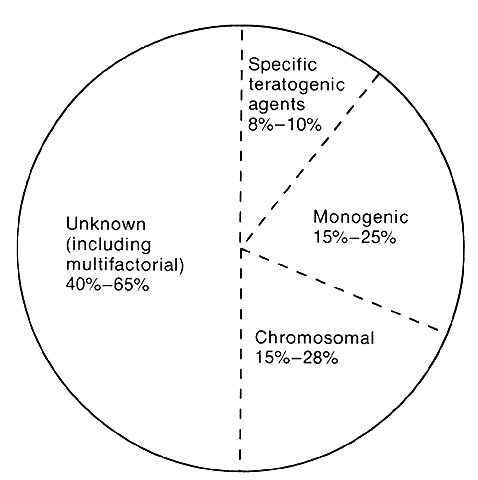
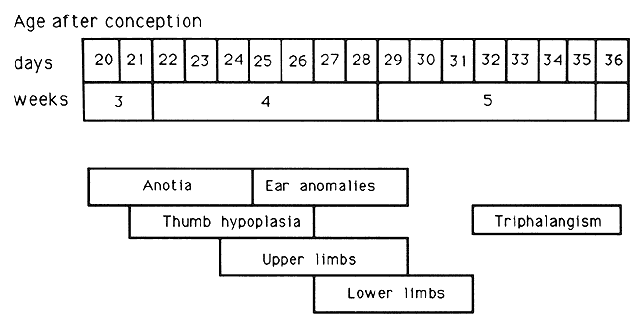
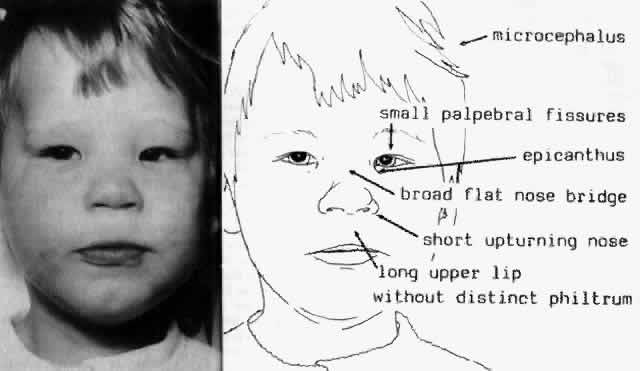
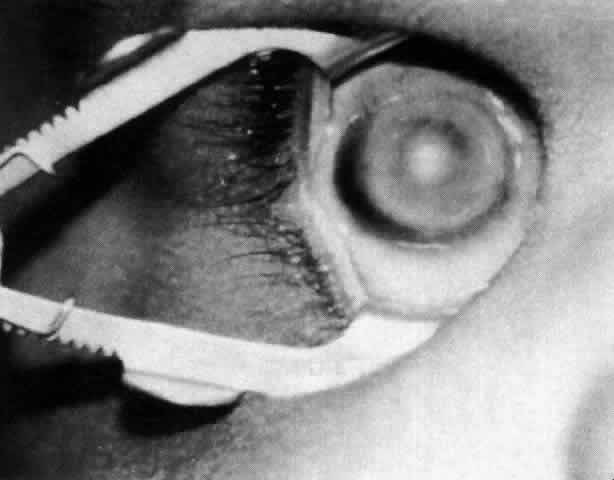
1. Wilson JG: Environment and Birth Defects. New York, Academic Press, 1973 2. Shepard TH: Teratogenicity of therapeutic agents. Curr Probl Pediatr 10:5, 1979 3. Dickens C: The Posthumous Papers of the Pickwick Club, p 459. London, Oxford
University Press, 1949 4. Warkany J: Congenital Malformations. Notes and Comments, p 6–19. Chicago, Year
Book Medical Publishers, 1971 5. Gregg NM: Congenital cataract following German measles in the mother. Trans Ophthalmol Soc Aust 3:35, 1941 6. Shepard TH: Catalog of Teratogenic Agents. Baltimore, Johns Hopkins University
Press, 1989 7. Shepard TH: Human teratogenicity. Adv Pediatr 33:225, 1986 8. Wilson JG, Fraser FC (eds): Handbook of Teratology 1, p 49–62. New
York, Plenum Press, 1977 9. Cahen RL: Experimental and clinical chemoteratogenesis. Adv Pharmacol 4:263, 1966 10. Axelrod LR: Drugs and nonhuman primate teratogenesis. In Wollam DHM (ed): Advances
in Teratology, pp 217–230. New York, Academic Press, 1970 11. Ueda K, Nishida Y, Oshima K, Shepard TH: Congenital rubella syndrome: Correlation of gestational age at time of
maternal rubella with type of defect. J Pediatr 94:763, 1979 12. Lenz K, Knapp K: Die Thalidomid-Embryopathie. Dtsch Med Wochenschr 87: 1232, 1962 13. Nowack E: Die sensible Phase bei der Thalidomid-Embryopathie. Humangenetik 1:516, 1965 14. Moore KL: The Developing Human. Philadelphia, WB Saunders, 1988 15. Strömland K, Miller M, Cook C: Ocular teratology. Surv Ophthalmol 35:429, 1991 16. Shepard TH: Human teratogens: How can we sort them out? Ann NY Acad Sci 447:105, 1986 17. Lammer EJ, Chen DT, Hoar RM et al: Retinoic acid embryopathy. N Engl J Med 313:837, 1985 18. Webster WS, Johnston MC, Lammer EJ, Sulik KK: Isotretinoin embryopathy and the cranial neural crest: An in vivo and in
vitro study. J Craniofac Genet Dev Biol 6:211, 1986 19. Willhite CC, Hill RM, Irving DW: Isotretinoin-induced craniofacial malformations in humans and hamsters. J Craniofacial Genet Dev Biol 2(suppl): 193, 1986 20. Dencker L, d'Argy R, Danielsson BRG et al: Saturable accumulation of retinoic acid in neural and neural crest derived
cells in early embryonic development. Dev Pharmacol Ther 10:212, 1987 21. Pratt RM, Abbott BD, Watanabe T, Goulding EH: Craniofacial malformations
induced by retinoids in mouse embryo culture. In McLachlan JA, Pratt
RM, Markert CL (eds): Banbury Report 26: Developmental toxicology: Mechanisms
and Risk, pp 227–242. New York, Cold Spring Laboratory, 1987 22. Cook CS, Sulik KK: Sequential scanning electron microscopic analyses of
normal and spontaneously occurring abnormal ocular development in C57BI/6J
mice. Scanning Electron Microscopy III: 1215, 1986 23. Webster WS, Germain MA, Edwards MJ: The induction of microphthalmia, encephalocele and other head defects following
hyperthermia during the gastrulation process in the rat. Teratology 31:73, 1985 24. Germain M-A, Webster W, Edwards MJ: Hyperthermia as a teratogen: Parameters determining hyperthermia-induced
head defects in the rat. Teratology 31:265, 1985 25. Spaeth GL, Nelson LB, Beaudoin AR: Ocular teratology. In Jakobiec FA (ed): Ocular
Anatomy, Embryology and Teratology, p 975. Philadelphia, Harper
and Row, 1982 26. Hale F: Relation of maternal vitamin A deficiency to microphthalmia in pigs. TX State J Med 33:228, 1937 27. Chamberlain JG, Nelson MM: Congenital abnormalities in the rat resulting from single injections of
a 6-aminonic-otinamide during pregnancy. J Exp Zool 153:285, 1963 28. Beaudoin AR: Teratogenicity of sodium arsenate in rats. Teratology 10: 153, 1974 29. Barr M: The teratogenicity of cadmium chloride in two stocks of Wistar rats. Teratology 7:237, 1973 30. Tassinari MS, Long SY: Normal and abnormal midfacial development in the cadmium-treated hamster. Teratology 25: 101, 1982 31. Clavert A: Etudes des malformations oculaires déterminées chez l'embryon
de Lapin par le cyclophosphamide. Arch Anat Histol Embryol 53:209, 1970 32. Singh S, Sanyal AK: Eye anomalies induced by cyclophosphamide in rat fetuses. Acta Anat (Basel) 94:490, 1976 33. DeMyer W: Cleft lip and jaw induced in fetal rats by vincristine. Arch Anat Histol Embryol 48: 180, 1965 34. Roux C: Action teratogene du triparanol chez l'animal. Arch Fr Pediatr 21:451, 1964 35. Clarren S, Bowden D: Fetal alcohol syndrome: A new primate model for binge drinking and its
relevance to human ethanol teratogenesis. J Pediatr 101:819, 1982 36. Sheller B, Clarren SK, Astley SJ, Sampson PD: Morphometric analysis of Macaca nemestrina exposed to ethanol during gestation. Teratology 38:411, 1988 37. Sulik K, Johnston M: Sequence of developmental alterations following acute ethanol exposure
in mice: Craniofacial feature of the fetal alcohol syndrome. Am J Anat 166:257, 1983 38. Pinazo-Duran MD: Efecto de la exposicion pre- y postnatal al alcohol sobre
