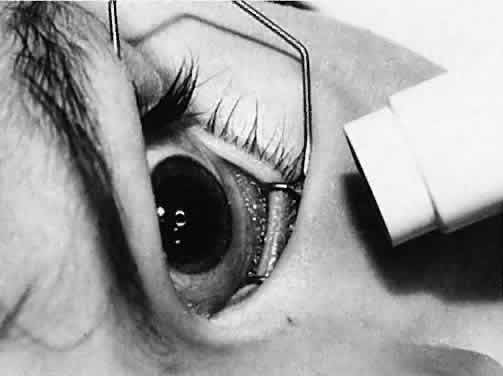
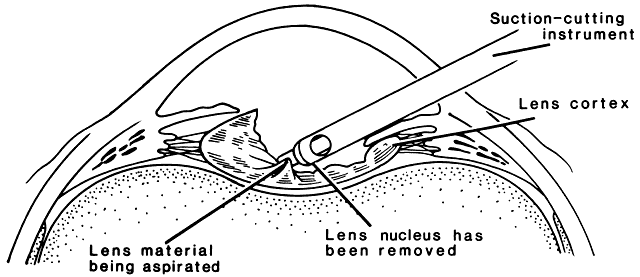
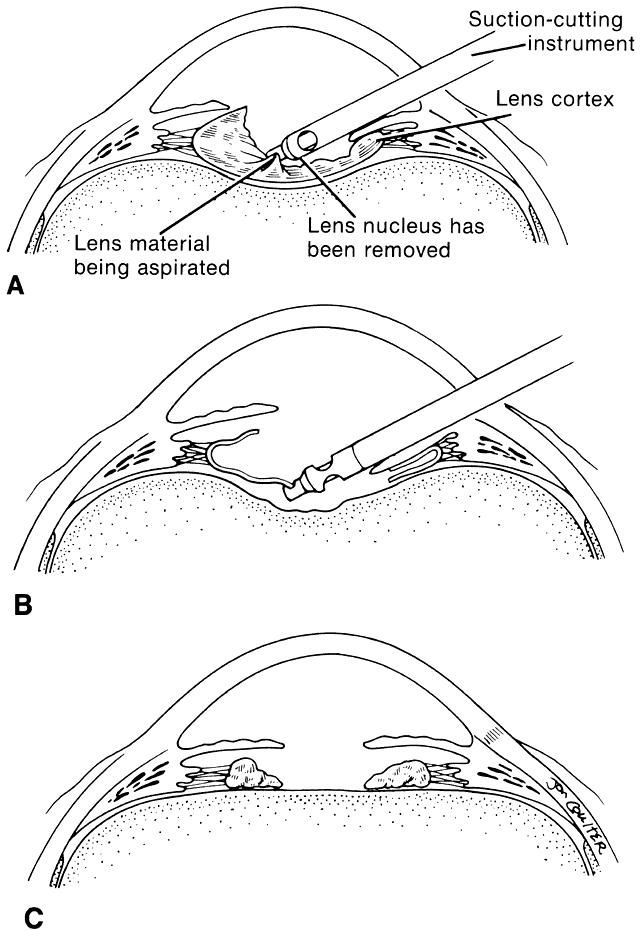
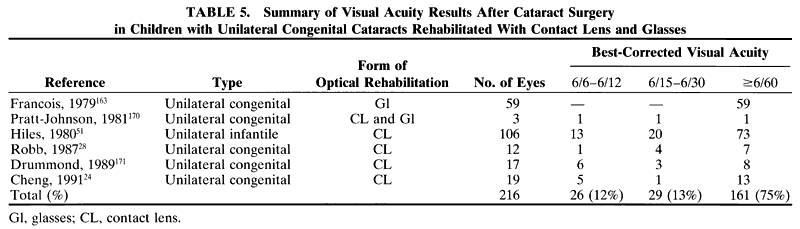
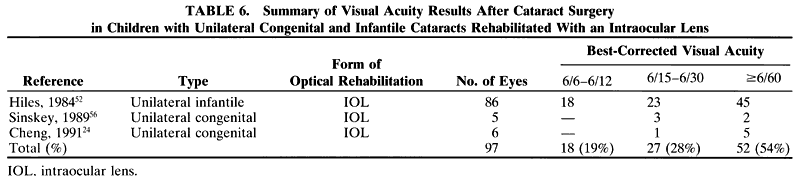
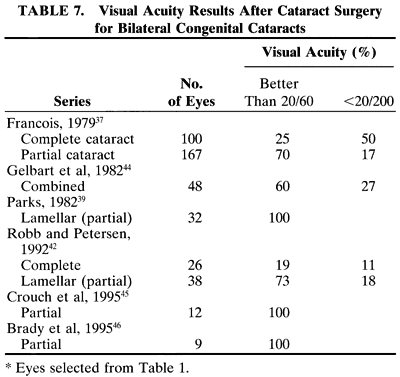
In a child, additional factors must be considered, such as the health of the child, the availability of resources for perioperative care, and the availability of anesthesiologists who are trained in the care of infants and young children. In adults, the surgery can be scheduled to accommodate the convenience of the patient. In children, the timing of surgery may be dictated by the degree of maturation of the visual system. Surgery may need to be performed in a timely fashion to facilitate the effective treatment of amblyopia. In infants with unilateral congenital cataracts, a critical period in visual development exists after birth, during which treatment should be initiated to optimize the possibility for promoting visual development in the affected eye. Evidence suggests that this critical period is before the first 6 to 17 weeks of age.1,2
