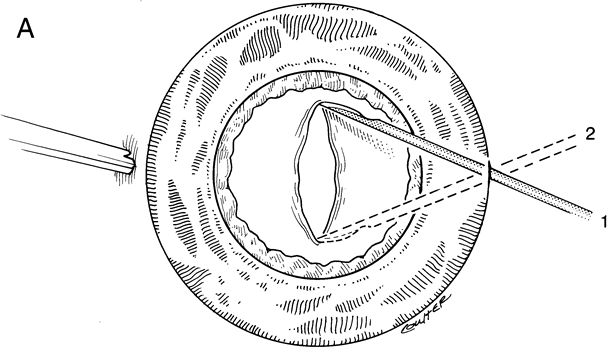
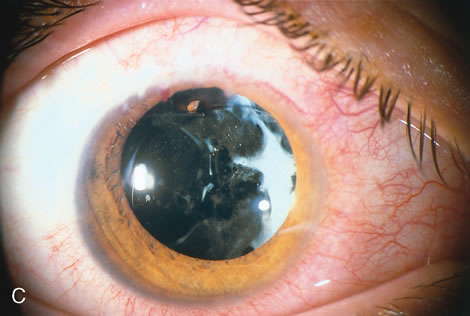
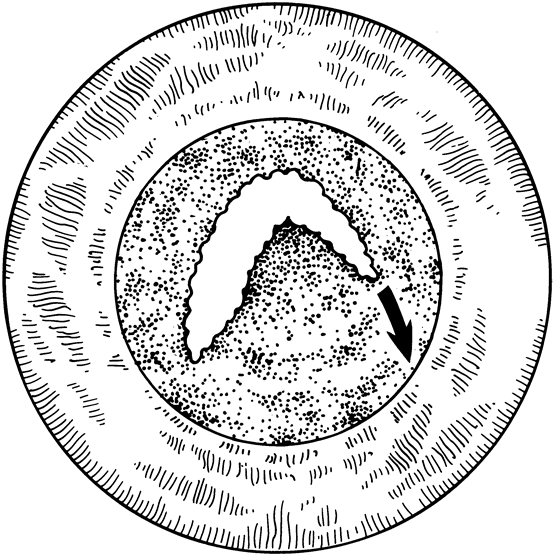
When phacoemulsification became available, it was quickly applied to the removal of children's cataracts because it provided the surgeon with better control of the flow of irrigating solutions and provided improved control of the aspiration flow and pressure.4 The instrument also added a new capability, that of being able to mechanically disrupt the lens nucleus and cortex to facilitate aspiration of the lens. Although the phacoemulsification instrument was helpful for removing lens cortex, it was ineffective in cutting or removing the posterior lens capsule. At the conclusion of the phacoemulsification procedure, the posterior capsule was left intact. When capsular opacification occurred, it was treated with a discission procedure, an operation that consisted of making a cut in the posterior capsule with a bent needle, a Ziegler knife, or a modification of the latter (Fig. 1). If the membrane was thick and resisted opening with a knife, an intraocular scissors was necessary to open the lens capsule.
