STRUCTURE
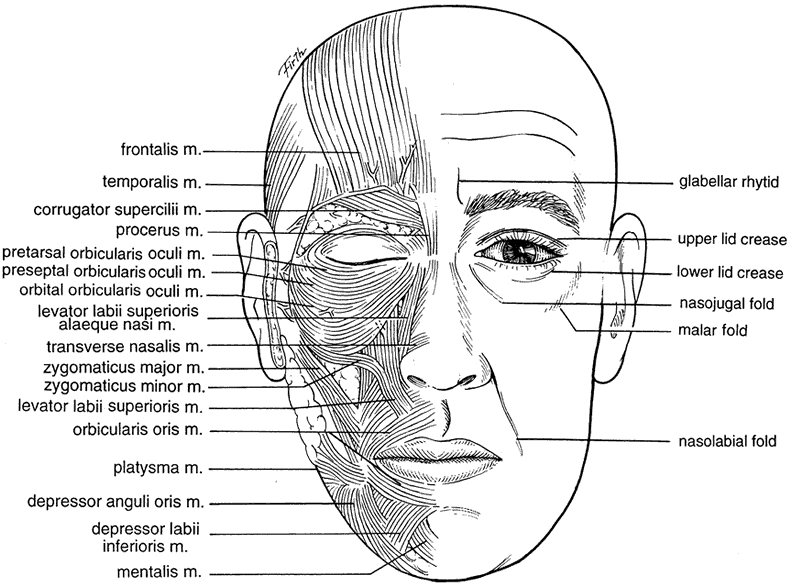
Located at the junction of the forehead and the upper eyelid, the eyebrow is a transverse elevation of hair that starts medially just inferior to the orbital margin and ends laterally above the orbital margin. The eyebrows are formed by the transverse elevation of the superciliary ridge of the frontal bone. The eyebrow layer consists of the skin, subcutaneous connective tissue, a muscular layer, a submuscular areolar layer, and the pericranium. The superciliary ridge is more prominent in males and often absent or less prominent in females. Because it extends only over the medial half to two thirds of the orbit, the lateral brow lacks this extra bony support. Laterally, the brow is supported by fascial attachments to the temporalis fascia. The eyebrow skin is thick and mobile and contains sebaceous glands. The subcutaneous tissue layer, like that of the scalp, consists of more fibrous tissue than fat. It is also correspondingly as thick as the eyebrow skin. The muscular layer is composed of the vertical fibers of the frontalis, the horizontal fibers of the orbicularis oculi, and the oblique fibers of the corrugator supercilii (Fig. 1).
|
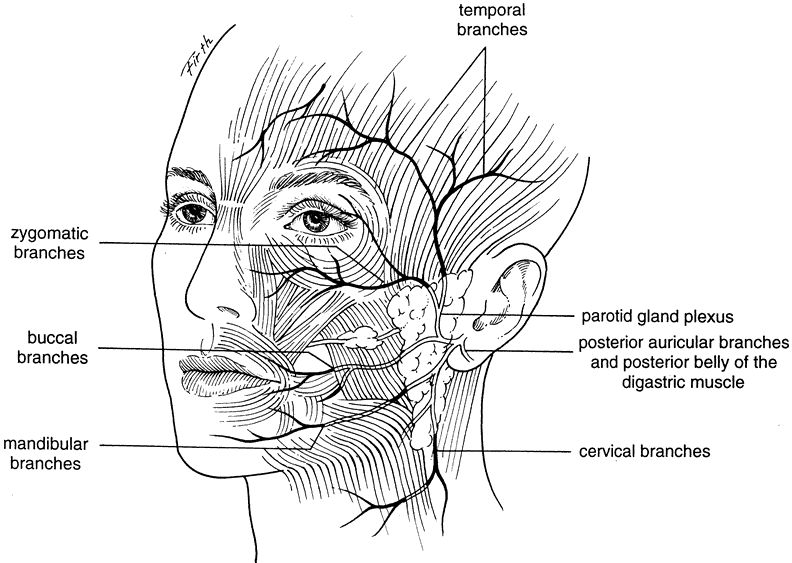
The corrugator supercilii muscle originates from the frontal bone near the superomedial orbital margin and inserts at the muscle and skin behind and immediately superior to the middle third of the eyebrow. The frontalis muscle and the orbicularis interdigitate in the eyebrow, a unique feature in the superficial muscle plane of the face. The corrugator motor nerve is usually seen traversing the orbital margin between the arcus marginalis and the supraorbital ridge on the posterior fat pad wall. It supplies the muscle on its lateral side temporal to the supraorbital nerve. Branches of the facial nerve provide motor innervation to the frontalis muscle. The supraorbital artery pierces the frontalis muscle 3 cm above the supraorbital ridge, whereas the supraorbital vein lies horizontally on the undersurface of the orbital orbicularis. The supraorbital vein is continuous with the angular, frontal, and superior orbital veins, as well as the deep preauricular plexus.