el desarrollo del nervio optico en la rata. Tesis doctoral, Universidad
de Valencia, 1991 39. Clarren SK, Astley S J, Bowden DM et al: Neuroanatomic and neurochemical abnormalities in non-human primate infants
exposed to weekly doses of ethanol during gestation. Alcohol Clin Exp Res 14:674, 1990 40. Cook CS, Nowotny AZ, Sulik KK: Fetal alcohol syndrome: Eye malformations in a mouse model. Arch Ophthalmol 105: 1576, 1987 41. Cook CS, Sulik KK: Keratolenticular dysgenesis (Peters' anomaly) as a result of acute embryonic
insult during gastrulation. J Pediatr Ophthalmol Strabismus 25:60, 1988 42. Wiley MJ, Cauwenbergs P, Taylor IM: Effects of retinoic acid on the development of the facial skeleton in hamsters: Early
changes involving cranial neural crest cells. Acta Anat 116:180, 1983 43. Shirai S: Eye abnormalities in mouse fetuses caused by simultaneous irradiation of
x-rays and ultrasound: II. Developmental abnormalities of the eye. Cong Anom 18:269, 1978 44. Shirai S, Ohshika S, Yuguchi S, Majima A: Ochratoxin A: I. Developmental eye abnormalities in mouse fetuses induced
by ochratoxin A. Acta Soc Ophthalmol Jpn 88:627, 1984 45. Shirai S, Ohshika S, Yuguchi S, Majima A: Ochratoxin A: III. Developmental abnormalities of the anterior segment
of the eye induced in mice by ochratoxin A. Acta Soc Ophthalmol Jpn 89:753, 1985 46. Källén B: Search for teratogenic risks with the aid of malformation registries. Teratology 35:47, 1987 47. Alford CA, Griffiths PD: Rubella, In Remington JS, Klein JO (eds): Infectious
Diseases of the Fetus and Newborn Infant, pp 69–103. Philadelphia, WB
Saunders, 1983 48. Parkman PD, Buescher EL, Artenstein MS: Recovery of rubella virus from army recruits. Proc Soc Exp Biol Med 111:225, 1962 49. Weller TH, Neva FA: Propagation in tissue culture of cytopathic agents from patients with rubella-like
illness. Proc Soc Exp Biol Med 111:215, 1962 50. Miller E, Cradock-Watson JE, Pollack TM: Consequences of confined rubella at successive stages of pregnancy. Lancet 2:781, 1982 51. Thompson KM, Tobin JO: Isolation of rubella virus from abortion material. Br Med J 2:264, 1970 52. Marks EO: Pigmentary abnormality in children congenitally deaf following maternal
German measles. Br J Ophthalmol 31:119, 1947 53. Krill AE: The retinal disease of rubella. Arch Ophthalmol 77:445, 1967 54. Kresky B, Nauheim JS: Rubella retinitis. Am J Dis Child 113:305, 1967 55. Gregg NM, Marks EO: Pigmentary abnormality in children congenitally deaf following maternal
German measles. Trans Ophthal Soc Aust 6: 122, 1946 56. Cooper LZ, Ziring PR, Ockerse AB et al: Rubella. Clinical manifestations and management. Am J Dis Child 118:18, 1969 57. Herrman KL: Rubella in the United States: Toward a strategy for disease control and
elimination. Epidemiol Infect 107:55, 1991 58. Kaplan KM, Cochi SL, Edmonds LD et al: A profile of mothers giving birth to infants with congenital rubella syndrome. Am J Dis Child 144:118, 1990 59. Koch FLP, Wolf A, Cowen D, Paige BH: Toxoplasmic encephalomyelitis. Arch Ophthalmol 29: 1, 1943 60. Remington JS: Toxoplasmosis and congenital infection. In Bergsma D (ed): Intrauterine
Infections. Birth Defects, Original Article Series 4, pp 47–56. New
York, The National Foundations-March of Dimes, 1968 61. Remington JS, Desmonts G: Toxoplasmosis. In Remington JS, Klein JO (eds): Infectious
Diseases of the Fetus and Newborn Infant, pp 143–263. Philadelphia, WB
Saunders, 1983 62. Pettapiece MC, Hiles DA, Johnson BL: Massive congenital ocular toxoplasmosis. J Pediatr Ophthalmol 13:259, 1976 63. Oniki S: Prognosis of pregnancy in patients with toxoplasmic retinochoroiditis. Jpn J Ophthalmol 27: 166, 1983 64. Kinney JS, Onorato IM, Stewart JA et al: Cytomegaloviral infection and disease. J Infect Dis 151:772, 1985 65. Alford CA, Stagno S, Pass RE, Britt WJ: Congenital and perinatal cytomegalovirus infections. Rev Infect Dis 12:745, 1990 66. Burns RP: Cytomegalic inclusion disease uveitis. Arch Ophthalmol 61:376, 1959 67. Weller TH, Hanshaw JB: Virological and clinical observation on cytomegalic inclusion disease. N Engl J Med 266:1233, 1962 68. McCarthy RW, Frenkel LD, Kollarits CR, Keys MP: Clinical anophthalmia associated with congenital cytomegalovirus infection. Am J Ophthalmol 90:558, 1980 69. Tarkkanen A, Merenmies L, Holmstrom T: Ocular involvement in congenital
cytomegalic inclusion disease, J Pediatr Ophthalmol 9:82, 1972 70. Miklos G, Orban T: Ophthalmic lesions due to cytomegalic inclusion disease. Ophthalmologica 148:98, 1964 71. Hittner HM, Desmond MM, Montgomery JR: Optic nerve manifestations of human congenital cytomegalovirus infection. Am J Ophthalmol 81:661, 1976 72. Christensen L, Beeman HW, Allen A: Cytomegalic inclusion disease. Arch Ophthalmol 57:90, 1957 73. Dvorak-Theobald G: Cytomegalic inclusion disease. Am J Ophthalmol 47:52, 1959 74. Hennis HL, Scott AA, Apple DJ: Cytomegalovirus retinitis. Surv Ophthalmol 34: 193, 1989 75. Alford CH: Chronic congenital infections of man. Yale J Biol Med 55:187, 1982 76. Frenkel LD, Keys MP, Hefferen SJ et al: Unusual eye abnormalities associated with congenital cytomegalovirus infection. Pediatrics 66:763, 1980 77. Nahmias AJ, Keyserling HL, Kerrick GM: Herpes Simplex. In Remington JS, Klein