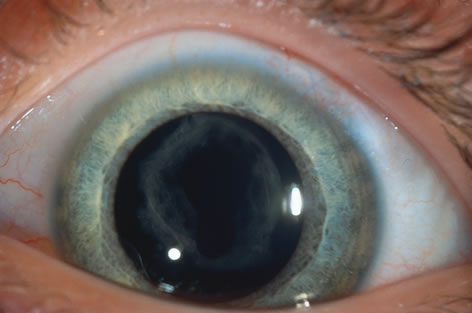
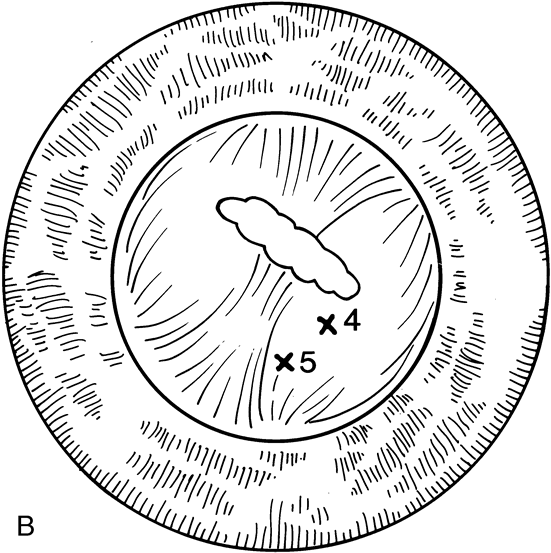
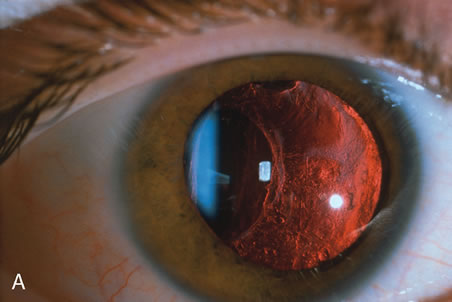
In children, opacification of the posterior capsule of the lens and a subsequent decrease in vision will always occur if the posterior lens capsule is left intact. The terms posterior capsule fibrosis, secondary membrane, and Elschnig's pearls are used interchangeably to describe these changes. Opacification of the posterior capsule results from proliferation and migration of residual lens epithelial cells.5 In adults, the extent and rapidity of capsular opacification can be minimized by reducing surgical trauma, by removing as many of the posterior lens capsule epithelial cells as possible by polishing the posterior capsule, and by selecting implants that reduce posterior capsule opacification.6–9 In children, even when these measures are taken, the lens epithelial cells rapidly proliferate. If a posterior capsulotomy is not performed, or if only a small opening in the capsule is made, the posterior capsule will opacify within weeks to months. The vitreous face is considered to serve as a scaffold for migration of the lens epithelial cells. However, even when this is disrupted with an anterior vitrectomy, the lens material can opacify the visual axis.10–11 Opacification of the posterior lens capsule causes difficulty in the visual habilitative process because it reduces the accuracy of retinoscopy and will cause light entering the eye to be diffracted. These factors will impede visual development and will make management of amblyopia difficult.
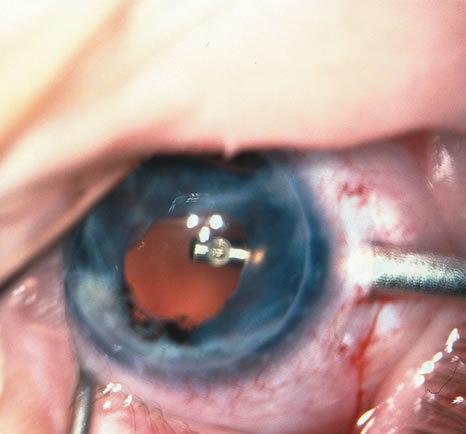
The advantage of leaving the posterior capsule of the lens intact after cataract surgery is that it retains a barrier between the anterior chamber and vitreous. This prevents the vitreous from entering the anterior chamber, and it theoretically preserves the ocular anatomic relationships after cataract surgery. The disadvantage of leaving the posterior lens capsule intact is that when the capsule opacifies, a second procedure is needed to re-establish a clear visual axis. To achieve this, a second anesthetic is administered and an incision is made into the clear cornea. The chamber is deepened with a viscoelastic material and a knife or other instruments are introduced into the anterior chamber to cut or tear the posterior capsule so that the visual axis can be cleared (Fig. 1).
