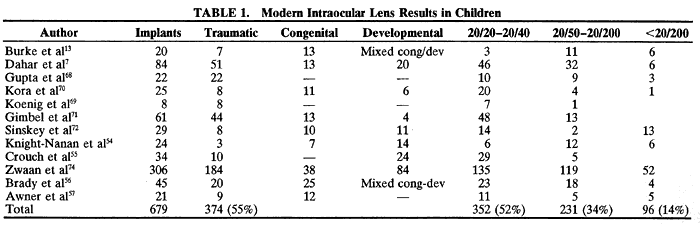
Lenses made of acrylic and silicone material, which can be folded to allow implantation through a smaller incision, have become popular with adult cataract surgeons and acrylic IOLs are currently the most commonly implanted IOLs in adult patients. Although long-term safety of these lenses is not yet as conclusively proven as that of PMMA lenses, the ACRYSOF acrylic IOL (Alcon Surgical) is being used commonly by pediatric cataract surgeons and no complications related to its material have been reported to date. In a pilot study on IOL implantation in infants, Lambert and colleagues used ACRYSOF and PMMA lenses in infants less than 6 months of age and found similar complications in both groups. None of the complications could be attributed to the IOL material used.2 Special care should be exercised in folding the high (greater than + 24.00 D) power lenses frequently needed in young children to avoid lens damage. Heating the lenses to slightly above room temperature with a warming lamp or by having the surgical assistants hold the packaged lens in their hands before use aids in folding the lens. Taking time to fold the lens particularly slowly is advantageous.
For primary lens implantation with placement within the capsular bag there may be some advantage to using a lens with more gently curved C-shaped loops with a shorter total diameter (12.5 mm) to theoretically produce more gentle and evenly distributed force on the capsular bag.27 A convex posterior surface or a so-called laser ridge theoretically produces a peripheral barrier to the migration of residual epithelial cells; however, opacification of the posterior capsule is unfortunately not prevented.38 A space between the lens and capsule is produced, which decreases the possibility of lens damage with nedodymium:yttrium-aluminum-garnet (Nd:YAG) laser capsulotomy. For lens implantation within the ciliary sulcus, it is slightly easier to direct the haptics of a modified J-shaped loop lens; however, the difference is not significant. Ideally, if the IOL is to be placed in the ciliary sulcus, the total diameter should be 13 to 14 mm.
For secondary PC lens implantation in the absence of capsular support, lens haptics should be modified with positioning holes at the vertex of each haptic to facilitate suture fixation of the lens.
AC lenses should be of the one piece, all-PMMA, flexible, open-haptic design originated by Choyce and Kelman, because the closed loop and small-diameter open-loop lens designs are clearly associated with many more complications.27,28,39 Both three-point and four-point fixation styles of open-haptic lens designs are available and appear to be equivalent in safety. The four-point fixation multiflex style of lens is more widely used and may be less subject to rocking or torsional movement. This style of lens is typically available in three diagonal-length sizes (S, M, and L) with fitting according to the so-called white-to-white + 1 mm corneal diameter. Smaller size lenses are avoided except in patients in whom corneal diameter is less than 10.5 mm to allow for ocular growth.
PRIMARY PC IOL IMPLANTATION
The procedure for primary PC IOL implantation in a pediatric patient is similar to that in an adult patient, although with several special considerations for the pediatric patient. A traditional fornix-based conjunctival flap and corneal-scleral incision or a clear-corneal incision are recommended. Because of the possibility of trauma or eye rubbing, if a scleral tunnel incision is used interrupted sutures should still be used for closure of the incision rather than leaving the wound unsutured. Children do not appear to be as prone to development of permanent incision-induced astigmatism as adult patients and the position, that is, temporal versus 12 o'clock, or construction of the incision do not appear to be as critical in children as in adult patients.
Capsulorrhexis is much more difficult in children younger than 5 years of age compared with adultsbecause of elasticity of the anterior capsule. If the tear begins to extend too far to the periphery of the lens, the technique should be abandoned rather than risk an area of zonulolysis. The anterior capsulectomy is then fashioned with a cutting irrigation/aspiration instrument (Ocutome), leaving a peripheral rim of anterior capsule and zonular attachments 1 to 2 mm wide. The integrity or resistance to tearing of anterior capsular openings created with this technique has been established.40,41 Phacoemulsification alone or in combination with irrigation/aspiration is similar to that in an adult patient, but the lens nucleus is seldom hard, and only a minimum of phacoemulsification power (if any) is used. Hydrodissection may be performed if desired, but in my experience it does not seem to be of any significant benefit. Removal of all, or as much as possible, of the lens cortex is required, even more so than in the adult, because of the vigorous inflammatory response of children to retained cortex and the rapid development of synechiae. If any appreciable cortex is retained, there is a significant risk of lens decentration or malposition because of the proliferation of lens epithelial cells and secondary membrane formation.
