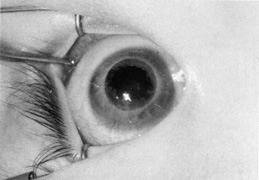
The donor should be at least 2 years of age. Younger corneas are less rigid, making tissue handling and suturing more difficult. They produce steeper postoperative corneas, which can reduce hyperopia from aphakia, but the postoperative refractive error is unpredictable and astigmatism appears to be a greater problem.15
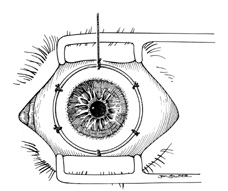
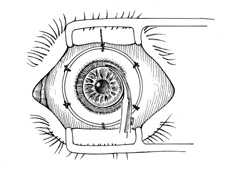
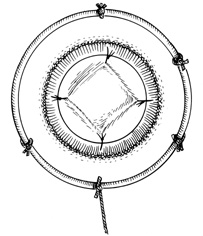
A Flieringa ring or McNeill-Goldmann blepharostat should be sutured to the globe to provide scleral support. One or two of the suture ends can be left long for fixation (Fig. 1). The center of the cornea and eight radial marks can be marked with a surgical pen and a radial keratotomy marker to guide suture placement. Alternatively, some trephines (e.g., Barron Recipient Trephine, Katena) are designed to create evenly spaced marks on the recipient cornea.
|
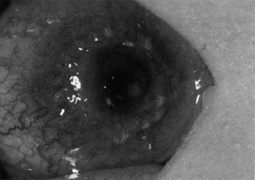
The diameter of the tissue to be removed is determined by the size and position of the pathology and the diameter of the recipient cornea. If possible, all thinned, necrotic, or infected tissue should be removed. The donor button should be at least 5.5 mm in diameter, and it is preferable that it be placed at least 2 mm from the limbus. The donor should be 0.5 to 1.0 mm larger than the recipient bed, to facilitate wound closure. Oversized grafts (>0.5 mm) tend to result in steeper postoperative corneas and therefore can be used to reduce hyperopia in aphakia. Similarly, they could lead to myopia in phakic eyes and should be avoided. Therefore, in infants, with a corneal diameter of 10 to 11 mm, typically a 7-mm donor is placed in a 6.5-mm bed. In a microphthalmic eye, particularly those with axial lengths of 13 mm or less, graft–host disparity of 1.5 mm appears to improve results.16
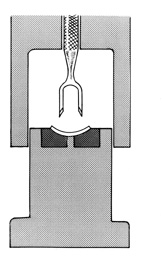
The donor corneoscleral rim is removed from the storage medium and placed in a trephine block, endothelial side up. The cornea is centered in the well, and excess medium is removed. A hollow shaft trephine is carefully centered and aligned vertically over the tissue and then firmly punched through the donor button (Fig. 2). A variety of trephines are available, some of which attempt to ensure centering and verticality of the cut, and some of which place four cardinal marks on the donor epithelium (e.g., Hanna trephine; Barron donor trephine). A drop of storage medium is placed on the endothelial surface, and the cornea is covered. The donor rim should be cultured.
|
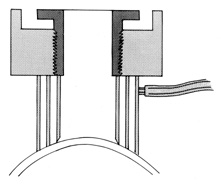
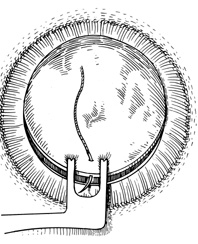
A hollow shaft trephine is also used to cut the recipient cornea. A suction trephine (e.g., Hanna trephine, Barron recipient trephine) facilitates centering and verticality of the cut, and may leave marks for suture placement (Figs. 3 and 4). The trephine is centered on the corneal mark, and a cut of approximately two-thirds depth is made. The anterior chamber is then entered with a knife at the 9 to 10 o'clock (for a right-handed surgeon) position. Right- and left-handed corneal scissors are used to extend the incision (Fig. 5). The scissors are tilted slightly to create a posterior ledge of host cornea, which helps seal the posterior wound. Care is taken to cut the full thickness of the cornea, so Descemet's membrane is not left in the eye, and to avoid damage to the iris and lens. Introduction of viscoelastic material into the anterior chamber may facilitate excision of the host cornea. It may be necessary to separate anterior synechiae, using blunt or sharp dissection. Vitreocorneal and corneolenticular adhesions are cut with scissors.
|
|
|
Usually the lens and iris move forward as soon as the eye is opened, and visualization and division of peripheral iris synechiae is difficult. Often it is best to close the wound first and lyse synechiae afterward. If iris-lens prolapse occurs, external pressure on the globe from the lids and speculum should be reduced as much as possible. Instead of a speculum, sutures can be placed in the lid margin before trephination, to obtain exposure. Hyperventilation of the patient also can help. Of course, the graft should be sutured in place as expediently as possible. If necessary, shallow sutures can be placed initially and replaced once more control is obtained. Pars plana vitrectomy can be performed to reduce vitreous pressure. Pars plana incisions in an infant should be 2 to 3 mm from the limbus and lens damage can occur during the procedure.