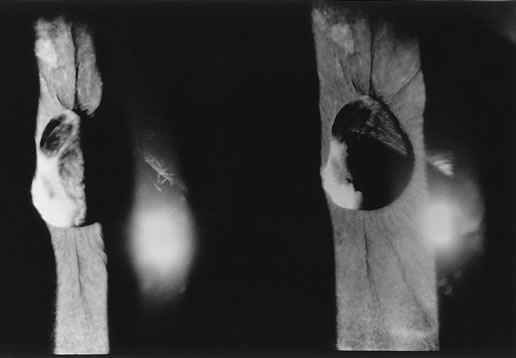
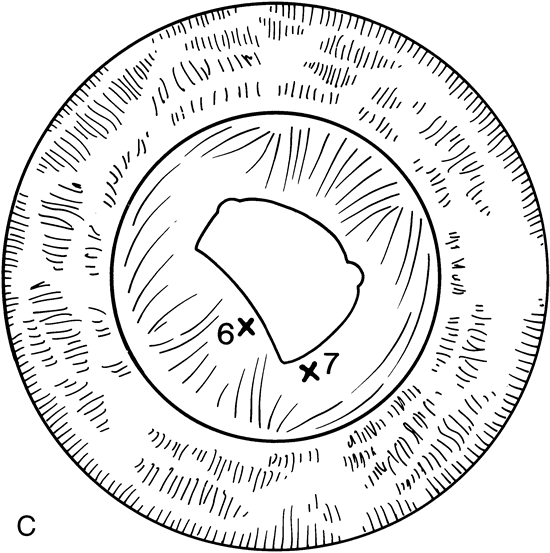
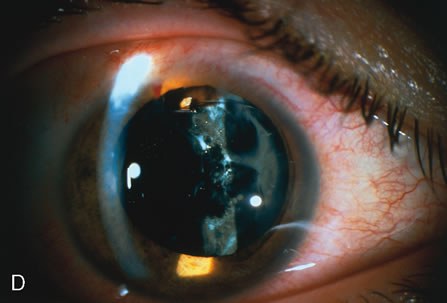
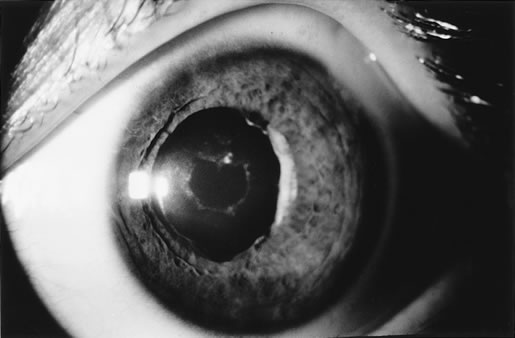
When the vitreous suction-cutting devices became available, they were quickly employed to remove cataracts in children. In addition to providing control of aspiration pressure and control of the flow of irrigation solutions, they also added the ability to remove some or all of the posterior lens capsule, even when the capsule had a thick fibrovascular stalk associated with persistent hyperplastic primary vitreous (PHPV) or a thick capsular plaque (Fig. 2). These fine-tip suction-cutting instruments provided sufficient control of the anterior chamber depth, thus permitting the surgeon to precisely open the posterior capsule and, if necessary, safely remove vitreous from the anterior chamber. Keech and co-workers,12 in a contemporaneous surgical series, showed that having the ability to remove the posterior lens capsule and perform an anterior vitrectomy reduced the need for secondary procedures from 75%, if the capsule was left intact, to 11% after capsulectomy and anterior vitrectomy. They found that when a large section of the posterior lens capsule was removed, it provided a lasting optical opening and reduced the requirement for additional surgery.
As techniques evolved, concerns were raised about the wisdom of removing a portion of the anterior vitreous with the capsulectomy. Occasional reports postulated that cystoid macular edema may occur with these techniques, but the actual risk of developing cystoid macular edema and the long-term results of an anterior vitrectomy on the eye of a child (and in particular on the retina) remain unknown.13 This relatively new capability of safely removing a portion of the lens capsule, or even the entire posterior lens capsule, gave rise to controversy. If only a small portion of the posterior capsule was removed, opacification could recur. Experience showed that the lens would develop pearls at the edge of the posterior capsulotomy, and these lens epithelial cells could migrate across a scaffold created by the anterior vitreous face. If the entire posterior lens capsule was removed, recurrence of posterior capsule opacification would usually not occur, but the barrier effect of the posterior lens capsule between the anterior chamber and vitreous was lost.