The procerus muscle arises from the nasal bone and the upper nasal cartilage. It travels superiorly to insert on the medial forehead skin.
The depressor supercilii muscle is distinct from the orbicularis oculi and corrugator muscles.7,8 Its origin is the frontal potion of the maxillary bone. It travels superiorly and inserts on the skin superior to the medial canthal tendon.7
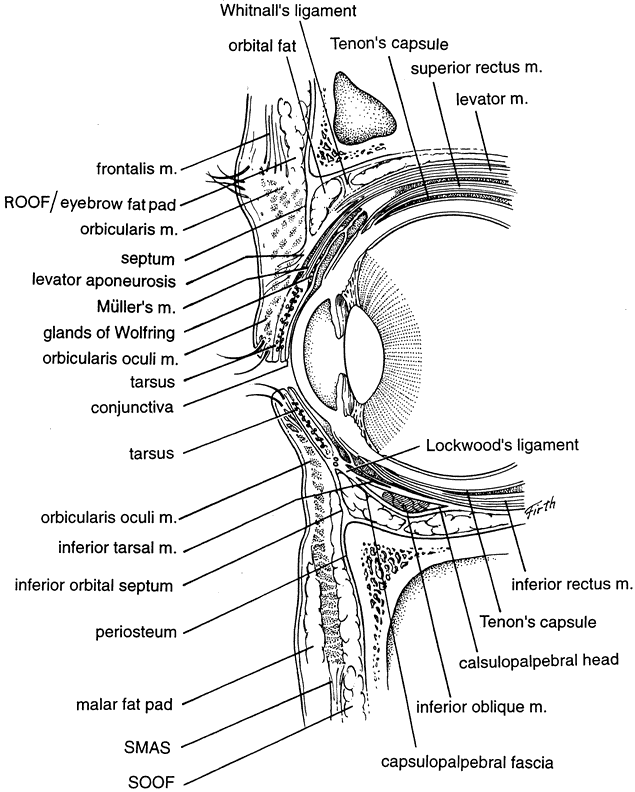
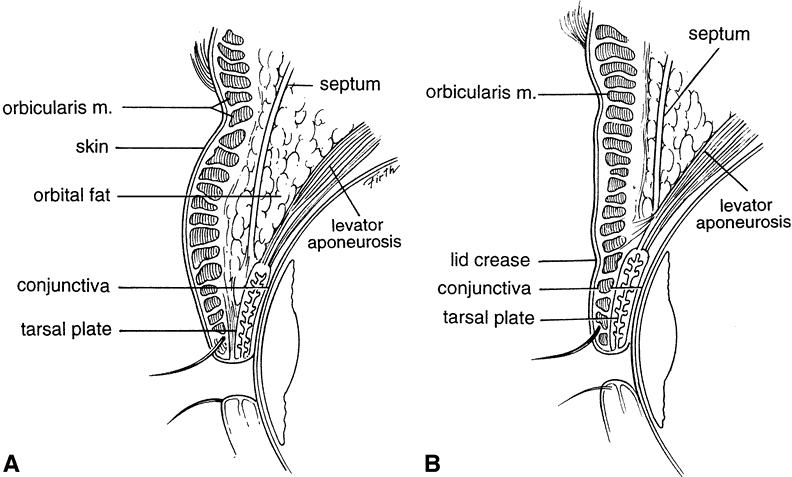
The galea aponeurosis joins the frontalis muscle anteriorly and splits around the frontalis muscle into a superficial and deep galea. The thinner superficial layer continues inferiorly as the anterior muscle sheath of the frontalis and orbicularis muscle, and the deep galea becomes the posterior muscle sheath of these two muscles. This deep galea layer divides inferiorly and encompasses the eyebrow fat pad. The eyebrow fat pad complex has also been referred to as the retro-orbicularis oculi fat (ROOF). The ROOF is bounded anteriorly by the posterior frontalis-orbicularis muscle fascia. Posteriorly, the fascial boundary of the ROOF is dense and fibrous and extends inferiorly to the eyelid as the orbital septum9 (Fig. 2). Eyebrow fat contains fibrous septa with interseptate fat-filled spaces. This fat pad contains the branches of the facial nerve. The ptosis surgeon must be cognizant of the ROOF, because the lower edge of the brow fat pad occasionally droops inferiorly into the eyelid and this eyebrow fat pad may be mistaken for the preaponeurotic fat pad. This error in judgment will cause the orbital septum to be misidentified as the levator aponeurosis and mistakenly advanced, resulting in marked lagophthalmos. Normally, the eyebrow fat pad is above the superior orbital rim, but hereditary and involutional changes can cause its descent. The eyebrow fat pad is continuous with the sub-superficial muscular aponeurotic system (SMAS) fat in the malar region and the postorbicularis layer in the lower eyelid.10 (see Fig. 2).
