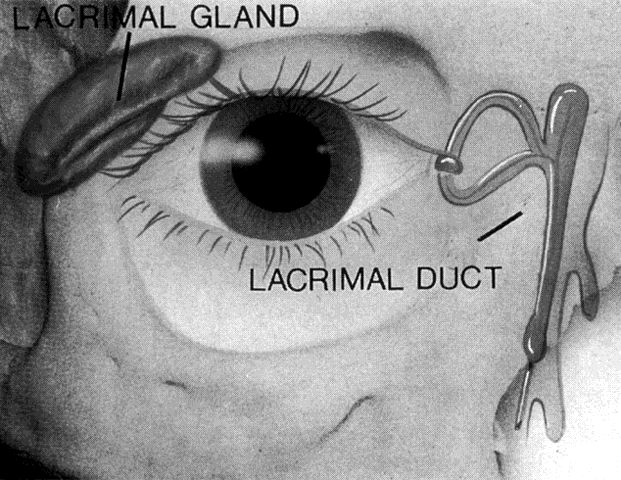
GROSS ANATOMY OF THE EXCRETORY SYSTEM The Membranous Passages Tears, secreted from the lacrimal gland in the superolateral region of
the conjunctiva, spread over the surface of the globe by two processes: (1) eyelid
movement; and (2) gravity. The tears then collect in the
lacrimal lake at the medial aspect of the conjunctival fornix. They then
pass by capillary attraction into the lacrimal puncta and the lacrimal
canaliculi. Aided by the lacrimal pump mechanism and gravity, fluid
then passes into the common canaliculus and the lacrimal sac. Fluid
continues downward into the nasolacrimal duct, emptying into the inferior
meatus of the nose under the inferior turbinate bone.2,18 Punctum The canaliculus begins as the punctum, surrounded by a strong, fibrous
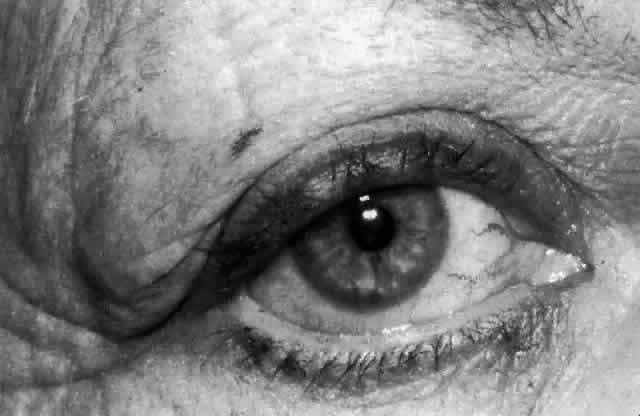
ring known as the lacrimal papilla. The papilla and the included punctum, which has an average diameter of 0.2 to 0.3 mm, are
located at the myocutaneous junction of the nasal
aspect of the eyelid margin. The papilla (Fig. 18) is slightly elevated relative to the surrounding tissue and becomes more
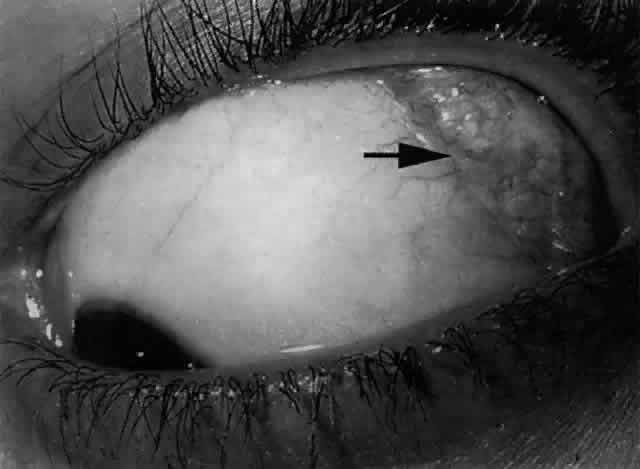
prominent with age because of atrophy of the encircling tissue.2,6 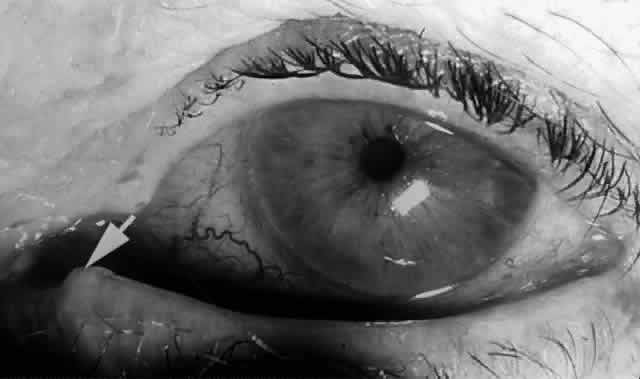 Fig. 18. The left lower papilla (arrow) is prominent in this elderly patient because of atrophy of the encircling
tissue. Fig. 18. The left lower papilla (arrow) is prominent in this elderly patient because of atrophy of the encircling
tissue.