In 1981, Taylor, and later, Parks14–15 reviewed these issues and concluded that it was best to remove the posterior lens capsule and combine this with a small anterior vitrectomy. It was considered that the risk of developing cystoid macular edema was relatively remote and unproved. There was a greater risk of decreased vision if the capsule became opaque and amblyopia became refractory to treatment. Following this, some surgeons began to remove as much of the lens capsule as possible to ensure that regrowth of the lens capsule and subsequent occlusion of the visual axis did not occur. When this technique is taken to its extreme, the entire posterior lens capsule is removed. This can also create a problem. If a child with a monocular cataract becomes intolerant to wearing a contact lens, and a complete lensectomy has been performed, there is insufficient support from the remaining lens remnants for placement of a secondary sulcus-fixated posterior chamber intraocular lens. These eyes pose difficult situations for the surgeon and the child. Optical rehabilitation can be achieved only by placing an anterior chamber IOL, or by suturing the IOL to the sclera in the posterior chamber.16,17
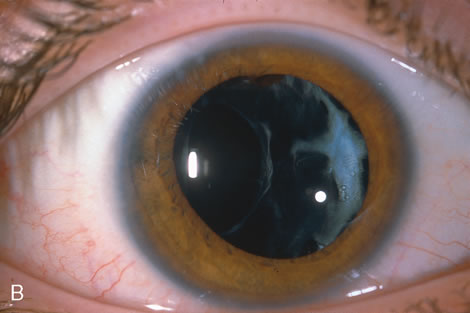
With the incremental acceptance of use of the IOL as the principal method of rehabilitating children's eyes, the issue of the formation of secondary membranes has become more complicated. Authors have advocated opening the posterior lens capsule at the time of the cataract surgery either by using vitrectors, or by performing a posterior lens capsule capsulorhexis, with or without prolapsing the lens optic through the posterior capsular opening.18–24 These measures however, do not ensure that the visual axis will not become opacified.25–27.
The pioneering work of Krasnov, Aron-Rosa, and Fankhauser in developing the neodymium (Nd>YAG laser and the refinement of its technology have resulted in an additional method for management of the posterior capsule.28
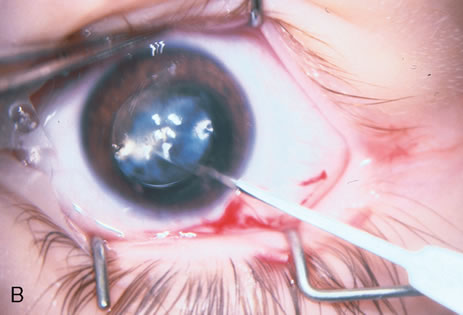
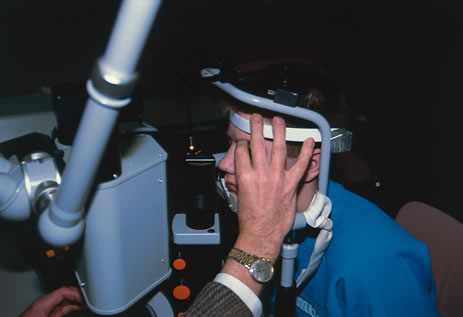
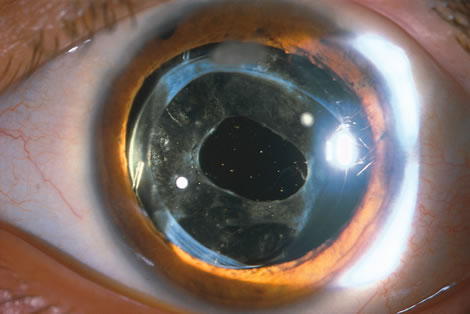
The Nd:YAG laser provides the ophthalmologist with the ability to open the posterior lens capsule without making a surgical incision. Because of this, the risk of infection and of complications related to making the incision and introducing instruments into the vitreous are reduced. In older cooperative children, the procedure can be performed in an office setting (Fig. 3).