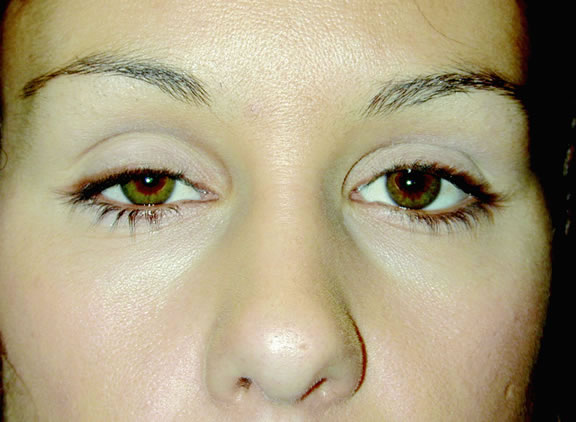
Although each individual has distinctive general form, size, direction of hair growth, and eyebrow color, the two eyebrows are mirror images of each other. Furthermore, there are differences between genders, with the eyebrow assuming a more arched appearance in females. The eyebrow hair in the older adult male is heavier and bushier than in the older adult female.
There are three types of hair in the eyebrow: (1) fine vellus hair; (2) the slightly larger and lightly pigmented hair; and (3) the large terminal hair, also known as the supercilia. The fine vellus hairs form an effective moisture barrier to keep sweat from running downward into the eye. The supercilia give the eyebrow its apparent color and configuration. The individual supercilia are relatively wide and may reach a length of 8 to 10 cm in later life. The supercilia are too large and too widely spaced to make a good moisture barrier.
The follicle of each supercilium is surrounded by a rich vascular network and abundant sensory nerve endings. The skin of the eyebrow and glabellar region contains numerous sebaceous glands. Eccrine sweat glands are sparse, except for in the tail of the brow. This predominance of sebaceous glands causes a greasy skin texture of the eyebrow and its skin. Along with the fine vellus hair in the eyebrow, this greasy texture produces an efficient barrier to fluids running down from the forehead. The fluid flow is redirected medially and laterally, away from the eye. Eyebrow cilia grow in different directions depending on their position.11 The upper rows grow down and laterally at an angle of less than 30 degrees from the vertical, whereas the lower most cilia grow up and laterally, also at an angle of less than 30 degrees. An abrupt reversal occurs when these cilia meet in the midline of the eyebrow. However, this reversal does not occur at the medial end of the eyebrow, where the eyebrow sweeps superolaterally. Shaving or cutting the eyebrow hair does not affect its subsequent growth. In fact, full regrowth of a shaven eyebrow occurs within 6 months.12
FUNCTION
The eyebrow, together with the eyelid, serves several diverse and complex functions in the visual system. Its primary function is the protection and maintenance of the anterior structures of the eye. Because of its position and curvature, the eyebrow shields the eyes from bright light coming from directly above, and it is an effective barrier to liquids running from the forehead into the eye. The large hairs of the eyebrow have abundant sensory innervation and are very sensitive to tactile stimulation. Accordingly, stimulation of the supercilia results in reflex blinking of both eyelids.
The eyebrows can be elevated, depressed, or drawn together. Their position is critical to facial configuration and expression. Maximal elevation of both medial and lateral portions of the eyebrows gives rise to the look of surprise. Depression of the medial portion of the eyebrow depicts anger or concern. Elevation of only one eyebrow portrays a quizzical or questioning expression. These expressions serve as nonverbal forms of communication to convey emotion.
Eyebrow elevation helps clear the visual axis and is a natural compensatory response to the forehead sagging and dermatochalasis that occur with aging. In maximal upgaze, the eyebrows also elevate to provide maximal clearance of the visual axis, in coordination with lid, globe, and forehead elevation.