JO (eds): Infectious Diseases of the Fetus and Newborn Infant, pp 636–678. Philadelphia, WB Saunders, 1983 78. Nahmias AJ, Visintine AM, Caldwell DR, Wilson LA: Eye infections with herpes simplex viruses in neonates. Surv Ophthalmol 21:100, 1976 79. Laforet EG, Lynch CL: Multiple congenital defects following maternal varicella. Report of a case. N Engl J Med 236:534, 1947 80. Srabstein JC, Morris N, Larke RPB et al: Is there a congenital varicella syndrome? J Pediatr 84:239, 1974 81. Charles NC, Bennett TW, Margolis S: Ocular pathology of the congenital varicella syndrome. Arch Ophthalmol 95:2034, 1977 82. Borzyskowski M, Harris RF, Jones RWA: The congenital varicella syndrome. Eur J Pediatr 137:335, 1981 83. Cotlier E: Congenital varicella cataract. Am J Ophthalmol 86:627, 1978 84. Brice JE: Congenital varicella resulting from infection during second trimester of
pregnancy. Arch Dis Child 51:474, 1976 85. Frey HM, Bialkin G, Gershon AA: Congenital varicella: Case report of a serologically proved long-term survivor. Pediatrics 59:110, 1977 86. Webster MH, Smith CS: Congenital abnormalities and maternal herpes zoster. Br Med J 2:1193, 1977 87. Savage MO, Moosa A, Gordon RR: Maternal varicella infection as a cause of fetal malformations. Lancet 1:352, 1973 88. Lambert SR, Taylor D, Kriss A et al: Ocular manifestations of the congenital varicella syndrome. Arch Ophthalmol 107:52, 1989 89. Mascola L, Pelosi R, Blount JH et al: Congenital syphilis. Why is it still occurring? JAMA 252:1719, 1984 90. Burkett G, Yasin S, Palow D: Perinatal implications of cocaine exposure. J Reprod Med 35:35, 1990 91. Wendel GD: Gestational and congenital syphilis. Clin Perinatol 15:287, 1988 92. Ricci JM, Fojaco RM, O'Sullivan MJ: Congenital syphilis: The University of Miami/Jackson Memorial Medical Center
Experience, 1986-1988. Obstet Gynecol 74:687, 1989 93. Duke-Elder S (ed): System of Ophthalmology, Vol 9, pp 306–321. London, Henry
Kimpton, 1977 94. Nabarro D: Congenital Syphilis. London, E Arnold, 1954 95. Hill RM, Knox JM: Syphilis. In Kelly VC (ed): Brenneman's practice
of pediatrics, Vol 2. Hagerstown, Harper and Row, 1972 96. Idsoe O, Guthe T, Willcox RR: Penicillin in the treatment of syphilis: The
experience of three decades. Bull WHO 47(suppl), 1972 97. Pedersen LM, Tygstrup IN, Pedersen J: Congenital malformations in newborn infants of diabetic women. Correlation
with maternal diabetic vascular complications. Lancet 1: 1124, 1964 98. Karlsson K, Kjellmer I: The outcome of diabetic pregnancies in relation to the mother's blood
sugar level. Am J Obstet Gynecol 112:213, 1972 99. Diamond MP, Salyer SL, Boehm FH, Vaughn WK: Congenital anomalies in offspring of insulin-dependent diabetic mothers. Diabetes Educator 12:272, 1987 100. Reece EA, Hobbins JC: Diabetic embryopathy: Pathogenesis, prenatal diagnosis and prevention. Obstet Gynecol Surv 41:325, 1986 101. Kitzmiller JL, Gavin LA, Gin GD et al: Preconception care of diabetes. Glycemic control prevents congenital anomalies. JAMA 265:731, 1991 102. Greene MF, Hare JW, Cloherty JP et al: First-trimester hemoglobin A1, and risk for major malformation and spontaneous
abortion in diabetic pregnancy. Teratology 39:225, 1989 103. Mills JL, Knopp RH, Simpson JL et al: Lack of relation of increased malformation rates in infants of diabetic
mothers to glycemic control during organogenesis. N Engl J Med 318:671, 1988 104. Mills JL, Simpson JL, Driscoll SG et al: Incidence of spontaneous abortion among normal women and insulin-dependent
diabetic women whose pregnancies were identified within 21 days of
conception. N Engl J Med 319:1617, 1988 105. Hanson U, Persson B, Thunell S: Relationship between haemoglobin A1c in early Type 1 (insulin-dependent) diabetic pregnancy and occurrence
of spontaneous abortion and fetal malformation in Sweden. Diabetologia 33: 100, 1990 106. Milunsky A, Alpert E, Kitzmiller JL et al: Prenatal diagnosis of neural tube defects. VIII. The importance of serum
alpha-fetoprotein screening in diabetic pregnant women. Am J Obstet Gynecol 142:1030, 1982 107. Soler NG, Walsh CH, Malins JM: Congenital malformations in infants of diabetic mothers. Q J Meal 45:303, 1976 108. Cousins LA: Congenital anomalies among infants of diabetic mothers. Etiology, prevention, prenatal
diagnosis. Am J Obstet Gynecol 147:333, 1983 109. Kucera J: Rate and type of congenital anomalies among offspring of diabetic women. J Reprod Med 7:61, 1971 110. Petersen RA, Walton DS: Optic nerve hypoplasia with good visual acuity and visual field defects. A