|
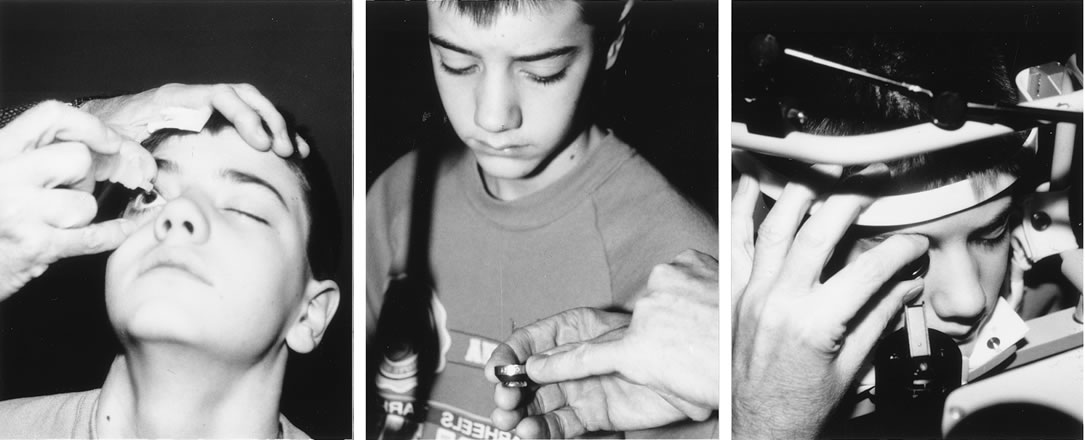
Until recently, application of laser technology for young or uncooperative children has been difficult because the instrumentation is bulky and difficult to transport. To some extent, these problems have been reduced with a reduction in the size of the laser. Use of the Nd:YAG laser to open the posterior lens capsule in young children has required that the patient be treated in a seated position. If the child is young or uncooperative, some surgeons have resorted to anesthetizing the child near the location of the Nd:YAG laser and have performed the laser treatment on the child while he or she is held in position for use of the laser delivery device. With this technique, there can be problems with positioning the eye, and if the child is treated at a location that is remote from the operating room, there may be difficulty in monitoring the child's anesthetic and providing emergency treatment if this would be required.
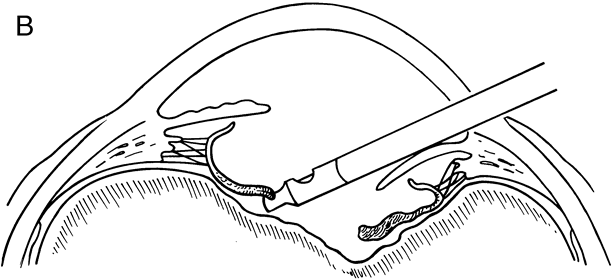
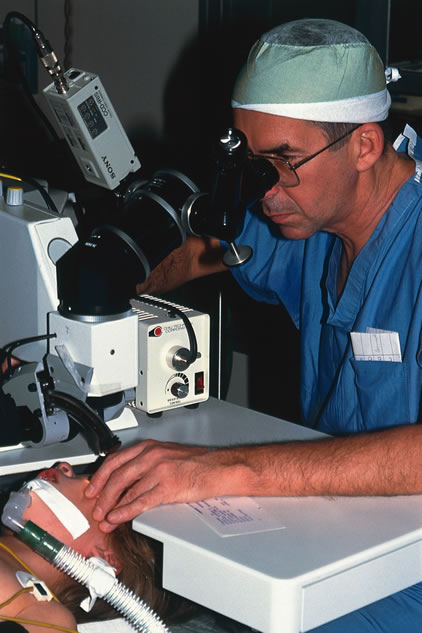
To overcome these difficulties, the Nd:YAG laser has been modified so that a posterior capsulotomy can be performed on a child in the supine position. The laser delivery systems now provide the surgeon with the ability to safely perform an Nd:YAG laser capsulotomy in children in the operating room with the assistance of sedation or, if necessary, general anesthesia (Fig. 4). In 1994, Atkinson and Hiles demonstrated the effectiveness of the Nd:YAG laser in children 3 months of age and older.29 The surgeon may elect to perform the Nd:YAG capsulotomy immediately after cataract surgery or at a later date, when the capsule has become tense or has begun to opacify.