|
The lacrimal papilla, together with its punctum, is surrounded by pretarsal
orbicularis muscle fibers that insert onto the posterior lacrimal
crest and more posteriorly onto the periosteum of the medial orbital
wall. Horizontal and posterior contraction of this muscle slightly inverts
the medial eyelid margin to place the punctum in apposition with
the lacrimal lake, allowing it to receive the tears.19 Canaliculi The canaliculi represent the palpebral portion of the lacrimal excretory
system. The canaliculi conduct tears from the conjunctival fornix to
the nasolacrimal sac. The initial 2 mm of each canaliculus is vertical, ending
in a 1-mm dilation called the ampulla. The ampulla is located on the anterior side of the tarsus and is thus
subjected to the compressive forces of the surrounding pretarsal orbicularis
muscle. The initial course of the canaliculus is vertical and slightly
anterior.2,19 At the ampulla, the canaliculus takes a horizontal course. Like the vertical
portion, the horizontal portion of each canaliculus is surrounded
by pretarsal orbicularis muscle fibers and is found close to the eyelid
margin. Because the horizontal portion of the canaliculus follows
the curved eyelid margin, it is not strictly horizontal. Medially, the
horizontal canaliculus makes an anterior bend, entering the lacrimal
sac nonperpendicularly. The medial boundary of the lacrimal lake is formed by the plica semilunaris and the caruncle, which prevent apposition of the medial aspect of the eyelids with the
eyeball. The longer lower canaliculus (10 mm) is directed into the lacrimal
lake lateral to the plica semilunaris, while the shorter superior
canaliculus (8 mm) collects fluid from the space between the plica semilunaris
and the caruncle.2,18,19 Lining the lacrimal fossa and surrounding the lacrimal sac is a dense, fibrous
membrane that Jones termed the lacrimal fascia.2 The upper and lower canaliculi narrow as they course medially to traverse
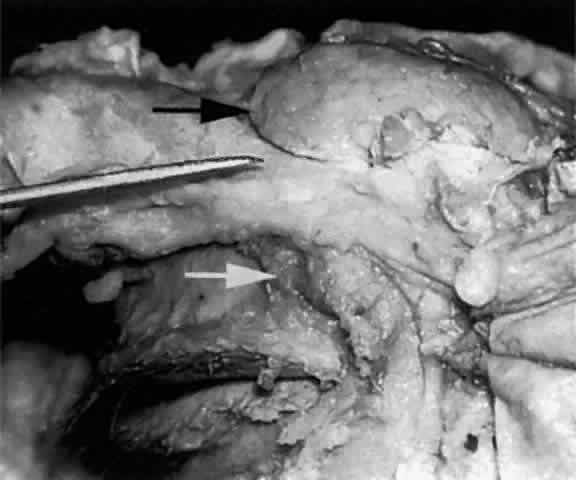
the lacrimal fascia individually before they join, in the majority
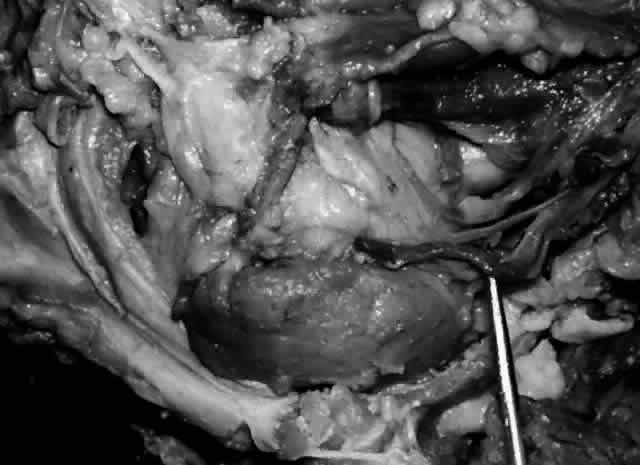
of cases, to form the common canaliculus (Fig. 19). The common canaliculus is located 2 to 3 mm posterior to the central portion of the medial canthal
tendon. Histologically, the common canaliculus represents a diverticulum
of the lacrimal sac and, if enlarged, is known as the sinus of
Maier. The point of common canalicular entrance into the lacrimal sac
is slightly above the midpoint of the sac on its lateral wall. In approximately 10% of
cases, the upper and lower canaliculi can be found
entering the lacrimal sac through separate openings, without the presence
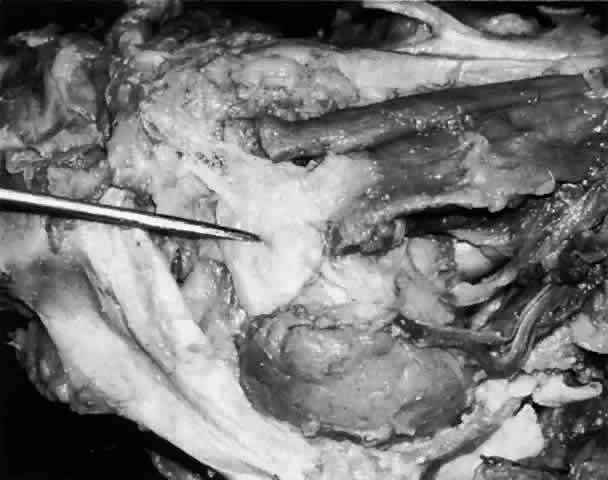
of a common canaliculus.20 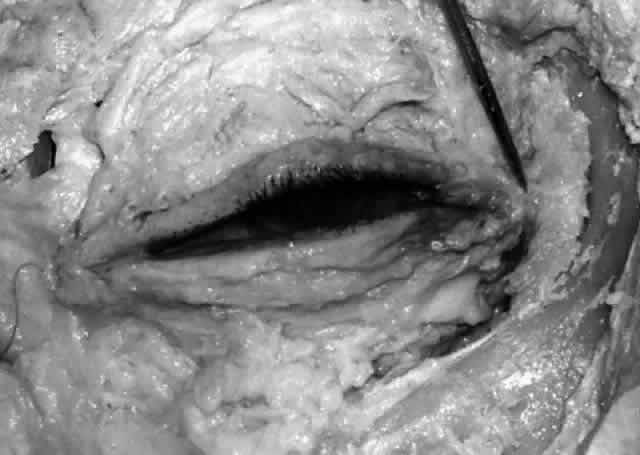 Fig. 19. The upper and lower canaliculus, in the majority of cases, join to form
the common canaliculus (pointer). The common canaliculus enters the lateral wall of the sac slightly above
the midpoint of the sac. Fig. 19. The upper and lower canaliculus, in the majority of cases, join to form
the common canaliculus (pointer). The common canaliculus enters the lateral wall of the sac slightly above
the midpoint of the sac.