study of children of diabetic mothers. Arch Ophthalmol 95:254, 1977 111. Kim RY, Hoyt WF, Lessell S, Narahara MH: Superior segmental optic hypoplasia. A sign of maternal diabetes. Arch Ophthalmol 107:1312, 1989 112. Donat JFG: Septo-optic dysplasia in an infant of a diabetic mother. Arch Neurol 38:590, 1981 113. Khoury MJ, Becerra JE, Cordero JF, Erickson JD: Clinical-epidemiologic assessment of patterns of birth defects associated
with human teratogens: Application to diabetic embryopathy. Pediatrics 84:658, 1989 114. Lamache MA: Communications: Réflexions sur la descendance des alcooliques. Bull Acad Nat Médecine 151:517, 1967 115. Lemoine P, Harousseau H, Borteyru JP, Menuet JC: Les enfants de parents alcooliques. Anomalies observées. A propos
de 127 cas. Ouest-Medical 21:476, 1968 116. Jones KL, Smith DW, Ulleland CN, Streissguth AP: Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1: 1267, 1973 117. Jones KL, Smith DW: Recognition of the fetal alcohol syndrome in early infancy. Lancet 2:999, 1973 118. Jones KL, Smith DW, Streissguth AP, Myrianthopoulos NC: Outcome in offspring of chronic alcoholic women. Lancet 1: 1076, 1974 119. Sokol RJ, Clarren SK: Guidelines for use of terminology describing the impact of prenatal alcohol
on the offspring. Alcoholism Clin Exp Res 13:597, 1989 120. Smith DF, Sandor GG, MacLeod PM et al: Intrinsic defects in the fetal alcohol syndrome: Studies on 76 cases from
British Columbia and the Yukon territory. Neurobeh Toxicol Teratology 3: 145, 1981 121. Goldstein G, Arulanantham K: Neural tube defect and renal anomalies in a child with fetal alcohol syndrome. J Pediatr 93:636, 1978 122. Aronson M, Kyllerman M, Sabel K-G et al: Children of alcoholic mothers. Developmental, perceptual and behavioural
characteristics as compared to matched controls. Acta Paediatr Scand 74:27, 1985 123. Streissguth AP, Aase JM, Clarren SK et al: Fetal alcohol syndrome in adoloscents and adults. JAMA 265:1961, 1991 124. Altman B: Fetal alcohol syndrome. J Pediatr Ophthalmol 13:255, 1976 125. Miller M, Israel J, Cuttone J: Fetal alcohol syndrome, J Pediatr Ophthalmol
Strabismus 18:6, 1981 126. Strömland K: Ocular abnormalities in the fetal alcohol syndrome. Acta
Ophthalmol 63(suppl 171 ), 1985 127. Chan T, Bowell R, O'Keefe M, Lanigan B: Ocular manifestations in fetal alcohol syndrome. Br J Ophthalmol 75:524, 1991 128. Miller M, Epstein R, Sugar J et al: Anterior segment anomalies associated with the fetal alcohol syndrome. J Pediatr Ophthalmol Strabismus 21:8, 1984 129. Garber JM: Steep corneal curvature: A fetal alcohol syndrome landmark. J Am Optom Assoc 55:595, 1984 130. Strömland K: Ocular involvement in the fetal alcohol syndrome. Surv Ophthalmol 31:277, 1987 131. Abel EL, Sokol RJ: Fetal alcohol syndrome is now leading cause of mental retardation. Lancet 2:1222, 1986 132. Strömland K: Contribution of ocular examination to the diagnosis of foetal alcohol syndrome
in mentally retarded children. J Ment Defic Res 34:429, 1990 133. Mena M, Nazal R, Fernandez E et al: Prevalence of fetal alcohol syndrome at foster homes of the Servicio nacional
de Menores VIII region, Chile. Rev Méd Chil 115:1218, 1987 134. Véghelyi PV, Osztovics M, Kardos G et al: The fetal alcohol syndrome: symptoms and pathogenesis. Acta Paediatr Acad Sci Hung 19:171, 1978 135. Vitéz M, Korányi G, Gönczy E et al: A semiquantitative score system for epidemiologic studies of fetal alcohol
syndrome. Am J Epidemiol 119:301, 1984 136. Kirchner M: Das embryonale Alkoholsyndrom. Kinderärztliche Praxis 41:574, 1979 137. Majewski F, Majewski B: Alcohol embryopathy: Symptoms, auxological data, frequency
among the offspring and pathogenesis. In Kuriyama K, Takada
A, Ishii H (eds): Biomedical and Social Aspects of Alcohol and Alcoholism, pp 837–844. Amsterdam, Elsevier Science Publishers BV, 1988 138. Sokolowski F, Sokolowski A, Majewski F: Risiken für die Nachkommen alkoholkranker Frauen. Pädiat Prax 38:373, 1989 139. Halliday HL, Reid MM, McClure G: Results of heavy drinking in pregnancy. Br J Obstet Gynecol 89:892, 1982 140. Dehaene P, Crepin G, Delahousse G et al: Aspects épidémiologiques du syndrome d'alcoolisme faetal. Nouv Presse Med 10:2639, 1981 141. Olegård R, Sabel KG, Aronsson M: Effects on the child of alcohol abuse during pregnancy: Retrospective and
prospective studies. Acta Paediatr Scand 275(suppl): 112, 1979 142. Quellete EM, Rosett HL, Rosman NP, Weiner L: Adverse effects on offspring of maternal alcohol abuse during pregnancy. N Engl J Med 297:528, 1977 143. Hanson JW, Streissguth AP, Smith DW: The effects of moderate alcohol consumption during pregnancy on fetal growth
and morphogenesis. J Pediatr 92:457, 1978 144. May PA, Hymbaugh KJ, Aase JM, Samet JM: Epidemiology of fetal alcohol syndrome among American Indians of the southwest. Soc Biol 30:374, 1983 145. Robinson GC, Conry JL, Conry RF: Clinical profile and prevalence of fetal alcohol syndrome in an isolated