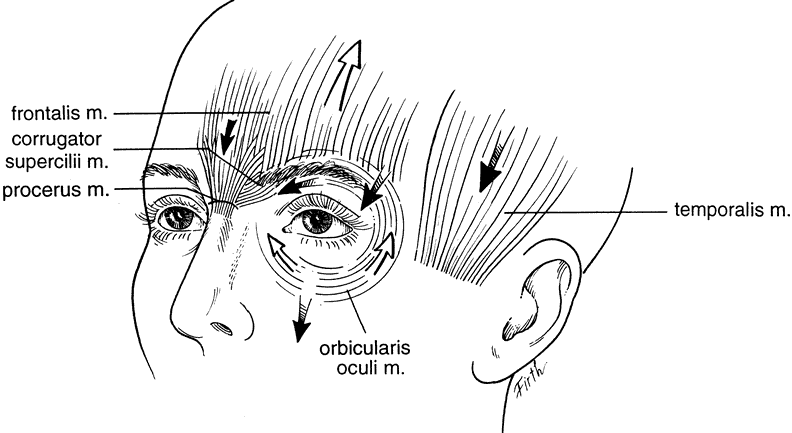
Eyebrow position is functionally dependent on an interplay of elevators and depressors.13 The main elevator is the frontalis muscle. The frontalis muscle elevates the forehead and eyebrows primarily medial to the conjoined fascia of the superficial temporalis fascia and the temporalis fascia proper. It also serves as an accessory elevator of the upper eyelid. Maximal action of the frontalis will result in an additional 3 to 5 mm of elevation of the upper eyelid.14 The depressors of the eyebrow are the corrugator supercilii, which draws the eyebrow inferomedially causing vertical glabellar folds, the procerus, which also depresses the medial brow and gives rise to the horizontal creases, and the depressor supercilii muscle, which depresses the medial brow.7,8 The orbicularis oculi depresses the eyebrow both medially and laterally. Eyebrow depressors are used during forceful eyelid closure. They are also active during visual concentration. Over time, gravity also asserts a downward force on the eyebrows and facial tissues (Fig. 3).
|
AGING AND DISEASE STATES
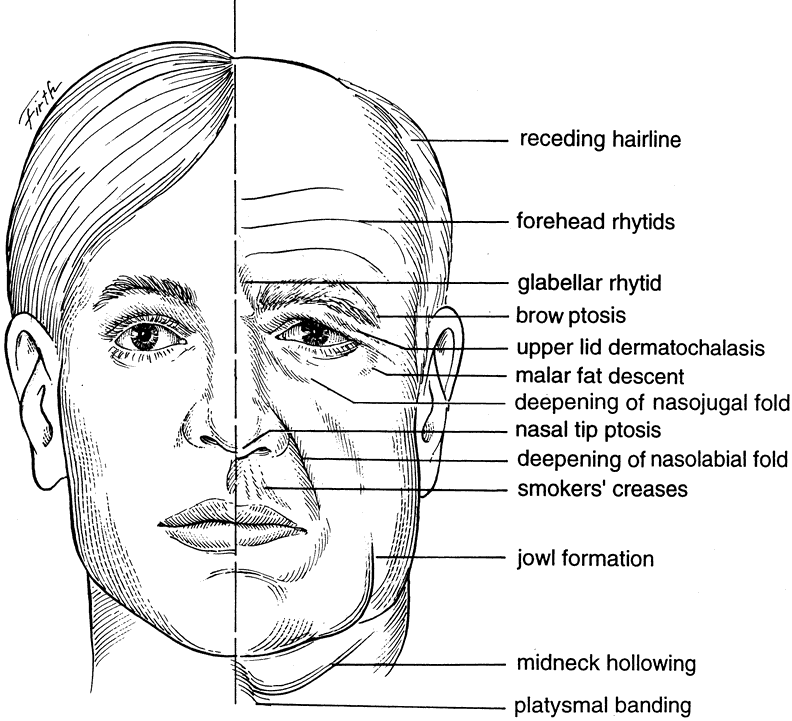
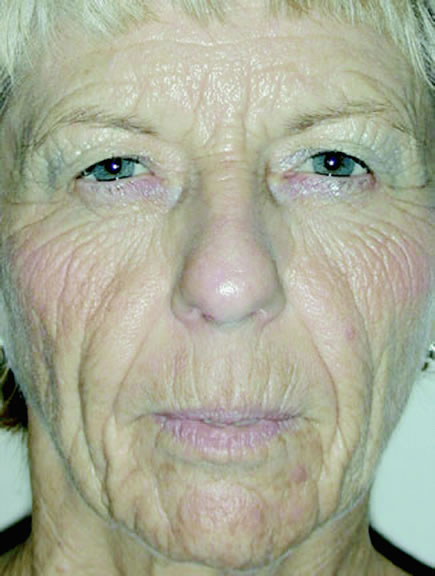
In aging, several changes are noted in the eyebrows, eyelids, and face. Among the common complaints of aging individuals are facial wrinkles known as rhytids and pigmentary abnormalities (dyschromia). These age-related facial changes are believed to be caused by three main factors: (1) photo-induced aging or ultraviolet (UV) damage, (2) mechanical influences such as gravity and muscle contraction, and (3) chronologic or intrinsic aging changes.