|
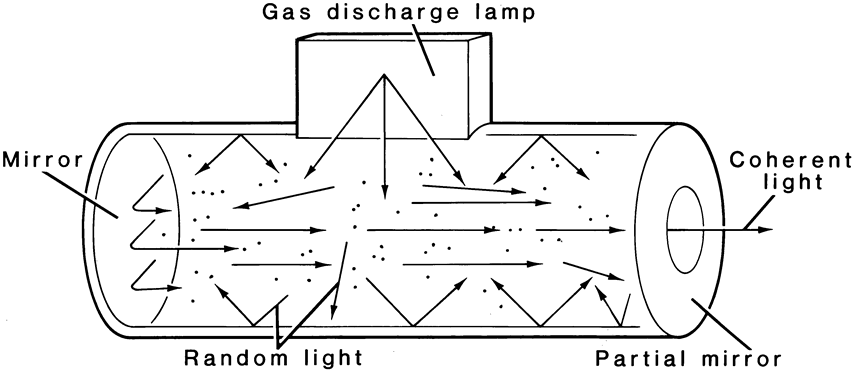
The term laser is an acronym that stands for “light amplification by a stimulated emission of radiation.” In 1917, Einstein postulated that a photon released from an excited atom could, upon interacting with a second similarly excited atom, trigger a second atom to be excited and release a photon. The photon released by the second atom would be identical in frequency, energy, direction, and phase to the triggering photon. These two photons could then proceed to trigger more atoms, causing an excited state or stimulated emission. Stimulated emission is achieved by “pumping” energy into a lasing medium. This is accomplished in the Nd:YAG by use of a gas discharge lamp. When an appropriate medium contains a great many excited atoms, the emission of light output will be randomized and equal in all directions. Once stimulated radiation is achieved, the energy can be given a preferential direction by placing a fully mirrored surface at one end of the optics cavity and a partial mirror at the opposite end (Fig. 5). The light energy reflected between these two mirrors collides with other excited atoms, causing more photons to be released and emit more light energy. The energy that escapes through the partial mirror is said to be coherent, which means that it has only one orientation or polarization. Other random light produced is trapped within the laser medium. The stimulated emission can be collimated and focused by a laser delivery system.
|
Light energy emitted by a laser differs from ordinary light sources. What makes lasers different is their ability to emit light-specific wavelengths. This light energy, the frequency of which is dependent on the laser medium, can be focused on a very small area, and the light can be discharged over very short periods of time. This makes it possible to concentrate light energy in an extremely small space without causing damage to surrounding tissues.
The energy produced is measured as the capacity to do work and is classified as a form of potential or kinetic energy. In the metric system, this is measured in joules (J). Power is a measure of the rate at which work is being done and is measured in watts (W). One watt equals one joule per second. The laser effect on tissue is dependent on the amount of energy, the number of pulses, and the time it takes to deliver the pulse.
The active medium of a Nd:YAG laser consists of neodymium embedded in a yttrium-aluminum garnet (YAG) medium. The advantage of the Nd:YAG laser is that the optical energy can be delivered in the Q-switched mode in a very brief period of time (nanoseconds), and it is readily absorbed by tissue. The Q-switched mode can be thought of as a very fast shutter that lets a pulse of coherent light exit from the laser into the delivery tube. The beam creates a very high temperature when it strikes tissue, and it will cause an acoustic pressure wave as well as thermal and optical breakdown of the tissue (photodisruption). If light energy is focused or concentrated on the posterior lens capsule, there will be destruction of the membrane. Secondarily, but related to these effects, energy is also released in the form of a pressure wave. This causes disruption of the secondary membrane. In addition to its use in disrupting the posterior lens capsule, the Nd:YAG laser may be used to perform peripheral iridotomy and cut fibrous adhesions, iris synechiae, and vitreous bands. In its thermal, continuous wave, or running mode, it may also be used to destroy the ciliary processes and lower intraocular pressure.
In this chapter we describe the use of the Nd:YAG laser to perform capsulotomy of the posterior lens capsule in children.