|
Lacrimal Sac The lacrimal sac and the nasolacrimal duct form one continuous structure (Fig. 20). The uppermost portion lies within the bony lacrimal sac fossa and is
termed the lacrimal sac. The middle part is contained within an osseous channel in the maxilla
and is termed the nasolacrimal duct. The inferior-most portion of the nasolacrimal duct courses for a short
distance beneath the nasal mucosa of the lateral wall of the inferior
meatus and is therefore known as the meatal portion. Individual variations in the membranous lacrimal sac and nasolacrimal
duct are due to variations in the surrounding bony configuration.2  Fig. 20. Skeletonized right lacrimal excretory system. With the bony canal removed, the
lacrimal sac (small arrow) is seen in continuum with the nasolacrimal duct (large arrow). The pointer is behind the upper and lower canaliculi. Fig. 20. Skeletonized right lacrimal excretory system. With the bony canal removed, the
lacrimal sac (small arrow) is seen in continuum with the nasolacrimal duct (large arrow). The pointer is behind the upper and lower canaliculi.
|
The lacrimal sac is found along the anterior aspect of the medial orbital
wall within a bony depression called the fossa for the lacrimal sac. The fossa is covered by the periorbita, which laterally splits at the
posterior lacrimal crest, one layer lining the bony fossa and the other
bridging straight across to reach the anterior lacrimal crest, sandwiching
the lacrimal sac in between. The latter layer attaches inferiorly
to the upper edge of the bony nasolacrimal canal. The layer of periorbita
that covers the lacrimal fossa is termed the lacrimal fascia. This fascia is pierced separately by the two canaliculi before forming
the common canaliculus. Usually, between the lacrimal fascia and the lacrimal sac there is a narrow
space that contains a fine venous plexus draining into the angular
or supraorbital vein. Fine branches of the infratrochlear nerve can
also be seen piercing the lacrimal fascia. Medially, and posteriorly, the
lacrimal sac is applied to the periosteal lining of the lacrimal fossa. Anteriorly, the
superior aspect of the lacrimal sac is covered by
the fibrous anterior limb of the medial canthal tendon (medial palpebral
ligament), which attaches to the upper part of the anterior lacrimal
crest. Below the anterior limb of the medial canthal tendon, the inferior
aspect of the lacrimal sac is bound anteriorly only by the orbital
septum, which also inserts onto the anterior lacrimal crest. It is
through this thinner covering that a distended sac may herniate. Above, below, and
behind the anterior limb of the medial canthal tendon, the
superficial head of the pretarsal orbicularis muscle can be found
inserting onto the lacrimal fascia.2,18,21 Posterior to the upper half of the lacrimal sac passes the posterior limb
of the medial canthal tendon. Deep to it lies Horner's muscle.22,23 This muscle arises from the upper part of the posterior lacrimal crest
and passes forward upon and laterally across the upper part of the lacrimal
sac to divide and run along each eyelid margin as the deep head
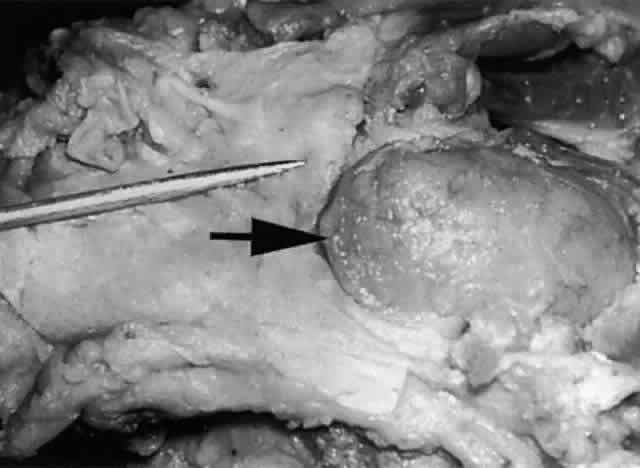
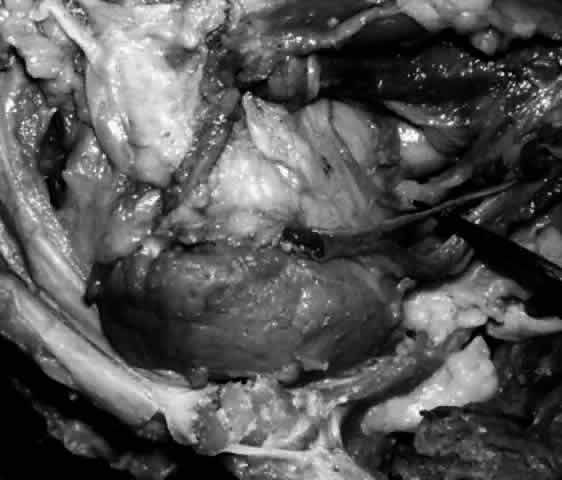
of the pretarsal orbicularis oculi muscle.2,22 The lacrimal sac is approximately 12 mm in height, 4 to 6 mm in depth, and 2 mm
wide. The 4-mm portion of the sac that projects above the medial
canthal tendon is somewhat pointed and is termed the fundus (Fig. 21). The larger portion below is termed the body. The walls of the sac are in apposition, unless the sac is filled with
lacrimal fluid. The sac is somewhat larger in male subjects.19,21 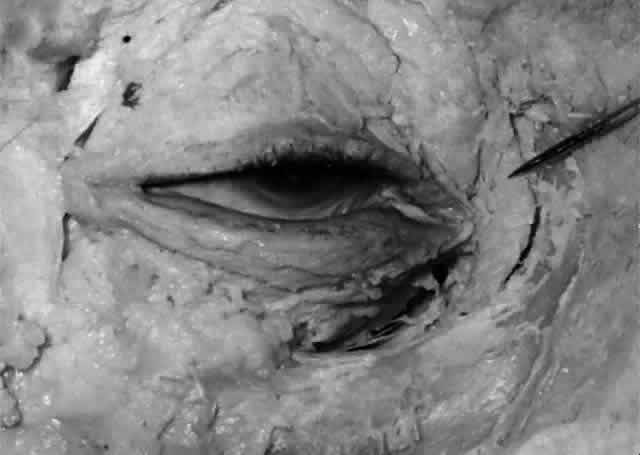 Fig. 21. The fundus of the lacrimal sac (pointer) is somewhat pointed and projects 4 mm above the entrance of the common
canaliculus. Fig. 21. The fundus of the lacrimal sac (pointer) is somewhat pointed and projects 4 mm above the entrance of the common
canaliculus.