community in British Columbia. Can Med Assoc J 137:203, 1987 146. Streissguth AP, Barr HM, Sampson PD: Moderate prenatal alcohol exposure: Effects on child IQ and learning problems
at age 7 years. Alcoholism Clin Exp Res 14:662, 1990 147. Plant ML, Plant MA: Maternal use of alcohol and other drugs during pregnancy and birth abnormalities: Further
results from a prospective study. Alcohol Alcohol 23:229, 1988 148. Wiedeman HR: Hinweis auf eine derzeitige Häufung hypo- und aplastischer Fehlbildungen
der Gliedmassen. Med Welt 37:1863, 1961 149. Kosenow W, Pfeiffer RA: Micromelia, haemangioma und duodenal stenosis. Monatsschr Kinderheilkd 109:227, 1961 150. McBride WG: Thalidomide and congenital abnormalities. Lancet 2:1358, 1961 151. Lenz W: Fragen aus der Praxis. Kindliche Missbildungen nach Medikament v Einnahme
während der Gravidität? Dtsch Med Wochenschr 86:2555, 1961 152. Smithells RW: Thalidomide and malformations in Liverpool. Lancet 1:1270, 1962 152a. Warkany J: Congenital malformations: Notes and Comments, p 84. Chicago, Year
Book Medical Publishers, 1971 153. Kajii T: Thalidomide experience in Japan. Ann Pediatr 205:341, 1965 154. Kida M (ed): Thalidomide embryopathy in Japan. Tokyo, Kodansha, 1987 155. Schott K: Conterganschaden und Augenmissbildung. Klin Monatsbl Augenheilkd 143:599, 1963 156. Gilkes MJ, Strode M: Ocular anomalies in association with developmental limb abnormalities of
drug origin. Lancet 1:1026, 1963 157. Papst W: Thalidomid und kongenitale Anomalien der Augen. Ber Dtsch Ophthalmol Ges 65:209, 1964 158. Schmidt JGH: Augenmuskelparesen bei Thalidomid-Embryopatie. Ber Dtsch Ophthalmol Ges 65:215, 1964 159. Cullen JF: Ocular defects in Thalidomide babies. Br J Ophthalmol 48: 151, 1964 160. d'Avignon M, Barr B: Ear abnormalities and cranial nerve palsies in thalidomide children. Arch Otolaryngol 80:136, 1964 161. Zetterstr6m B: Ocular malformations caused by thalidomide. Acta Ophthalmol 44:391, 1966 162. Miller M, Strömland K: Ocular motility in thalidomide embryopathy. J Pediatr Ophthalmol Strabismus 28:47, 1991 163. Strömland K, Miller M: Thalidomide embryopathy clarifies developmental anomalies of the ocular
system. Teratology 44: 17A, 1991 164. Strömland K, Miller M: Refractive evaluation of thalidomide embryopathy. Graefes Arch Clin Exp Ophthalmol 230: 140, 1992 165. Meadow SR: Anticonvulsant drugs and congenital abnormalities. Lancet 2:1296, 1968 166. Speidel BD, Meadow SR: Maternal epilepsy and abnormalities of the fetus and newborn. Lancet 2:839, 1972 167. Fedrick J: Epilepsy and pregnancy: A report from the Oxford record linkage study. Br Med J 2:442, 1973 168. Lowe CR: Congenital malformations among infants born to epileptic women. Lancet 1:9, 1973 169. Bertollini R, Källén B, Mastroiacovo P, Robert E: Anticonvulsant drugs in monotherapy. Effect on the fetus. Eur J Epidemiol 3:164, 1987 170. Martinez-Frias ML: Clinical manifestation of prenatal exposure to valproic
acid using case reports and epidemiologic information, Am J Med Genet 37:277, 1990 171. Hanson JW, Myrianthopoulos NC, Harvey MAS, Smith DW: Risks to the offspring of women treated with hydantoin anticonvulsants
with emphasis on the fetal hydantoin syndrome. J Pediatr 89:662, 1976 172. Hanson JW: Teratogen update: Fetal hydantoin effects, Teratology 33:349, 1986 173. Nakane Y, Okuma T, Takahashi R et al: Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic
drugs: A report of a collaborative study group in Japan. Epilepsia 21:663, 1980 174. Holmes LB: The effects of exposure to phenytoin in utero. Proc Greenwood Genet Center 4:92, 1985 175. Hanson JW, Smith DW: The fetal hydantoin syndrome. J Pediatr 87:285, 1975 176. Hampton GR, Krepostman JI: Ocular manifestations of the fetal hydantoin syndrome. Clin Pediatr 20:475, 1981 177. Wallar PH, Genstler DE, George CC: Multiple systemic and periocular malformations associated with the fetal
hydantoin syndrome. Ann Ophthalmol 10: 1568, 1978 178. Wilson RS, Smead W, Char F: Diphenylhydantoin teratogenicity: Ocular manifestations and related deformities. J Pediatr Ophthalmol Strabismus 15: 137, 1978 179. Hoyt CS, Billson FA: Maternal anticonvulsants and optic nerve hypoplasia. Br J Ophthalmol 62:3, 1978 180. Bartoshesky LE, Bhan I, Nagpaul K, Pashayan H: Severe cardiac and ophthalmologic malformations in an infant exposed to