Another difference is the frequent presence of structural abnormalities of the child's globe and visual pathways. These abnormalities can influence the visual result that is achieved. Inherent defects in the available methods of optical correction and compliance with the correction of aphakia and amblyopia therapy affect the visual result. Additionally, persistence with treatment of strabismus, glaucoma, and other related defects is needed to maintain a successful surgical outcome.
Because of the difficulties with correction of aphakia in children, surgery may not be advisable in some cases. For example, observation of a monocular partial lens opacity that decreases visual acuity to 20/40 in an amblyopia-susceptible 2-year-old child may be preferable to making that child aphakic and intolerant of contact lenses or even pseudophakic without the ability to accommodate, and intolerant of glasses with bifocal lenses. The reverse also can be true. Irreversible deprivation amblyopia can be caused by a partial lens opacity, for example, posterior lenticonus, in an infant or a young child. In these cases, the opportunity to achieve high levels of central vision may be permanently lost if surgery is delayed.3 Because of these factors, the accepted 95% success rate for excellent vision after cataract surgery in adults may not be achieved in children.
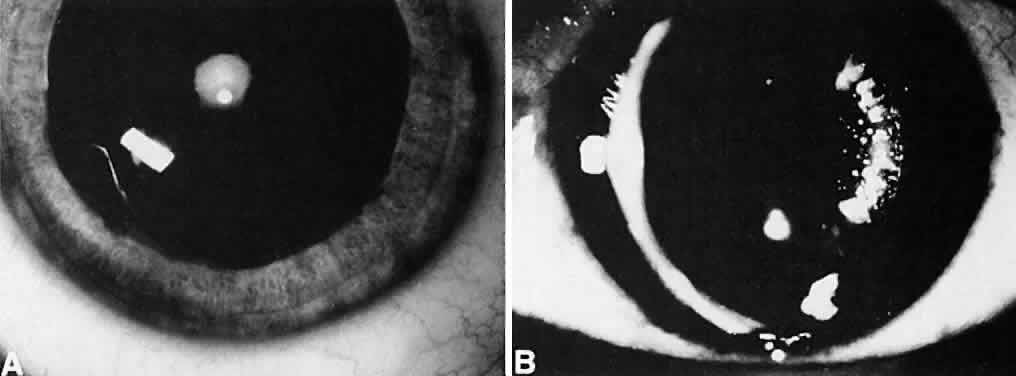
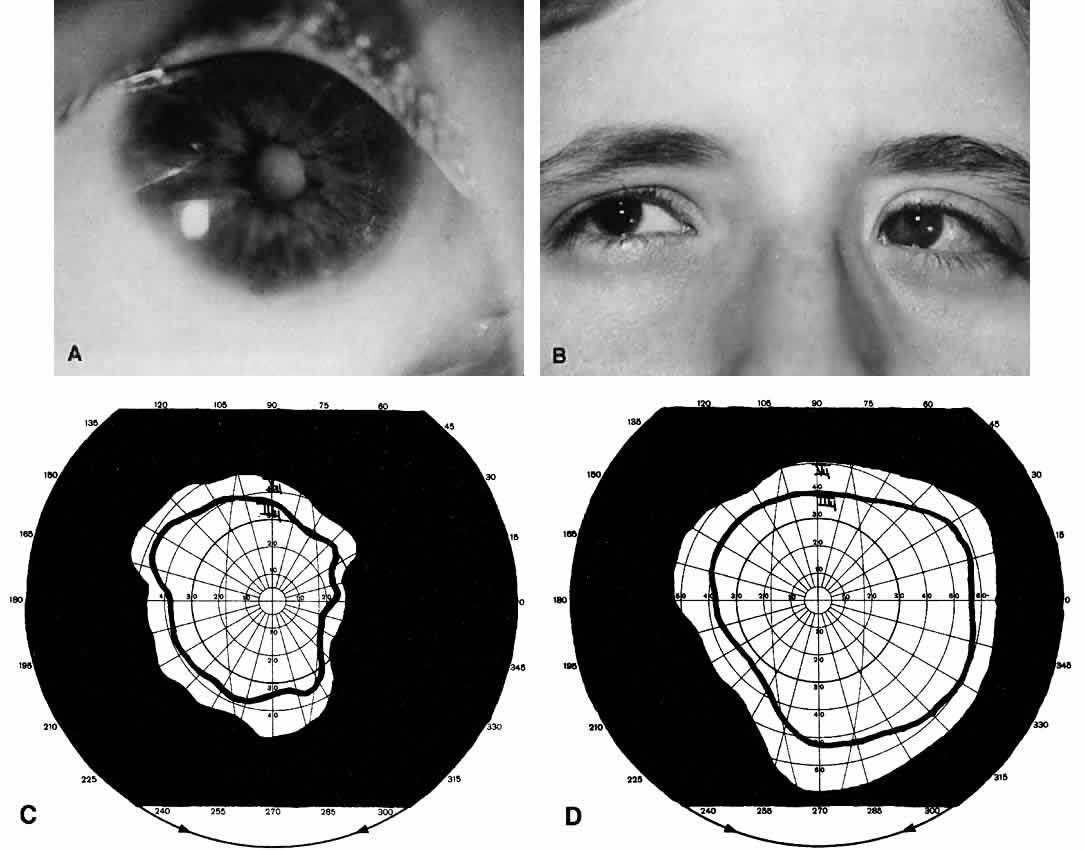
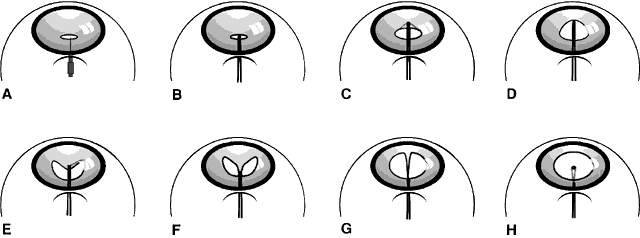
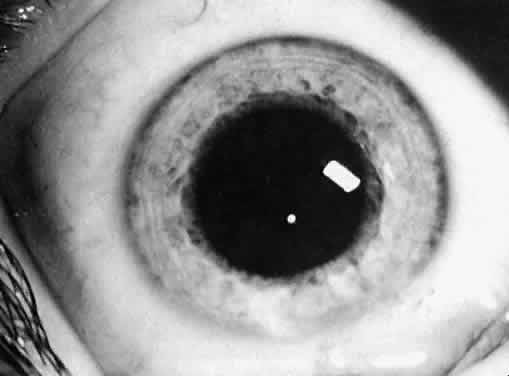
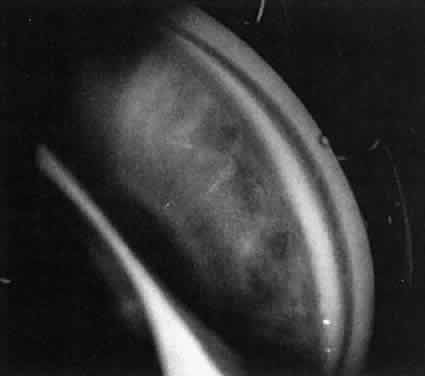
Not all cataracts require surgical treatment. Eyes with punctate or small anterior polar cataracts and others with partial opacification of the lens, such as posterior lenticonus, which only slightly interferes with the refraction of light, are best followed and not surgically treated (Fig. 1). In some cases, it is difficult to determine whether the presence of a partial cataract is responsible for a decrease in visual acuity or whether the refractive error or optical distortion produced by the cataract has produced a mild, reversible form of deprivation amblyopia. In these situations, correction of the refractive error and a trial of occlusion therapy should be attempted. If the visual acuity improves, it may be deduced that amblyopia was responsible for the loss of visual acuity and that the optical distortion produced by the partial cataract is not yet surgically significant.