|
Nasolacrimal Duct The nasolacrimal duct (see Fig. 20) is an inferiorly directed continuation of the nasolacrimal sac. The nasolacrimal
duct measures 3 to 4 mm in diameter in adults and 2 mm in
infants. The nasolacrimal duct measures approximately 12.5 mm in vertical
length and usually terminates as a 5-mm extension into the inferior
nasal meatus. The upper and mid portions conform in shape and direction
with the surrounding bony nasolacrimal canal, whereas the lower or
meatal part is usually buried in the mucous membrane of the lateral wall
of the inferior meatus. The inferior opening (ostium) is variable in shape and position. It may be round, linear, or punctiform
and may be bridged by a valve, flap, diaphragm, or threads of mucous
membrane.2,19 In some cases, the nasolacrimal duct may empty directly into the roof of
the inferior meatus, but in the majority, the opening is on the side
wall of the inferior meatus (Fig. 22), 30 mm behind the lateral margin of the anterior nares. In children and
infants, the opening is found 20 mm behind the lateral margin of the
anterior nares. In a small number of cases, the nasolacrimal duct is
found to course beneath the mucosa of the lateral nasal wall without
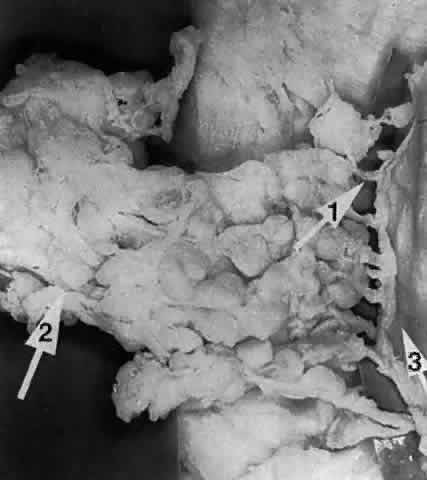
opening into the nose.24,25 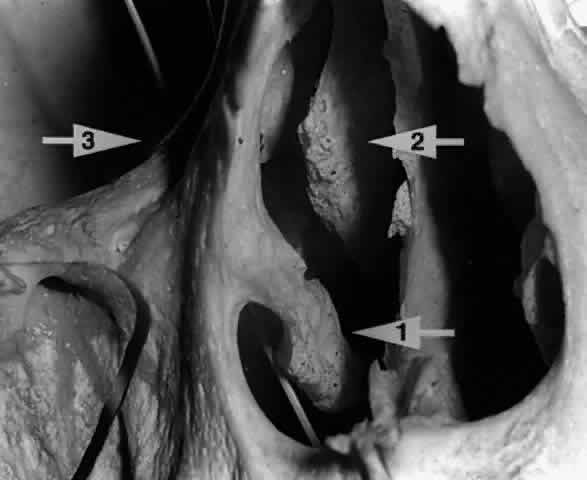 Fig. 22. A twig placed in the right nasolacrimal fossa of this skull is seen exiting
below the inferior turbinate (arrow 1). The middle turbinate (arrow 2) is seen lying medial to the nasolacrimal fossa (arrow 3). Fig. 22. A twig placed in the right nasolacrimal fossa of this skull is seen exiting
below the inferior turbinate (arrow 1). The middle turbinate (arrow 2) is seen lying medial to the nasolacrimal fossa (arrow 3).
|
The mucosal lining of the nasolacrimal duct contain crypts and folds that
usually disappear by adulthood. Some may persist, however, and if under
the influence of raised intranasal air pressure, may develop into
valves, sinuses, or diverticula (Fig. 23). These would include the sinus of Arlt, Béraud's valve, the
spiral valve of Hyrtl, and Taillefer's valve. The most significant
fold is the one situated at the meatal opening of the nasolacrimal
duct called the valve of Hasner. In approximately 30% of full-term neonates, there
persists a delicate membrane that, within 6 months after
birth, usually undergoes spontaneous perforation. However, in approximately 4% of
infants, the distal end of the nasolacrimal duct will remain
closed. Massage or lacrimal probing may be necessary to permit patency.25,26 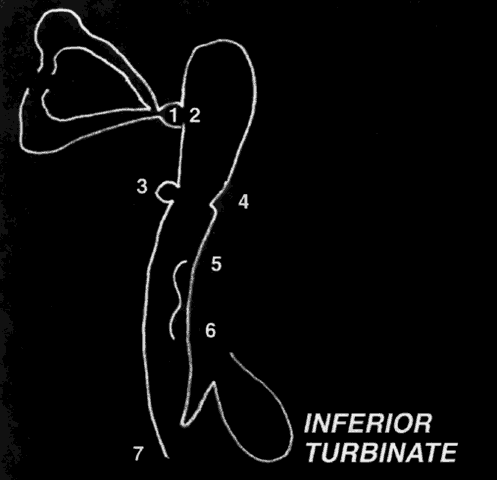 Fig. 23. Diagram of the valves and sinuses of the nasolacrimal passages. 1 = Maier's sinus; 2 = Rosenmüller's valve; 3 = Arlt's sinus; 4 = Kraus's or Béraud's valve; 5 = spiral valve of Hyrtl; 6 = Taillefer's valve; 7 = Hasner's valve. Fig. 23. Diagram of the valves and sinuses of the nasolacrimal passages. 1 = Maier's sinus; 2 = Rosenmüller's valve; 3 = Arlt's sinus; 4 = Kraus's or Béraud's valve; 5 = spiral valve of Hyrtl; 6 = Taillefer's valve; 7 = Hasner's valve.