Bleeding from the cornea or iris usually ceases with time and irrigation. Dilute solutions of epinephrine (e.g., 3 mL 1:10,000 in 500-mL saline) may be helpful. If iris bleeding continues, wet field cautery or a retinal diathermy unit can be applied to the vessel.
If cataract extraction is necessary, an anterior capsulorrhexis is optimum. This is started with a cystotome and completed with Utrata forceps. However, the capsulorrhexis often extends peripheral and capsulotomy must be performed with a needle, knife, or scissors. The soft lens nucleus is expressed or removed by a mechanical cutting and suction instrument, and cortical material is removed by irrigation and aspiration. A central posterior capsular opening is created.
Anterior vitrectomy is performed with a mechanical suction and cutting device. Constant irrigation is not necessary. Vitrectomy is continued until the remaining vitreous is well posterior to the iris plane. The anterior iris surface is dabbed with cellulose sponges, to ensure removal of all vitreous strands.
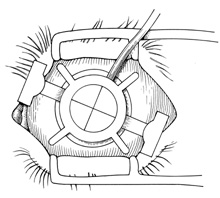
The anterior chamber is re-formed with balanced salt solution, and viscoelastic material is placed centrally over the lens, if present, and on the peripheral recipient rim. The donor cornea is transferred to the bed and sutured into place with four interrupted 10-0 nylon or 9-0 silk sutures (Fig. 6). A double-pronged forceps (e.g., Pollock) facilitates placement of the initial 12 o'clock suture in the donor (Fig. 7). Care must be taken to minimize endothelial damage. The graft is stabilized against the recipient opening superiorly by pulling the tissue with the forceps. The needle is passed radially between the tips of the two-pronged forceps, minimizing movement of the donor tissue.
|
|
Alignment of the second suture at the 6 o'clock position is crucial to ensure proper distribution of the tissue in the bed. Donor and recipient marks created with the trephines can facilitate suture placement. Anterior iris adhesions can be severed once four to eight cardinal sutures are in place. This is often best done with the chamber filled with a viscoelastic material, particularly in phakic eyes. The peripheral angle is swept with an iris spatula, avoiding contact with the posterior surface of the donor cornea. In some cases sharp dissection is required. If viscoelastic material is not sufficient to maintain the chamber, then additional interrupted sutures or part of the continuous suture is placed before the dissection is performed.
A continuous suture is preferable in most cases because it facilitates rapid wound closure, induces less postoperative inflammation, and is easier to remove when the wound is healed. Interrupted sutures are used when there is localized corneal vascularization (present in some quadrants and not in others) or there is some other reason to expect uneven wound healing. In these cases interrupted sutures can be removed from healed areas while leaving sutures in unhealed areas. Continuous sutures are indicated when symmetric healing is expected, the cornea is totally avascular, or all quadrants are vascularized to a similar extent. Interrupted sutures can also be used when the child is old enough to cooperate with selective suture removal at the slit-lamp to reduce astigmatism.
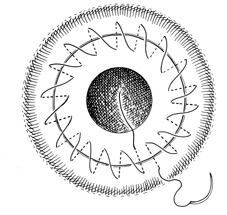
The continuous suture consists of 16 to 24 bites placed in the posterior quarter of the cornea (Fig. 8). The two suture ends are tied on the graft surface with one triple throw, and the interrupted sutures are removed. The chamber is filled with saline solution. The continuous suture is tightened with non-toothed forceps, and the wound is checked for leakage, using topical application of fluorescein dye if necessary. If leakage is present, the running suture is tightened further. The knot then is completed with two additional single throws. If leakage is detected at this point, superficial interrupted sutures are placed as necessary.
The scleral fixation ring is removed and subconjunctival injections of antibiotic (e.g., gentamicin, 20 mg) and long-acting corticosteroid (e.g., methylprednisolone acetate, 40 mg) are given. Antibiotic ointment is placed in the eye, which is then covered with a pressure patch and shield. If necessary, elbow restraints are placed on young children to avoid eye manipulation.
The following technique can be helpful in eyes with severe posterior pressure: When excising the host cornea a small hinge is left uncut (on the left for right-handed surgeons). The cornea is lifted temporarily to lyse synechiae and inject viscoelastic, and then reattached with one 7-0 silk suture on the side opposite the hinge. A thick layer of viscoelastic is placed on top of the recipient's cornea, and the donor cornea is placed on top and sutured into place with two 9-0 nylon cardinals. The recipient cornea is then cut free and slid out from underneath.