|
TREATMENT GUIDELINES
When a cataract is extensive or if a partial cataract is present and the vision does not improve with a trial of occlusion therapy, cataract surgery should be considered. The guidelines for cataract surgery given in this section are based on the extent of the lens opacity and whether the cataract is isolated to one eye or both. Specific indications for cataract surgery, however, should be considered individually for each child.
Unilateral Partial Cataract
Children who have monocular partial cataracts should undergo cataract surgery when visual acuity in the affected eye is judged to be less than 20/70, when there is a loss of central fixation, or when visual deprivation is sufficient to cause strabismus. In questionable cases or when the cataract is small or only partially obstructs the visual axis, a trial of amblyopia treatment along with optical correction of anisometropia and dilation of the pupil, if necessary, to provide a clear visual axis3 should be performed before the decision is made to remove a partial cataract.
Unilateral Complete Congenital Cataract
Infants and children younger than 8 years of age who have monocular complete cataracts that are present from birth should have cataract surgery performed as soon as they are medically stable (preferably before 17 weeks of age). A child who is older than 8 years of age may have a complete monocular cataract removed to visualize the fundi or to improve peripheral vision. Treatment of amblyopia with improvement of central vision in children who have had complete congenital cataracts after 2 years of age usually is unsuccessful. If there was a possibility that the cataract was partial during infancy, with some opportunity for stimulation of the macula with clearly formed images, the cataract should be removed at any age to regain central visual acuity.
Bilateral Partial Cataracts
Children younger than 8 years of age who have bilateral partial cataracts should undergo surgery when vision in either eye is less than 20/70, if visual deprivation is producing strabismus, or if there appears to be a risk for the development of nystagmus as a result of visual deprivation.
Bilateral Complete Cataracts
If the lens opacity is complete and involves both eyes, the cataract should be removed in the eye with the most complete lens opacity. The fellow eye should be treated as soon as the first eye recovers from surgery. This interval should be brief (5 to 10 days) in very young children. If a health problem precludes repeated anesthetic sessions, consideration may be given to performing the surgery on both eyes during the same anesthetic session, with the use of different instrumentation and separate operative fields.4,5
OTHER CONTRIBUTING FACTORS
Decisions to perform cataract surgery can be difficult when young or developmentally delayed children cannot provide subjective visual acuity responses. In these situations, the decision to remove a cataract depends on the size, density, type, location, and progression of the opacity and the estimated effect of these factors on vision. Estimates of the effect of a partial cataract on vision can at times be difficult, even for experienced examiners; in difficult cases, second opinions can be helpful.6
Size and Density
Cataracts that are central in location and larger than 1.5 to 2 mm obstruct light entering the eye sufficiently to reduce visual acuity and impede development of the visual system. In this situation, a trial of dilating the pupil with topical applications of 2.5% phenylephrine drops three times a day to allow light to enter around the opacity may result in an improvement in visual acuity. Pupils that dilate poorly with phenylephrine may respond to 0.5% atropine eyedrops; however, the accompanying cycloplegia necessitates the use of bifocals to correct the cycloplegic refractive error and the lack of accommodation. Frequent monitoring of visual acuity and pupil size at different times of the day and under different lighting conditions is recommended. Improvements in acuity may be temporary if the lens opacity progresses in size.