|
The upper portion of the nasolacrimal duct can be separated easily from
the surrounding periosteum that lines the bony canal. As it passes inferiorly, however, the
two become more fused and tightly adherent to the
bony canal. If a probe is inadvertently passed through the lacrimal
sac wall during instrumentation, it can be advanced inferiorly outside
the nasolacrimal duct within the bony canal until the duct becomes adherent
to the bone. VASCULAR SUPPLY TO THE EXCRETORY SYSTEM The medial canthus is an extremely vascular area. The nasolacrimal passages
receive its vascular supply from (1) the ophthalmic artery, (2) the
angular artery, and (3) the infraorbital artery. The ophthalmic artery is a branch of the internal carotid artery. In the orbital apex, the ophthalmic
artery lies lateral to the optic nerve. It then passes over
the optic nerve to course anteriorly and medially within the orbit. The
ophthalmic artery terminates as the dorsal nasal artery, from which
emanates the superior medial palpebral branches that supply the lacrimal
sac. The facial branch of the external carotid artery crosses the mandible diagonally
toward the nasolabial fold. It passes between the levator labii
superioris and levator ala nasi muscles. The facial artery lies beneath
the orbicularis muscle 6 to 8 mm medial to the inner canthus and 5 mm
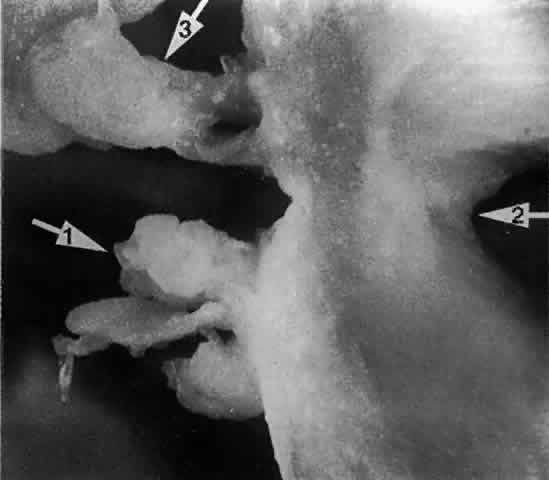
anterior to the lacrimal sac, where it is known as the angular artery (Fig. 24). The angular artery perforates the superior orbital septum above the
medial canthal tendon to send branches to the lacrimal sac as well as
to the duct. 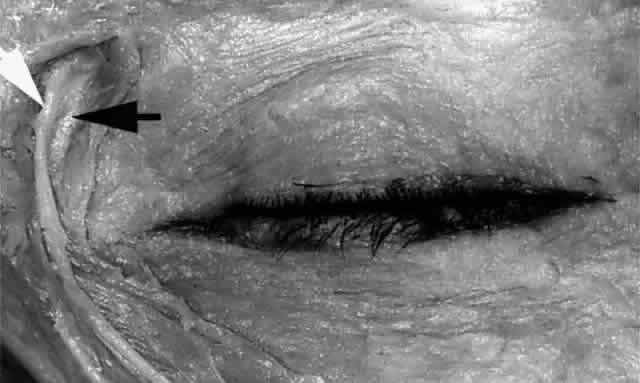 Fig. 24. The angular artery (white arrow) and angular vein (black arrow) are seen 6 to 8 mm medial to the inner canthus (left eye). Fig. 24. The angular artery (white arrow) and angular vein (black arrow) are seen 6 to 8 mm medial to the inner canthus (left eye).
|
The infraorbital artery sends branches to the lower lid that eventually pierce the lateral margin
of the upper nasolacrimal canal to supply the sac as well as the duct. The
inferior portion of the nasolacrimal duct receives arterial supply
from the nasal branch of the sphenopalatine artery, which is a branch
of the internal maxillary artery.1,2,6 The superior aspect of the venous plexus that surrounds the nasolacrimal
duct drains into the angular vein and infraorbital vein. The inferior aspect of the venous plexus that surrounds the nasolacrimal
ducts drains into the nasal cavity, through the sphenopalatine veins
into the pterygoid plexus and the internal maxillary vein. The angular
vein (see Fig. 24) lies superficial and lateral to the angular artery. Venous drainage passes
downward into the facial vein and eventually into the internal jugular
vein. Alternatively, venous drainage can pass supramedially into
the orbit via the supraorbital vein into the superior ophthalmic vein, which
eventually passes posteriorly into the cavernous sinus. The angular
vein and artery are important surgical landmarks in dacryocystorhinostomy
surgery. The lymphatic drainage from the sac accompanies the facial vein and drains
into the submaxillary nodes. The lymphatic drainage from the lower
aspect of the nasolacrimal duct joins the lymphatic vessels of the inferior
nasal meatus, which drain anteriorly toward the anterior nares
and into the submaxillary nodes or posteriorly into the deep cervical
