INCIDENT HISTORY
First, a complete account of the accident should be obtained. In some situations parents or baby-sitters have not witnessed the accident and may be unable to give an exact accounting. The child should be questioned and may be able to provide valuable information regarding the accident. Isolating the child from the parents may reveal a more accurate history of the events. From the history, the nature and extent of the injury can be determined, which aids in how extensive an evaluation is needed. Knowing the type of trauma may alert one to look for secondary ocular effects, such as fat emboli from a crush injury. Injuries caused by large blunt objects may produce contusions to the periorbital region and would favor contrecoup retinal problems and blowout fractures of the orbit along with rim fractures. Missiles or sharp objects would raise the suspicion of a perforated globe or retained foreign bodies in soft tissues or the orbit.
Because of the potential for general anesthesia, pertinent past surgical history of the patient and drug allergies are important to obtain along with current immunizations. Although the incidence of tetanus is rare, in the United States booster doses of tetanus toxoid should be received every 5 years to maintain adequate immunity and should be administered in “dirty” cases.1 A dose of 0.5 ml of tetanus toxoid is appropriate for immunized children. If the child has not been immunized, the tetanus immunization series should be initiated with a 0.5-ml dose of diphtheria-pertussis-tetanus vaccine.
MEDICAL HISTORY
Even in the pediatric age group, a complete medical history is necessary. Although the incidence of transmissible disease is low, the potential exists and one should not hesitate to obtain permission for testing for human immunodeficiency virus and hepatitis. Medical conditions should be considered with particular attention to hematologic and bleeding disorders. Knowing the sickle cell status in black patients is important before general anesthesia is administered.
EXAMINATION
Examination should be as extensive as the injury permits. If there are other injuries that must be addressed, the examination and treatment may be modified as determined by the severity and extent of those injuries. Recording visual acuity should be attempted on all patients with ocular and periocular trauma. Various methods of evaluating the vision include Snellen letters, random Es, Allen pictures, finger counting, and light perception with or without projection. One must consider the child's age and the severity of the injury when determining the appropriate visual acuity test. The severity of ocular injury can be assessed by its affect on vision. Initial visual acuity is known to be a prognostic indicator of final outcome.
External
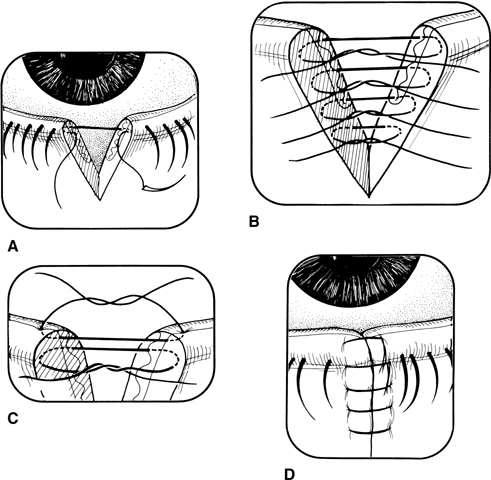
The external examination should consist of looking for puncture wounds of the lids and brow, which could be through and through lacerations or punctures that could involve the globe. If there is ever any question of an open globe, a shield should be placed on the eye until the child is placed under general anesthesia for thorough evaluation and possible repair. Lacerations of the eyelid margin must be attended to with particular attention to the nasolacrimal system and its integrity. Palpation of the orbital rim helps to rule out fractures with possible displacement.
Ocular motility, ductions, and versions must be evaluated because of the possibility of muscle entrapment, laceration, or possible paralysis.
When there has been ocular trauma, one should always ask about diplopia. A small child often does not understand the meaning of double vision, and the clinician needs to explain this problem in very simple terms.