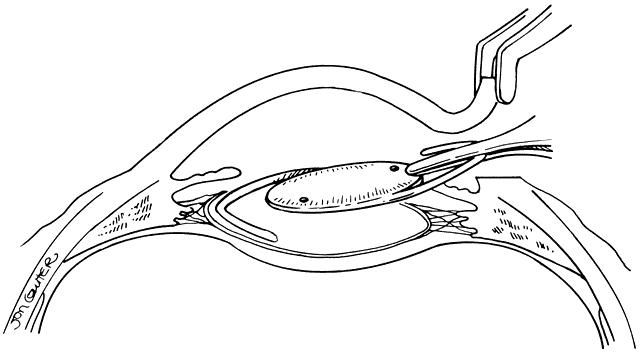
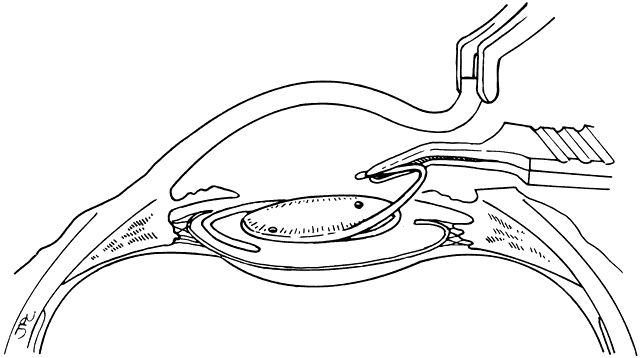
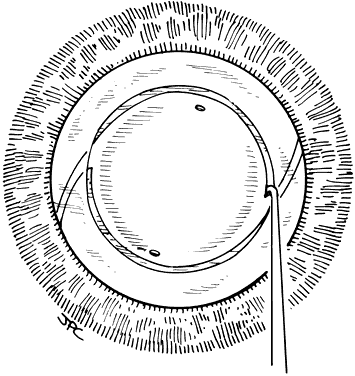
Lens implantation is then performed with capsular or so-called in-the-bag haptic placement (Figs. 1 through 5). Special care must be taken to ensure that both haptics are within the capsular sac. If haptic placement is asymmetric, with one loop in the capsular sac and one loop in the sulcus, lens decentration may occur. In the pediatric patient, the vigorous proliferation of residual lens epithelial cells leads to a greater tendency for uneven force on the lens haptics and a greater chance for lens decentration. Having both haptics in the ciliary sulcus is probably preferable to asymmetric placement. If a foldable Acrysof IOL is used, the so-called moustache style of lens fold, with both haptics placed down on the posterior capsule to unfold into the capsular bag, should not be used in cases of posterior lenticonus where the posterior capsule abnormality may catch one or both of the haptics, which would lead to posterior capsule rupture.
|
A peripheral iridectomy is performed in all patients as an added safety precaution and viscoelastic is removed before the wound is closed. In older children able to cooperate at the slit lamp microscope for suture removal, the wound is closed with interrupted 10-0 nylon sutures; suture removal is planned for 5 to 6 weeks after surgery. In young or uncooperative children, the sutures may be removed with an examination under anesthesia, also affording the opportunity for detailed examination of the eye, or interrupted 9-0 Polyglactin absorbable sutures may be used. The activity level of the young patient and possible eye rubbing are kept in mind during wound closure, and extra sutures are placed for added strength if there is any question about the integrity of the wound.
A decision must then be made regarding the posterior capsule, PC opacification after extracapsular cataract surgery is caused by proliferation and migration of residual lens epithelial cells.42 This occurs as an age-related tendency, and virtually all pediatric patients develop capsular opacification over time.43 In an infant less than 1 year of age this may occur within the first several postoperative weeks. In an older child, opacification may take years to develop. One study of IOL use in children reported an average time to opacification of 2 years, regardless of patient age at the time of surgery and suggested primary capsulectomy-anterior vitrectomy at the time of implant surgery for children who are not expected to be candidates for YAG capsulotomy within 18 months of surgery.44 In patients less than 2 years of age, the risk of developing a thick membrane on which it would be difficult to perform YAG capsulotomy is significant and all these patients should have primary posterior capsulotomy-anterior vitrectomy procedures performed. If the patient can be expected to be cooperative enough for subsequent Nd:YAG laser capsulotomy, or if the facilities are available for performing YAG laser procedures on supine anesthetized patients, the capsule is left intact to avoid the potential complications of intraocular capsulotomy. It should be emphasized here that if this is the strategy taken, YAG capsulotomy must be performed in a timely fashion to avoid problems with amblyopia induced by the visual deprivation of capsular opacification and to prevent development of a thick membrane that may be resistant to YAG laser disruption using a reasonable amount of energy.