nodes. NERVE SUPPLY OF THE EXCRETORY SYSTEM Sensory nerve supply to the nasolacrimal sac is derived from the infratrochlear
nerve, which is the terminal branch of the nasociliary nerve, a
branch of the ophthalmic division of the fifth cranial nerve (V1). The lower portion of the nasolacrimal duct receives sensation from the
anterior superior alveolar branch of the maxillary division of the
fifth cranial nerve (V2). There may be a physiologic relationship between the innervation of the
lacrimal gland (lacrimal nerve) and the lacrimal sac (infratrochlear nerve), both
being branches of the ophthalmic division of the fifth cranial
nerve. This may explain why destruction of the sac leads to a decrease
in tear secretion and why the epiphora of dacryocystitis may be
caused in part by reflex irritation from the diseased sac as well as from
obstruction.2,11 HISTOLOGY OF THE MEMBRANOUS EXCRETORY PASSAGES The membranous portion of the nasolacrimal system is lined with mucous
membrane continuously from the conjunctiva at the lacrimal puncta, to
the nasal mucosa at the valve of Hasner beneath the inferior turbinate. The
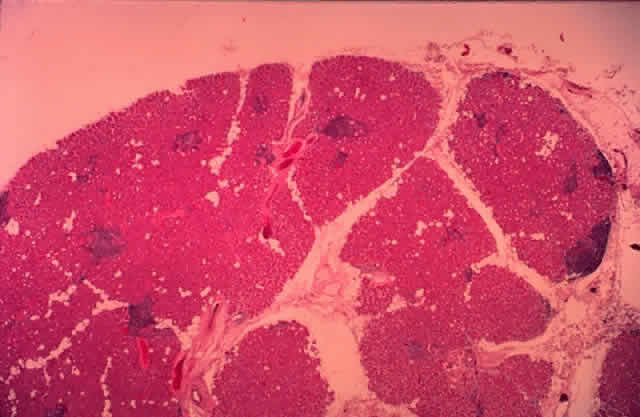
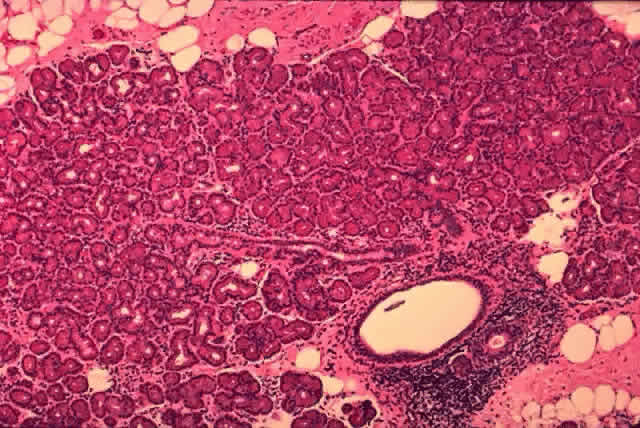
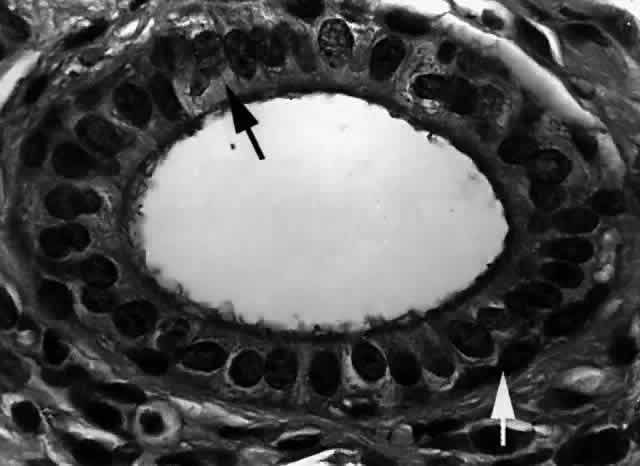
histology shows a transition from a nonkeratinizing stratified squamous
epithelium in the lacrimal puncta and canaliculi (Fig. 25) to a columnar epithelium in the nasolacrimal sac to an erectile venous
plexus containing mucoperiosteum near the valve of Hasner. Whereas the
canaliculi are lined by 6 to 12 layers of nonkeratinizing squamous
epithelium, the lacrimal sac and nasolacrimal duct are lined by a dual
layer of columnar epithelium, which gradually assumes the characteristics
of the nasal mucosa distally as it approaches the nasal cavity. Mucous
glands and an occasional lacrimal secretory gland can be seen within
the nasolacrimal duct. Atrophic or vasomotor nasal mucosal changes
occur in the distal portion of the nasolacrimal duct, especially in
the meatal portion.2,19 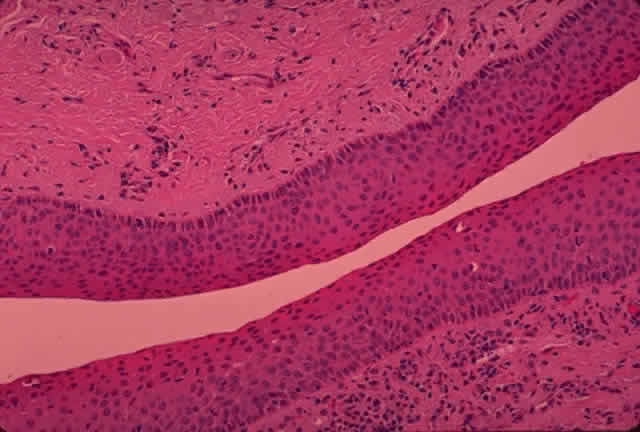 Fig. 25. The canaliculus. The canaliculus is lined by 6 to 12 layers of nonkeratinizing
stratified squamous epithelium. (H & E, original magnification × 25; Courtesy of Ralph Eagle, MD, Philadelphia, PA) Fig. 25. The canaliculus. The canaliculus is lined by 6 to 12 layers of nonkeratinizing
stratified squamous epithelium. (H & E, original magnification × 25; Courtesy of Ralph Eagle, MD, Philadelphia, PA)
|
Orbicularis oculi muscle fibers surround the base of the papilla in a sphincter-like
fashion. The substantia propria consists of dense elastic
tissue in the regions of the papilla and the canaliculi, becoming more
fibrous in the region of the lacrimal sac. The fibroelastic layer of
the nasolacrimal duct contains a venous plexus that is well developed
in the meatal portion.2,19 In general, the dense elastic tissue is found to decrease from the punctum
inferiorly, whereas the venous plexus and thickness of the walls
increase.1,2,19 LACRIMAL EXCRETORY OSTEOLOGY The osseous lacrimal passages consist of (1) the fossa for the lacrimal
sac and (2) the nasolacrimal canal. The Fossa for the Lacrimal Sac The fossa for the lacrimal sac (Fig. 26) is a broad groove found near the lower two thirds of the medial orbital
margin. It measures approximately 16 mm high, 4 to 8 mm wide, and 2 to 3 mm
deep.27,28 It is shallower above, extending to the frontal bone and becomes deeper