diphenylhydantoin in utero. Pediatrics 69:202, 1982 181. German J, Kowal A, Ehlers KH: Trimethadione and human teratogenesis. Teratology 3:349, 1970 182. Zackai EH, Mellman WJ, Neiderer B, Hanson JW: The fetal trimethadione syndrome. J Pediatr 87:280, 1975 183. Feldman GL, Weaver DD, Lovrien EW: The fetal trimethadione syndrome. Am J Dis Child 131:1389, 1977 184. Robert E, Guibaud P: Maternal valproic acid and congenital neural tube defects. Lancet 2:937, 1982 185. DiLiberti JH, Farndon PA, Dennis NR, Curry CJR: The fetal valproate syndrome. Am J Med Genet 19:473, 1984 186. Ardinger HH, Atkin JF, Blackston RD et al: Verification of the fetal valproate syndrome phenotype. Am J Med Genet 29:171, 1988 187. Verloes A, Frikiche A, Gremillet C et al: Proximal phocomelia and radial ray aplasia in fetal valproic syndrome. Eur J Pediatr 149:266, 1990 188. Tunnessen WW, Lowenstein EH: Glaucoma associated with the fetal hydantoin syndrome. J Pediatr 89: 154, 1976 189. Jäger-Roman E, Deichl A, Jakob S et al: Fetal growth, major malformations, and minor anomalies in infants born
to women receiving valproic acid. J Pediatr 108:997, 1986 190. Rosa FW: Teratogenicity of isotretinoin. Lancet 2:513, 1983 191. Benke PJ: The isotretinoin teratogen syndrome. JAMA 251:3267, 1984 192. Braun JT, Franciosi RA, Mastri AR et al: Isotretinoin dysmorphic syndrome. Lancet 1:506, 1984 193. de la Cruz E, Sun S, Vangvanichyakorn K, Desposito F: Multiple congenital malformations associated with maternal isotretinoin
therapy. Pediatrics 74:428, 1984 194. Hill RM: Isotretinoin teratogenicity. Lancet 1:1465, 1984 195. Lammer EJ, Chen DT, Hoar R et al: Retinoic acid embryopathy. N Engl J Med 313:837, 1985 196. Hansen LA, Pearl GS: Isotretinoin teratogenicity. Acta Neuropathol 65:335, 1985 197. Rosa FW: Retinoic acid embryopathy. N Engl J Med 314:262, 1986 198. Happle R, Traupe H, Bounameaux Y, Fisch T: Teratogene Wirkung von Etretinat beim Menschen. Dtsch Med Wochenschr 109:1476, 1984 199. Lammer EJ: Embryopathy in infant conceived one year after termination of maternal
etretinate. Lancet 2:1080, 1988 200. DiSaia PJ: Pregnancy and delivery of a patient with a Starr-Edwards mitral valve prosthesis. Report
of a case. Obstet Gynecol 28:469, 1966 201. Pettifor JM, Benson R: Congenital malformations associated with the administration of oral anticoagulants
during pregnancy. J Pediatr 86:459, 1975 202. Shaul WL, Hall JG: Multiple congenital anomalies associated with oral anticoagulants. Am J Obstet Gynecol 127:191, 1977 203. Holmes B, Moser HW, Halldorsson S et al: Mental Retardation: An Atlas of
Diseases with Associated Physical Abnormalities, pp 136–137. New
York, Macmillan Company, 1972 204. Becker MH, Genieser NB, Finegold M et al: Chondrodysplasia punctata. Am J Dis Child 129:356, 1975 205. Warkany J: Warfarin embryopathy. Teratology 14:205, 1976 206. Kleinebrecht J: Zur Teratogenität von Cumarin-Derivaten. Dtsch Med Wochenschr 107: 1929, 1982 207. Baillie M, Allen ED, Elkington AR: The congenital warfarin syndrome: A case report. Br J Ophthalmol 64:633, 1980 208. Hall JG, Pauli RM, Wilson KM: Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med 68:122, 1980 209. Kaplan LC: Congenital Dandy Walker malformation associated with first trimester warfarin: A
case report and literature review. Teratology 32:333, 1985 210. Iturbe-Alessio I, Fonseca MC, Mutchinik O et al: Risks of anticoagulant therapy in pregnant women with artificial heart
valves. N Engl J Med 315:1390, 1986 211. Schardein JL: Chemically Induced Birth Defects. New York, Marcel Dekker, 1985 212. Stettner E: Ein weiterer Fall einer Schädigung einer menschlichen Frucht durch
Röntgenbestrahlung. Jahrb Kinderh 95:43, 1921 213. Zappert J: Uber Roentgenogene fetale Microcephalie. Mschr Kinderheilkd 34:490, 1926 214. Murphy DP: The outcome of 625 pregnancies in women subjected to pelvic radium or roentgen
irradiation. Am J Obstet Gynecol 18:179, 1929 215. Goldstein L, Murphy DP: Microcephalic idiocy following radium therapy for
uterine cancer during pregnancy. Am J Obstet Gynecol 18:189,281, 1929 216. Goldstein L, Murphy DP: Etiology of ill health in children born after maternal pelvic irradiation. II. Defective
children born after postconceptional maternal irradiation. AJR 22:322, 1929 217. Goldstein L: Radiogenic microcephaly. Arch Neurol Psychiatr 24:102, 1930 218. Dekaban AS: Abnormalities in children exposed to X-radiation during various stages
of gestation: tentative timetable of radiation injury to the human fetus, part
I. J Nucl Med 9:471, 1968 219. Plummer G: Anomalies occurring in children exposed in utero to the atomic bomb in
Hiroshima. Pediatrics 10:687, 1952 220. Otake M, Schull WJ: In utero exposure to A-bomb radiation and mental retardation: A reassessment. Br J Radiol 57:409, 1984 221. Mole RH: Consequences of pre-natal radiation exposure for post-natal development. A
review. Int J Radiat Biol 42: 1, 1982 222. Neel JV, Schull WJ, Awa AA et al: The children of parents exposed to atomic bombs: estimates of the genetic