Type and Location
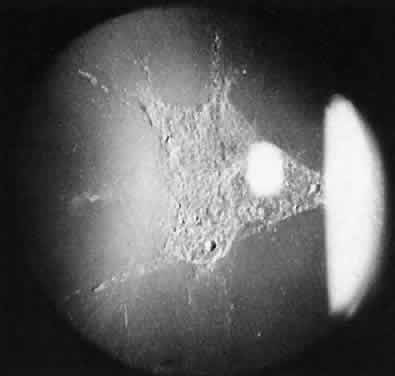
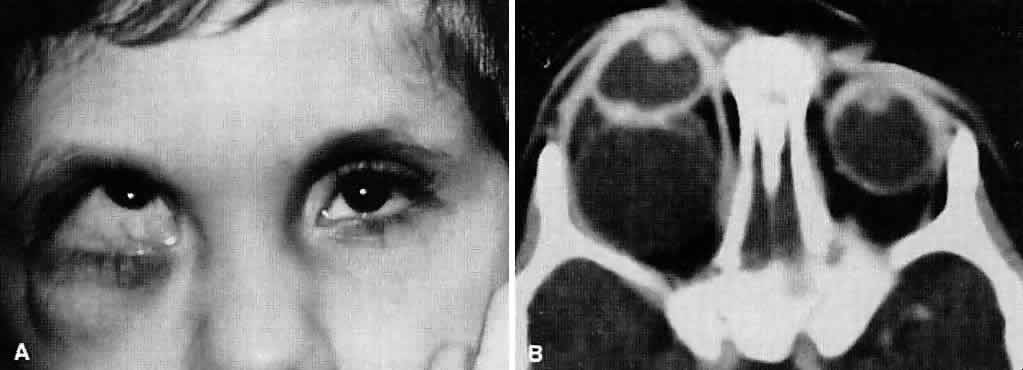
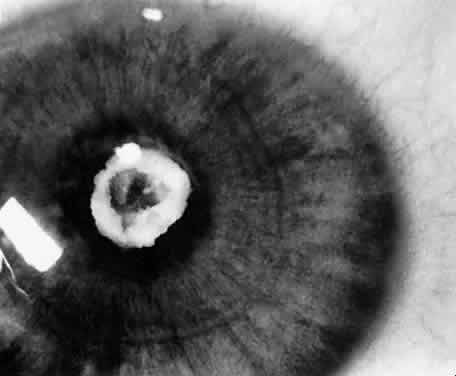
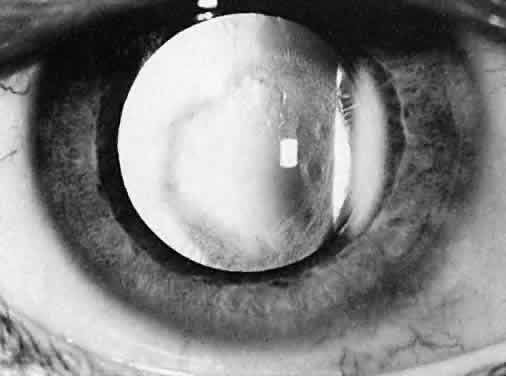
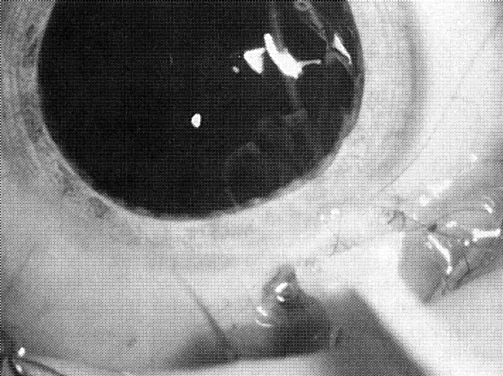
Usually, anterior polar cataracts are visually insignificant and allow normal visual development (see Fig. 1). However, some affect vision, and all require careful monitoring.7 Centrally located cataracts that are on or near the posterior lens capsule have a greater effect on the refraction of light and visual acuity (Fig. 2). Nuclear cataracts associated with metabolic disorders or prenatal infections produce double refracting systems that cause optical distortion and significantly decrease visual acuity (Fig. 3).
|
Progression
Not all cataracts in children increase in size or density. Premature neonates may have partial opacification (transient neonatal opacities) that may disappear over the first 1 or 2 weeks of life.8 Other cataracts that may regress are those due to galactosemia. These cataracts have the morphologic features of oil droplets. If early dietary restriction of galactose is observed, the opacity may regress.9 Anterior polar cataracts typically remain stable in size and density.