If posterior capsulectomy-anterior vitrectomy is to be performed at the time of implant surgery, after the implant wound is closed, a vitreous cutting instrument is used from the pars plana, posterior to the posterior capsule and IOL.45 An infusion cannula is placed into the AC and a stab incision is made for the cutting instrument 1.5 to 2 mm posterior to the limbus. A central posterior capsulectomy is then made with removal of the anterior vitreous. Generous vitrectomy should be performed in infants and young children.
SECONDARY PC IOL IMPLANTATION
Capsular Support Present
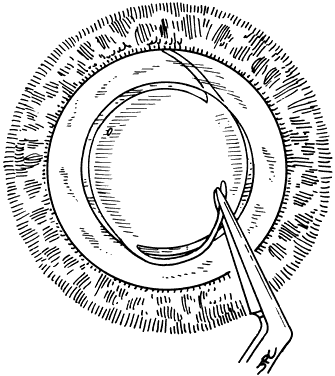
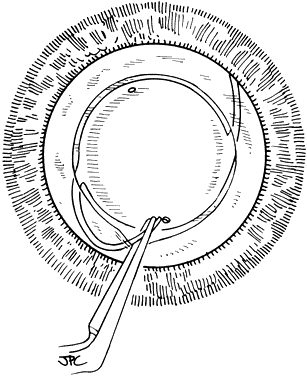
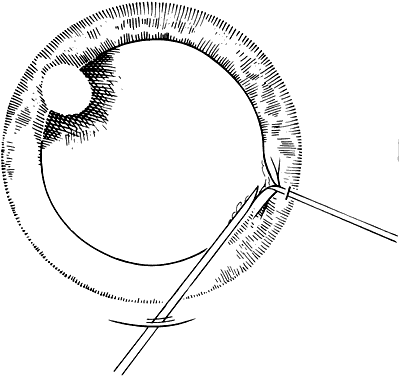
If the cataract surgery has been performed, leaving a rim of capsule present, secondary implantation of a PC IOL into the ciliary sulcus is then performed. The anterior and posterior capsular flaps having sealed together will thus form a so-called shelf to help guide and support the haptics in the ciliary sulcus.46,47 Preoperative evaluation of pupillary dilation, the integrity of this capsular shelf, and examination for iridocapsular synechiae are important. If pupillary dilation is seen to be poor or if synechiae are present, a paracentesis tract should be planned. An iris or Sinskey hook is used for retraction of the iris to aid in examination of the iridocapsular relationships. Synechiae may be lysed with viscoelastic, an iris spatula, or, frequently, they may need to be cut with a discission knife (Fig. 6).
A technique for in-the-bag secondary IOL implantation is selected patients has been described by Wilson and coworkers.48 In this technique, if Sommering's ring is present, with a ring of reproliferated cortex present between the lens equator and the fused anterior and posterior capsular leaflets, the capsular bag may be reopened to allow placement of the haptics within the bag. Those authors suggest limiting the anterior and posterior capsulectomy performed during the initial cataract surgery to a smaller than usual 4 to 5 mm and the performance of generous anterior vitrectomy to aid in preventing closure of the capsulectomy. The success of this technique in reliably producing the required capsular conditions without producing problems with synechiae or secondary membranes has not yet been demonstrated.
Capsular Support Absent
Several techniques have been advocated for suture fixation of PC IOLs into the ciliary sulcus since the concept was first described by Girard.34,35,46–59 Important improvements are the use of an implant with holes at the apex of each haptic to make fixation of the sutures to the haptics easier and less likely to malposition, and incorporation of conjunctival-scleral flaps to sequester the suture knot to prevent erosion and the increased possibility of endophthalmitis. Fixation of the haptics at slightly oblique meridians helps avoid the major arterial vessels of the iris and ciliary body and also decreases the chances for hemorrhage with the passage of the fixation sutures.24
SECONDARY AC IOL IMPLANTATION
Secondary AC IOL implantation in pediatric patients is performed in the same manner as that used in adults. There may be some advantage to a horizontal orientation of the haptics over a vertical orientation. If a peripheral iridectomy is present superiorly, horizontal orientation avoids it. There is also some suggestion that chronic discomfort after AC IOL implantation, particularly if too large a lensis implanted, may be avoided with horizontal placement.