doubling dose of radiation for humans. Am J Hum Genet 46:1053, 1990 223. Nefzger MD, Miller R J, Fujino T: Eye findings in atomic bomb survivors of Hiroshima and Nagasaki: 1963-1964. Am J Epidemiol 89:129, 1968 224. Smith DW, Clarren SK, Harvey MAS: Hyperthermia as a possible teratogenic agent. J Pediatr 92:878, 1978 225. Layde PM, Edmonds LD, Erickson JD: Maternal fever and neural tube defects. Teratology 21: 105, 1980 226. Fraser FC, Skelton J: Possible teratogenicity of maternal fever. Lancet 2:634, 1978 227. Spraggett K, Fraser FC: Teratogenicity of maternal fever in (wo)man-a retrospective study. Teratology 25:78A, 1982 228. Saxén L, Holmberg PC, Nurminen M, Kuosma E: Sauna and congenital defects. Teratology 25:309, 1982 229. Nicholson HO: Cytotoxic drugs in pregnancy. J Obstet Gynaecol Br Commonw 75:307, 1968 230. Garber JE: Long-term follow-up of children exposed in utero to antineoplastic agents. Semin Oncol 16:437, 1989 231. Catanzarite VA, Ferguson JE: Acute leukemia and pregnancy: A review of management and outcome, 1972-1982. Obstet Gynecol Surv 39:663, 1984 232. Avilés A, Niz J: Long-term follow-up of children born to mothers with acute leukemia during
pregnancy. Med Pediatr Oncol 16:3, 1988 233. Reynoso EE, Shepherd FA, Messner HA et al: Acute leukemia during pregnancy: The Toronto leukemia study group experience
with long-term follow-up of children exposed in utero to chemotherapeutic
agents. J Clin Oncol 5:1098, 1987 234. Kirshon B, Wasserstrum N, Willis R et al: Teratogenic effects of first trimester cyclophosphamide therapy. Obstet Gynecol 72:462, 1988 235. Hiilesmaa VK, Teramo K, Granström M-L: Fetal head growth retardation associated with maternal antiepileptic drugs. Lancet 2:165, 1981 236. Jones KL, Lacro RV, Johnson KA, Adams J: Pattern of malformations in the children of women treated with carbamazepine
during pregnancy. N Engl J Med 320:1661, 1989 237. Rosa FW: Spina bifida in infants of women treated with carbamazepine during
pregnancy, N Engl J Med 324:674, 1991 238. Schroer RJ, Elhassani S: Multiple congenital anomalies with prenatal carbamazepine exposure. Proc Greenwood Genet Center 8:31, 1989 239. Seip M: Growth retardation, dysmorphic facies and minor malformations following
massive exposure to phenobarbitone in utero. Acta Paediatr Scand 65:617, 1976 240. Rating D, Nau H, Jäger-Roman E et al: Teratogenic and pharmacokinetic studies of primidone during pregnancy and
in the offspring of epileptic women. Acta Paediatr Scand 71:301, 1982 241. Gustavson EE, Chen H: Goldenhar syndrome, anterior encephalocele, and aqueductal stenosis following
fetal primidone exposure. Teratology 32: 13, 1985 242. Safra MJ, Oakley GP Jr: Association between cleft lip with or without cleft palate and prenatal
exposure to diazepam. Lancet 2:478, 1975 243. Saxén I: Associations between oral clefts and drugs taken during pregnancy. Int J Epidemiol 4:37, 1975 244. Laegreid L, Olegård R, Walström J, Conradi N: Teratogenie effects of benzodiazepine use during pregnancy. J Pediatr 114: 126, 1989 245. Strömland K: Ocular malformations in children exposed to drugs during gestation. Clin Pediatr 27:257, 1988 246. Nelson LB, Ehrlich S, Calhoun JH et al: Occurrence of strabismus in infants born to drug-dependent women. Am J Dis Child 141:175, 1987 247. Marion RW, Wiznia AA, Hutcheon RG, Rubinstein A: Human T-cell lymphotropic virus type III (HTLV-III) embryopathy. Am J Dis Child 140:638, 1986 248. Joshi VV, Oleske JM, Connor EM: Morphologic findings in children with acquired immune deficiency syndrome: Pathogenesis
and clinical implications. Pediatr Pathol 10: 155, 1990 249. Curless RG: Congenital AIDS: Review of neurologic problems. Childs Nerv Syst 5:9, 1989 250. Hoyme HE, Jones KL, Dixon SD et al: Prenatal cocaine exposure and fetal vascular disruption. Pediatrics 85:743, 1990 251. Dominguez R, Vila-Coro AA, Slopis JM, Bohan TP: Brain and ocular abnormalities in infants with in utero exposure to cocaine
and other street drugs. Am J Dis Child 145:688, 1991 252. Good WV, Ferreiro DM, Golabi M, Kabori JA: Abnormalities of the visual system in infants exposed to cocaine. Ophthalmol 99:341, 1992 253. Margolis S, Martin L: Anophthalmia in an infant of parents using LSD. Ann Ophthalmol 12: 1378, 1980 254. Apple DJ, Bennett TO: Multiple systemic and ocular malformations associated with maternal LSD
usage. Arch Ophthalmol 92:301, 1974 255. Bogdanoff B, Rorke LB, Yanoff M, Warren WS: Brain and eye abnormalities. Am J Dis Child 123: 145, 1972 256. Hoyt CS: Optic disc anomalies and maternal ingestion of LSD. J Pediatr Ophthalmol 15:286, 1978 257. Matsumoto H, Koya G, Takeuchi T: Fetal Minamata disease. A neuropathological study of two cases of intrauterine
intoxication by a methyl mercury compound. J Neuropathol Exp Neurol 24:563, 1965 258. Murakami U: The effect of organic mercury on intrauterine life. Adv Exp Biol Med 27:301, 1972 259. Samples JR, Meyer SM: Use of ophthalmic medications in pregnant and nursing women. Am J Ophthalmol 106:616, 1988 260. Kooner KS, Zimmerman TJ: Antiglaucoma therapy during pregnancy-Part I. Ann Ophthalmol 20: 166, 1988 261. Kooner KS, Zimmerman TJ: Antiglaucoma therapy during pregnancy-Part II. Ann Ophthalmol 20:208, 1988 262. Frishman WH, Chesner M: Beta-adrenergic blockers in pregnancy. Am Heart J 115:147, 1988 263. Lustgarten JS, Podos SM: Topical timolol and the nursing mother. Arch Ophthalmol 101:1381, 1983 264. Worsham GF, Beckman EN, Mitchell EH: Sacrococcygeal teratoma in a neonate. Association with maternal use of