inferiorly, where it continues as the bony nasolacrimal canal. The fossa
is formed by two bones: (1) the frontal process of the maxilla forming
the anterior lacrimal crest; and (2) the lacrimal bone forming the
posterior lacrimal crest. The vertically placed “maxillary-lacrimal” suture, between the two bones, runs the depth of the lacrimal
fossa and parallel to the axis of the nasolacrimal canal. The extent
to which the two bones participate in the formation of the fossa
varies considerably. In most, the suture is located approximately in the
center of the fossa. In some, the maxillary-lacrimal suture may be
located more anteriorly, indicating a more major contribution by the lacrimal
bone; in others, the suture may be located more posteriorly, indicating
a larger contribution of the maxillary bone.2,19 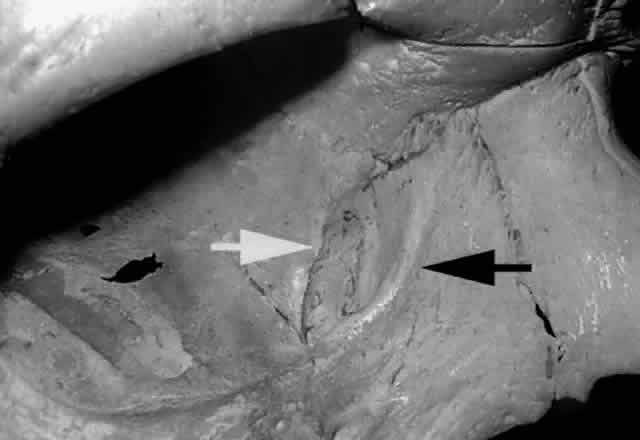 Fig. 26. The fossa for the lacrimal sac is formed by the frontal process of the
maxillary bone anteriorly (black arrow) and lacrimal bone, posteriorly (white arrow) (right orbit). The vertically placed “maxillary-lacrimal” suture
is seen in the middle of the fossa. Fig. 26. The fossa for the lacrimal sac is formed by the frontal process of the
maxillary bone anteriorly (black arrow) and lacrimal bone, posteriorly (white arrow) (right orbit). The vertically placed “maxillary-lacrimal” suture
is seen in the middle of the fossa.
|
Clinically, this variation is important because the thin lacrimal bone
is more easily broken with blunt instrumentation when initiating an osteotomy
during dacryocystorhinostomy surgery. The lacrimal bone is pneumatized by anterior ethmoidal air cells (agger
nasi bullae), which occasionally pneumatizes the anterior lacrimal crest. In
cases where the lacrimal bone contribution to the lacrimal fossa
is dominant (Fig. 27), ethmoidal air cells are more likely to be found in the lacrimal fossa
and nasal cavity.19,28 Clinically these air cells separating the upper half of the lacrimal fossa
from the nasal cavity, if present, must be opened or removed in order
to enter the nasal cavity during dacryocystorhinostomy surgery. 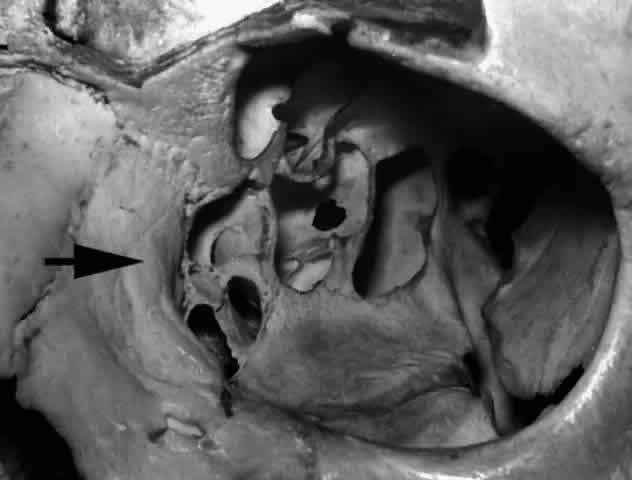 Fig. 27. Ethmoidal air cells extend to the anterior lacrimal crest (arrow), demonstrating a dominant ethmoidal and lacrimal bone contribution to
the lacrimal fossa (left orbit). Fig. 27. Ethmoidal air cells extend to the anterior lacrimal crest (arrow), demonstrating a dominant ethmoidal and lacrimal bone contribution to
the lacrimal fossa (left orbit).
|
The rounded anterior lacrimal crest is poorly defined above (see Fig. 26), but becomes well defined inferiorly as it continues with the infraorbital
margin (Fig. 28). The medial canthal tendon attaches superiorly on the anterior lacrimal
crest, where a small tubercle is sometimes found. Inferiorly, the orbital
septum and some superficial orbicularis oculi fibers attach to
the anterior lacrimal crest. The anterior lacrimal crest is stronger than
the posterior lacrimal crest. 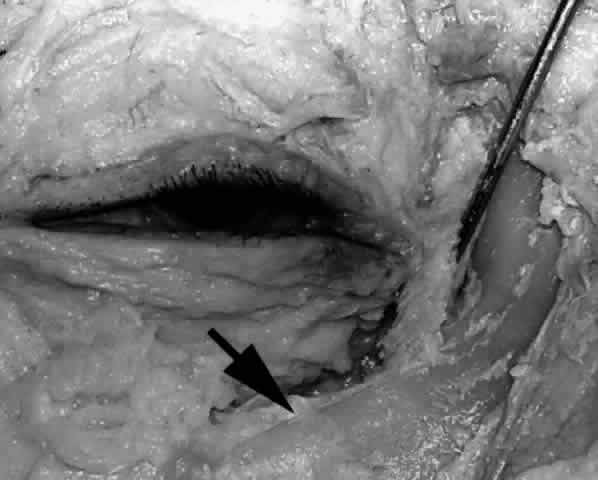 Fig. 28. With the medial canthal tendon removed and the nasolacrimal sac reflected
laterally (pointer), the anterior lacrimal crest is better defined
inferiorly as it continues as the inferior orbital rim (arrow). Fig. 28. With the medial canthal tendon removed and the nasolacrimal sac reflected
laterally (pointer), the anterior lacrimal crest is better defined
inferiorly as it continues as the inferior orbital rim (arrow).