Other types of cataracts characteristically progress in size and density. Cataracts caused by rubella, uveitis, diabetes, exposure to radiation, and use of corticosteroids usually show progression with time.
Visual Acuity and Signs of Visual Function
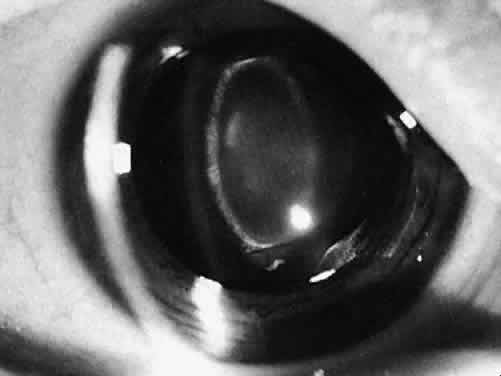
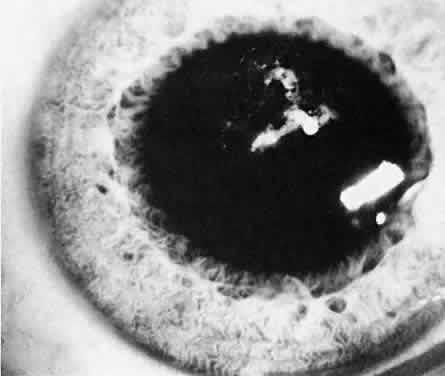
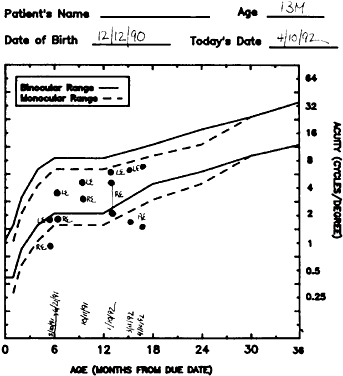
Ultimately, it is the measured or estimated level of visual acuity or acuity potential that determines the need for cataract surgery. If either the measured or the estimated decrease in visual acuity produced by the cataract is sufficient to prevent adequate visual development or if signs of significantly decreased visual acuity, such as strabismus or poor central fixation, are present, cataract surgery is indicated. Use of the Teller visual acuity cards at periodic intervals can help to measure visual acuity.10 In some children, visual acuity that is below normal or decreasing can be documented accurately to support the decision to perform cataract surgery (Figs. 4 and 5). Care must be taken in interpreting Teller visual acuity data because the normal levels in young infants and children are low and span a wide range. The Teller acuity card measurement of grating visual acuity may severely underestimate the level of visual loss in patients with cataracts and amblyopia.11
Coexisting Ocular Defects
Infants with cataracts may have coexisting defects of the globe or visual system that influence the decision to proceed with cataract surgery. If a cataract exists in an eye with other major structural anomalies and the fellow eye is normal, the benefits of proceeding with surgery are negligible, and the child should not be subjected to the risk of anesthesia and perioperative morbidity. If both eyes have major structural defects and complete cataracts, cataract surgery may be indicated to allow the child some opportunity for the development of vision, even if it is limited.
Health of the Child
An assessment of the general health of a child is important when considering cataract surgery because general anesthesia is required for the operation. Premature infants with severe bronchopul-monary dysplasia may have unacceptable risksassociated with the use of general anesthesia. Other children who have systemic diseases such as galactosemia, homocystinuria, congenital heart disease, and Marfan's syndrome or who have experienced trauma have additional risks associated with general anesthesia. For these children, appropriate consultation is needed to assess the risks of the procedure before proceeding with surgery.
Social Situation
Because the success or failure of visual rehabilitation of an infant or young child with a cataract depends on postoperative care, acceptance of optical correction, compliance with occlusion therapy, and integrity and capabilities of the family unit and its support structures must be considered. For example, if one parent has bilateral familial cataracts and poor vision and the other parent also has a visual impairment, delivery of postoperative care may be problematic. If the delivery of adequate postoperative care is likely to be a problem, cataract surgery must be considered carefully because the risk for amblyopia and visual loss produced by uncorrected aphakic refractive errors or pseudophakia may outweigh the amblyopia and visual loss produced by a partial cataract.