Interior Examination
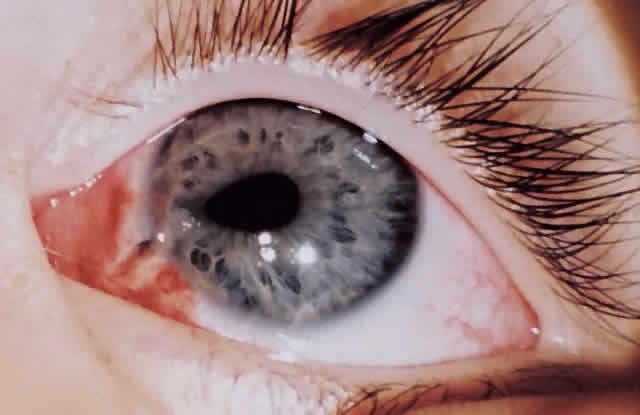
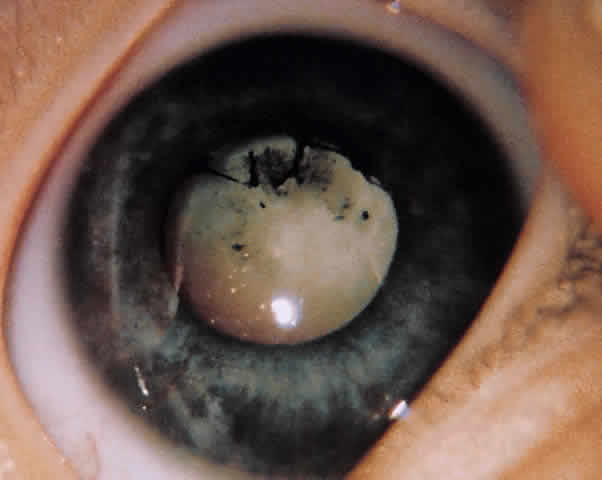
ANTERIOR SEGMENT. A slit-lamp or penlight examination should assess the clarity of the cornea, the anterior chamber depth, distortion of the pupil, and the presence of a hyphema (Fig. 1).
|
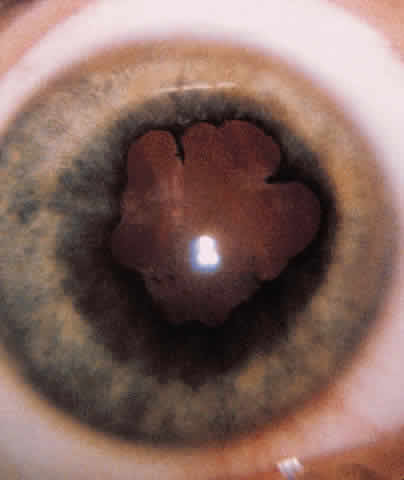
PUPILS. The size and shape of the pupil can aid in evaluation of an open globe. The reaction of the pupil, depth of the anterior chamber, consensual pupillary response, and afferent defect are critical in the initial evaluation.
RETINA. If possible, the retina must be evaluated for tears, hemorrhage, perforation, and retained foreign bodies. No retinal examination or manipulation of the globe should be attempted if there is a concern about a lacerated globe. The nature of the injury would also direct the need for appropriate diagnostic tests: roentgenography, computed tomography (CT), or ultrasonography.
If the retina is unable to be visualized and there is no obvious penetrating injury to the globe, B-scan ultrasonography can help in assessing the status of the retina. This plays a role in patients with hyphemas and vitreous hemorrhages. If a retained foreign body is suggested, plain films or CTs are helpful in localizing metallic foreign bodies. Magnetic resonance imaging is preferred when localizing glass but should never be ordered if one suspects a metallic foreign body.
PLAN OF ACTION
After all information has been gathered, a definitive plan of action can be considered. If surgery is needed for a rupture, repair must be made as soon as possible. Correction of external problems involving the eyelids and lacrimal system can be delayed for 24 to 48 hours without increased risk of infection.
After a thorough history and examination have been performed, it is important to document all this information to help in the management of the patient and also be available if any legal action regarding the injury is taken.