|
The posterior lacrimal crest is more sharply defined than the anterior lacrimal crest (see Fig. 26) and may present as a prolonged plate of bone curving forward over the
fossa. Superiorly, it is continuous with the medial orbital rim. The
posterior lacrimal crest is the strongest component of the lacrimal bone. The
posterior lacrimal crest is stronger and flatter superiorly, where
the deep head of the pretarsal orbicularis oculi muscle inserts. The
orbital septum also inserts onto the posterior lacrimal crest and
continues inferiorly to cover the pars lacrimal muscle of Horner on its
posterior surface. Just anterior to the posterior lacrimal crest, the
bone becomes quite thin and is aerated by ethmoidal air cells. Just
behind the posterior lacrimal crest, the lacrimal bone may again thin
and extend posteriorly, or it may immediately meet the ethmoid bone. The middle turbinate is an outcropping of the ethmoid bone and may project anteriorly into
the nasal cavity to lie medial to the lacrimal fossa19,28 (see Fig. 22). Clinically, on occasion, the anterior portion of the middle turbinate
must be excised during dacryocystorhinostomy to establish an unobstructed
osteotomy. The Nasolacrimal Canal The nasolacrimal canal is a short, bony tube extending inferiorly, laterally, and
posteriorly from the lacrimal fossa toward the inferior meatus
of the nose. It contains the membranous nasolacrimal duct. The maxillary
bone forms the anterior, lateral, and posterior walls of the canal. The
medial wall of the nasolacrimal canal is formed superiorly by
the descending process of the lacrimal bone, which articulates with the
ascending processing of the inferior turbinate bone below. In some
cases, the medial wall of the nasolacrimal canal is almost entirely formed
by the maxilla (Fig. 29), with a corresponding decrease in contribution from the lacrimal and
inferior turbinate bones. This results in a narrowing of the nasolacrimal
canal and corresponding nasolacrimal duct.29 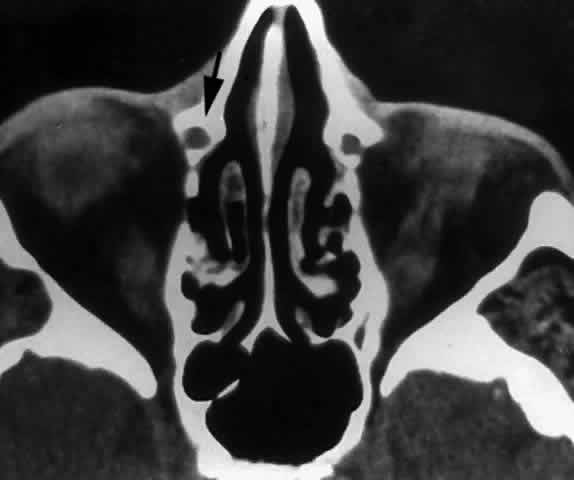 Fig. 29. In this computerized tomographic scan of the orbit, the medial wall of
the nasolacrimal canal is almost entirely formed by the maxilla (arrow). Fig. 29. In this computerized tomographic scan of the orbit, the medial wall of
the nasolacrimal canal is almost entirely formed by the maxilla (arrow).
|
The caliber, length, and course of the nasolacrimal canal vary considerably. The
canal is slightly oval, having the greatest dimensions in the
anteroposterior plane. The length of the canal is 12 to 13 mm with a
slightly lateral convexity. The canal inclines posteriorly (Fig. 30) approximately 15° from vertical as the canal descends from the lacrimal
fossa to the nose.28 Clinically, the lateral descent of the nasolacrimal canal can be estimated
by drawing a line between the tear sac and the ala nasae. Persons
who have narrow interorbital distances and wide noses have the greatest
lateral descent, whereas those with wide interorbital distances and
narrow noses show a more vertical descent.1,2  Fig. 30. A twig in the nasolacrimal canal demonstrates its posterior inclination
as it passes inferiorly. Fig. 30. A twig in the nasolacrimal canal demonstrates its posterior inclination
as it passes inferiorly.
|
The nasolacrimal canal is seen in relief (Fig. 31) on the medial wall of the maxillary sinus and sometimes on the lateral
wall of the middle meatus of the nose. In the usual circumstance where
the maxillary bone provides the major contribution to the nasolacrimal
canal, the elevation is greater on the maxillary sinus side. 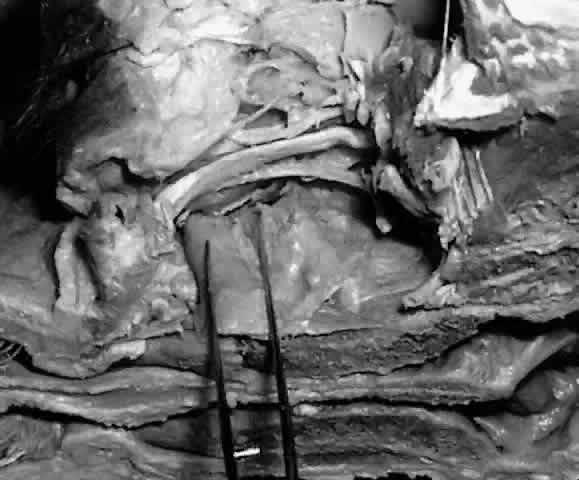 Fig. 31. With the lateral wall of the maxillary sinus removed, the nasolacrimal
canal is seen in relief on the medial wall of the maxillary sinus (within forceps). Fig. 31. With the lateral wall of the maxillary sinus removed, the nasolacrimal
canal is seen in relief on the medial wall of the maxillary sinus (within forceps).
|
|