Photo-induced aging or UV damage comes from habitual sunlight exposure to skin. Eighty percent of UV-related photo-induced aging occurs before the age of 20, and photo-induced aging alone accounts for more than 90% of age-related skin changes.15 Skin changes are characterized clinically by elastosis, irregular pigmentation, roughness or dryness, teleangiectasia, atrophy, deep wrinkling, and a variety of neoplasms.16
Mechanical influences from gravity and contraction of the muscles of facial expression cause a decrease in skin elasticity resulting from constant stretch and tension.
Chronologic skin aging is the histologic and physiologic changes seen in sun-protected skin of most older individuals. Clinical changes seen in the skin are laxity, dryness, and fine wrinkling. Histologic changes are also noted in the aging skin. In the epidermis, there is a flattened dermal-epidermal junction, giving the appearance of atrophy and cellular heterogeneity. The melanocyte density declines, and the melanocytes tend to cluster focally, producing lentigines. The Langerhans cells also decrease in number with advancing age. Changes in the dermis include attenuation in number and diameter of elastic fibers in the papillary dermis, an increase in number and thickness of elastic fibers in the reticular fibers, and a coarsening of collagen fibers with an increase in density of the collagen network.17
Eyebrow ptosis may occur in young or aged individuals. It might be genetic or result from a combination of gravity, loss of tissue elasticity, decreased subcutaneous tissue, and progressive bony resumption. Clinical observations show that the lateral eyebrow segment usually becomes ptotic earlier because it receives less support from the deeper structures than the medial eyebrow. The eyebrow fat pad complex promotes mobility and gravitational descent of the eyebrow, particularly the lateral eyebrow segment.13 The three forces acting on the lateral eyebrow are (1) frontalis muscle resting tone, which elevates the eyebrow segment medial to the temporal fusion line of the skull; (2) gravity, which causes the soft tissue mass lateral to the temporal line to descend over the temporal fascia plane; and (3) corrugator supercilii muscle activity in conjunction with lateral orbicularis oculi muscle action, which counteract frontalis muscle activity to cause depression of the brow.13 These forces produce downward displacement of the lateral eyebrow. In acquired eyebrow ptosis, there are usually accompanying deep forehead wrinkles as a result of compensatory frontalis overaction. This frontalis contraction, however, does not extend far laterally because of lack of lateral frontalis fibers. Repetitive frontalis muscle contraction is noted in aging patients with symptoms of visual obstruction and a feeling of eyelid heaviness or fullness. Visual obstruction may be caused by brow ptosis, blepharoptosis, dermatochalasis, or any combination of the three.
Synophrys is a condition in which hypertrophy of the eyebrows result in fusion at the midline glabellar area. Synophrys can occur as an isolated finding or may be associated with a generalized hypertrichosis. A congenital absence of eyebrow is usually associated with generalized alopecia, whereas isolated absence is extremely rare.
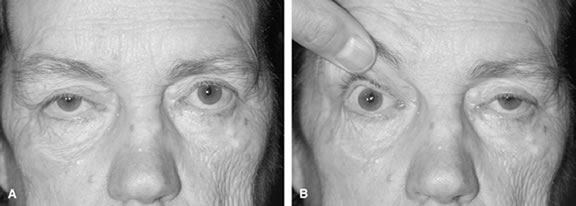
The eyebrow is a helpful indicator of certain localized and systemic disease conditions. Paralysis of the levator palpebrae muscle causes upper eyelid ptosis. As a result, the ipsilateral frontalis muscle elevates the brow in an effort to lift the eyelid. The brow can provide as much as an additional 3 to 5 mm of eyelid elevation.13 In peripheral facial nerve palsy, the ipsilateral frontalis muscle loses muscle tone and the eyebrow assumes a lower position. Hence, eyebrow position may be used to distinguish a third nerve palsy from a seventh nerve palsy.