acetazolamide. JAMA 240:251, 1978 265. Layton WM, Hallesy DW: Deformity of forelimb in rats: Association with high doses of acetazolamide. Science 149:306, 1965 266. Layton WM: Teratogenic action of acetazolamide in golden hamsters. Teratology 4:95, 1971 267. Altman B: Ocular effects in the newborn from maternal drugs. In Leopold
IH, Burns RP (eds): Symposium on ocular therapy, Vol 11, pp 97–98. New
York, John Wiley & Sons, 1979 268. Pitel M, Lerman S: Studies on the fetal rat lens. Effects of intrauterine Adrenalin and noradrenalin. Invest Ophthalmol 1:406, 1962 269. Heinonen OP, Slone D, Shapiro S: Birth defects and drugs in pregnancy. Littleton, MA, Publishing
Sciences Group, 1977 270. Boehm FH, Growdon JH: The effect of scopolamine on fetal heart rate baseline variability. Am J Obstet Gynecol 120: 1099, 1974 271. Shenker L: Clinical experiences with fetal heart rate monitoring of one thousand patients
in labor. Am J Obstet Gynecol 115:1111, 1973 272. Lahti A, Antila E, Saxén L: The effect of hydrocortisone on the closure of the palatal shelves in two
inbred strains of mice in vivo and in vitro. Teratology 6:37, 1972 273. Kraus AM: Congenital cataract and maternal steroid ingestion. J Pediatr Ophthalmol 12: 107, 1975 274. Ballard PD, Hearney EF, Smith MB: Comparative teratogenicity of selected glucocorticoids applied ocularly
in mice. Teratology 16: 175, 1977 275. Gasset AR, Itoi M, Ishii Y, Ramer RM: Teratogenicities of ophthalmic drugs. II. Teratogenicities and tissue accumulation
of thimerosal. Arch Ophthalmol 93:52, 1975 276. Schardein JL: Chemically induced birth defects. New York, Marcel Dekker, 1985 277. Leroux ML: Existe-t-il une surdite congenitale acquise due a la streptomycine? Ann Otolaryngol 67: 194, 1950 278. Robinson GC, Cambon KG: Hearing loss in infants of tuberculous mothers treated with streptomycin
during pregnancy. N Engl J Med 271:949, 1964 279. Warkany J: Antituberculous drugs. Teratology 20:133, 1979 280. Roy AS: Ocular malformation following ethambutol, rifampicin, isoniazide in the
first trimester of pregnancy. Indian J Pediatr 57:730, 1990 281. Oberheuser F: Praktische Erfahrungen mit Medikamenten in der Schwangerschaft. Therapiewoche 31:2198, 197 l 282. Itoi M, Gefter JW, Kaneko N et al: Teratogenicities of ophthalmic drugs. I. Antiviral ophthalmic drugs. Arch Ophthalmol 93:46, 1975 283. Simpson WJ, Calif LL: A preliminary report on cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol 73:808, 1957 284. Ericson A, Källén B, Westerholm P: Cigarette smoking as an
etiologic factor in cleft lip and palate. Am J Obstet Gynecol 135:348, 1979 285. Kline J, Stein ZA, Susser M, Warburton D: Smoking: A risk factor for spontaneous abortion. N Engl J Med 297:793, 1977 286. Werler MM, Pober BR, Holmes LB: Smoking and pregnancy. Teratology 32:473, 1985 287. Christianson RE: The relationship between maternal smoking and the incidence of congenital
anomalies. Am J Epidemiol 112:684, 1980 288. Barr HM, Streissguth AP: Caffeine use during pregnancy and child outcome: A 7-year prospective study. Neurotoxicology and Teratology 13:441, 1991 289. MRC Vitamin Study Research Group: Prevention of neural tube defects: results
of the medical research council vitamin study. Lancet 338:131, 1991 290. Hale F: Pigs born without eye balls. J Hered 24:105, 1933 291. Wilson JG, Roth CB, Warkany J: An analysis of the syndrome of malformations induced by vitamin A deficiency. Effects
of restoration of vitamin A at various times during gestation. Am J Anat 92: 189, 1953 292. Giroud A, Tuchmann-Duplessis H: Malformations congenitales. Role des facteurs exogenes. Pathol Biol 10:119, 1962 293. Geelen JA: Hypervitaminosis: an induced teratogenesis. Crit Rev Toxicol 6:351, 1979 294. Rosa FW, Wilk AL, Kelsey FO: Vitamin A congeners. In Sever JL, Brent RL (eds): Teratogen
Update: Environ-mentally Induced Birth Defect Risks. New
York, Alan R. Liss, 1986 295. Wagner LK, Hayman LA: Pregnancy and women radiologists. Radiology 145:559, 1982 296. Ericson HA, Källén AJ: Hospitalization for miscarriage and delivery outcome among Swedish nurses
working in operating rooms 1973-1978. Anesth Analg 64:981, 1985 297. Källén B, Malmquist G, Mortiz U: Delivery outcome among physiotherapists: Is non-ionizing radiation a fetal
hazard? Arch Environ Health 37:81, 1982 298. Ericson A, Källén B: An epidemiological study of work with video screen and pregnancy outcome. Am J Indust Med 9:447, 1986 299. Spaeth GL, Nelson LB, Beaudoin AR: Ocular teratology. In Jakobiec FA (ed): Ocular
Anatomy, Embryology and Teratology, p 963. Philadelphia, Harper
and Row, 1982 |