular pressure of about 2 mmHg, whereas pressure on the eyelid caused by holding the eyelids open may result in much larger increases.41
Attempts to move the globe in a direction opposite to a mechanically restricted extraocular muscle raises the intraocular pressure. It is thought that this pressure rise is due to compression of the eye between the restricted muscle and the contracting muscle. This occurs, for example, when upgaze is attempted and the inferior rectus muscle is entrapped by an orbital floor fracture. With dysthyroid involvement of the inferior rectus muscle, the intraocular pressure on attempted upgaze may rise 3 to 10 mmHg.42,43 Intraocular pressure in normal individuals does not rise more than 2 mmHg with upgaze. Therefore, this phenomenon can be a helpful diagnostic sign when recognized. If unrecognized in a thyroid patient, it can cause an overestimation of resting intraocular pressure.
A similar increase in intraocular pressure is seen when the extraocular muscles co-contract, as in Duane's syndrome, or after aberrant regeneration of a previously injured third nerve.4 Intraocular pressure may increase after intravenous succinylcholine, a muscle-depolarizing agent commonly used by anesthesiologists to paralyze the skeletal muscles. Succinylcholine is generally thought to raise intraocular pressure by causing tonic extraocular muscle contraction; therefore, clinicians have avoided its use in patients with open eye injuries to avoid extrusion of global contents.44,45 Kelly and coworkers,46 however, have shown that succinylcholine raises intraocular pressure even when the extraocular muscles are detached from the globe. They attribute the ocular hypertensive effect of succinylcholine mainly to its cycloplegic action and to a lesser degree on its effect on EVP and choroidal blood volume. Nondepolarizing muscle relaxants, such as pancuronium or atracurium, do not raise the intraocular pressure.47,48
Exercise can decrease the intraocular pressure, perhaps because of the acidosis produced by short-term physical exertion, although this mechanism has been disputed.49,50 Even brief physical exertion can cause a mild lowering of intraocular pressure, possibly through sympathetic constriction of choroidal vessels and reduction in uveal volume.51 Continuous strenuous exercise can significantly reduce intraocular pressure, probably through increased plasma osmolarity.52 Physical exertion of longer duration appears to have less effect on the intraocular pressure but exercise conditioning has been reported to lead to a small decrease in baseline intraocular pressure.53 This effect may be more pronounced in patients with higher baseline intraocular pressures.54
Blunt ocular trauma usually has a biphasic effect on intraocular pressure: there is initially a transient rise, followed by a more prolonged decline until the injury resolves.1 After trauma, inflammatory debris or swelling of the trabecular beams may obstruct aqueous outflow. Prostaglandins may also play a role. In rabbits, topical application of prostaglandin-E2 causes a rise in intraocular pressure, which is prevented by pretreatment with prostaglandin synthetase inhibitors such as aspirin and indomethacin.55 The role of humoral factors is supported by the observation of consensual responses in the contralateral eye after ocular trauma and topical prostaglandin application.56 The late, more prolonged decrease in intraocular pressure after trauma is probably due to the decrease in aqueous secretion usually seen in inflammatory conditions.
The level of accommodation has a small effect on the intraocular pressure. Accommodative effort decreases the intraocular pressure, whereas relaxation of accommodation returns the intraocular pressure to baseline values.57,58 These changes parallel the larger effects of parasympathomimetic and parasympatholytic agents.
The intraocular pressure is depressed by general anesthesia, irrespective of the type of anesthetic.47,59 Possible mechanisms include muscle relaxation, decreased blood pressure, increased blood carbon dioxide levels, and a direct central effect of the anesthetic agent.59,60 One important exception is ketamine, which may transiently raise intraocular pressure when given intravenously.44 Retrobulbar anesthesia causes a considerable drop in intraocular pressure due to inhibition of the ciliary body and loss of extraocular muscle tone.1 A large volume of anesthetic instilled into the retro- or peribulbar space may initially compress the globe and raise intraocular pressure, however.
Changes in plasma osmolality profoundly affect the intraocular pressure.4 Water passes freely across the blood-aqueous and blood-vitreous interfaces in either direction, but solute molecules such as ions exchange at much slower rates. If the total concentration of solute molecules in the blood exceeds the concentration in the aqueous and vitreous, water from the vitreous and probably aqueous is drawn into the plasma. This loss of water from the eye decreases the intraocular pressure. Interestingly, the empirically measured water loss is less than osmolality calculations predict, suggesting that there are limiting factors in the eye in addition to compensatory mechanisms.4 After several hours, the concentration of solute increases in the intraocular fluids, and the intraocular pressure returns toward baseline. It may even exceed baseline, resulting in a “rebound” phenomenon.61
The ocular response to plasma hyperosmolality is used clinically in situations such as acute angle closure glaucoma, in which the intraocular pressure has risen to dangerous levels. Hyperosmolar agents are given to raise plasma osmolality and lower intraocular pressure. An effective hyperosmolar agent must penetrate the eye relatively slowly. If osmotic equilibrium is obtained too quickly, the intraocular pressure effect is short-lived.5,45 Useful and relatively nontoxic agents include intravenous urea and mannitol, and oral glycerol and isosorbide. Oral ethanol can also raise plasma osmolality and reduce intraocular pressure indirectly by reducing the production of antidiuretic hormone.62
Conversely, if the concentration of solute in the plasma is lower than that of the intraocular fluids, water enters the eye from the plasma and raises intraocular pressure.4,63 This pressure increase may be considerable in glaucoma patients and forms the basis for the water-provocative test.5 In this test, a patient rapidly drinks a liter of water, lowering the plasma osmolality. Patients exhibiting an intraocular pressure increase of more than 8 mmHg are thought to have glaucoma. The water provocative test, however, has a high false-positive rate and limited diagnostic value.5 Nevertheless, the sensitivity of the intraocular pressure to hypo-osmolality in glaucoma patients should be considered, particularly when there are alterations in systemic fluids and electrolytes. Intraocular pressure, for example, may increase significantly during hemodialysis. Whether or this effect is mediated by changes in plasma osmolality remains controversial.64,65
A drop in body temperature causes a decrease in the intraocular pressure, probably by inhibition of aqueous secretion. A relatively large change in temperature (more than 1°) is necessary to produce significant pressure lowering, however; it is therefore unlikely that temperature plays a major role in physiologic variations of intraocular pressure.66
Finally, blood pH affects intraocular pressure, with systemic acidosis lowering intraocular pressure. In addition to inhibiting carbonic anhydrase in the ciliary body, oral and parenteral carbonic anhydrase inhibitors lower intraocular pressure to some extent by producing a systemic metabolic acidosis.67