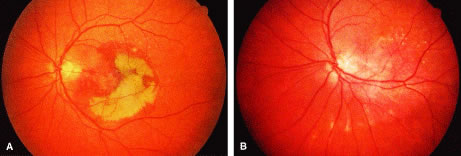
In the literature, most patients who underwent macular translocation have been those with exudative AMD. The various methods of macular translocation may be divided into two broad categories. One category involves large retinal incisions. This group is further subdivided based on whether or not a 360-degree retinotomy is performed. The other major category involves only punctate retinotomies. Subdivisions are based on the absence or presence of chorioscleral shortening, either infolding (imbrication) or outfolding (outpouching).5 These different methods are described briefly.
Macular translocation involving curvilinear retinotomies relies on the principle of detaching the retina from its anterior connection, either at the ora serrata or peripheral retina. The detached retina remains tethered to the optic nerve and is rotated about this axis.
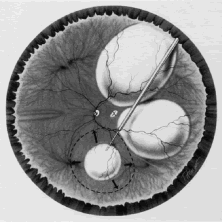
In 1993, Machemer and Steinhorst described the technique of pars plana vitrectomy with a 360-degree retinotomy at the peripheral edge of the retina. After the artificial detachment of the entire retina, it was rotated around the optic nerve. The retina was rotated by 30 to 80 degrees with removal of the CNV (Fig. 1A and B). This allowed the fovea to be displaced onto areas of intact RPE. The greatest degree of translocation is achieved with this method. However, postoperative cyclotropia and proliferative vitreoretinopathy occurred in two of the three patients. Nonetheless, this pioneering work succeeded in generating tremendous interest in this technically difficult surgery.6
Eckardt and colleagues further refined the method of pars plana vitrectomy with peripheral 360-degree retinotomy. The use of perfluorocarbons and wide-angle viewing systems (BIOM, Moeller-Wedel) greatly facilitated this procedure of macular translocation. Primary and secondary strabismus surgery is performed to correct torsional diplopia.
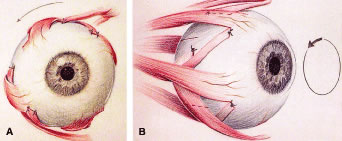
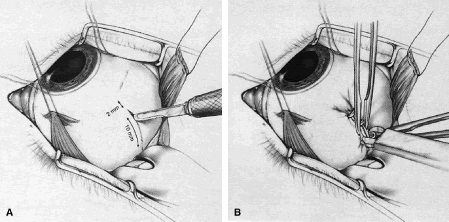
Before performing the vitreoretinal surgery, primary torsional surgery may be performed. This involves recessing the superior oblique by 12 mm while the inferior oblique receives a 12-mm tuck. Muscle strips measuring 1.5 to 2.0 mm are then fashioned from two to four recti muscles. These strips of split recti muscle are then passed underneath its respective muscle and reattached adjacent to the insertion of the adjacent recti muscle (Fig. 2A and B). This reduces the cyclotropia induced by macular translocation. Secondary strabismus surgery is then performed on the operated eye or fellow eye based on postoperative findings.
In the method described by Eckardt and colleagues, phakic patients underwent phacoemulsification via a corneoscleral wound before the vitrectomy. The intraocular lens was placed during a separate surgery at a later time in most cases. In addition to the standard sclerotomies for a three-port pars plana vitrectomy, a fourth sclerotomy is made at the 12 o'clock position to accommodate a chandelier light source. A core vitrectomy is performed. Then the posterior hyaloid face is detached if not already detached. The vitrectomy is then extended to the far periphery with meticulous trimming of the vitreous base. A retinotomy is then placed at the equator at the 6 o'clock position and BSS-Plus solution is infused in the subretinal space to detach the retina. Perfluorocarbon fluid (PFC) is used to hold the retina in place, while a 360-degree retinotomy at the ora serrata is performed. The PFC is then removed. Complete detachment of the retina is facilitated by a bimanual technique using a blunt knob spatula and an atraumatic retina forceps. Once complete detachment was achieved, the CNV is removed with forceps or endolasered. The retina is then rotated in the superotemporal direction with two spatulas or backflush instruments. The fovea is rotated on average by 39 degrees. PFC is reinjected to again hold the retina in place while endolaser is applied to the 360-degree retinotomy. An inferior iridectomy is performed. Then silicone oil is used to replace the PFC. The silicone oil is then removed 2 to 16 weeks later.
Of the 30 eyes in this series, adequate rotation of the retina was not achieved in three patients. A serious complication involves PFC in the subretinal space noted in the postoperative period. Subretinal perfluorocarbon may cause irreversible local retinal damage. Other complications included proliferative retinopathy in three eyes, retinal detachments in five eyes, chronic macular edema in four eyes, and recurrence of CNV in three eyes. Sixty percent of patients (18/30) had visual acuities of 20/50 or better.7 Faude and colleagues8 advocate the use of calcium- and magnesium-free solutions to facilitate retinal detachment for macular translocation. Work on animal models has demonstrated that retina-RPE interface adhesiveness is reduced by low pH, low calcium, low magnesium, and dark adaptation.9
Fujikado and colleagues10 have used the macular translocation technique described by Eckardt with success in patients with high myopia and CNV. Muscle surgery is first performed, which involves recessing the superior oblique muscle and tucking the inferior oblique by 9 mm. The inferior oblique is secured posterior and superior to the lateral rectus insertion. A corneoscleral approach is used for cataract extraction leaving a capsular bag in place. They irrigate the vitreous cavity with calcium-free and magnesium-free balanced salt solution (BSS) for 20 minutes to facilitate retinal detachment. A 39-gauge needle is then used to infuse subretinal BSS-Plus solution to detach the retina. The first site is superior to the optic nerve just before the first bifurcation of the superotemporal vascular arcade. Other sites along the equator are chosen for subretinal infusion. An air-fluid exchange facilitates the connection of the areas of detached retina. The 360-degree retinotomy is then performed. Then a small amount of perfluorocarbon is infused to gently tamponade the macula while the retina is rotated clockwise, displacing the fovea onto a bed of healthy RPE. Endolaser is then applied to the edge of the retinotomy. A perfluorocarbon-silicone oil exchange is then performed. The silicone oil is removed and an artificial lens placed 2 to 8 weeks later. The retina is rotated an average of 23 degrees. The average foveal displacement was 1.5 disc diameters.
Visual results were encouraging. Seventy-two percent (8/11) of patients experienced visual improvement of 0.2 logarithm of the minimum angle of resolution (logMARs), with 64% of patients having a visual acuity of 20/50 or better. Of the three patients who developed rhegmatogenous retinal detachments, one developed proliferative vitreoretinopathy. These complications were successfully managed with further surgery. Fujikado and colleagues felt that patients with CNV benefit more from 360-degree retinotomy because of the greater amount of foveal displacement compared with limited macular translocation. The greater distance protected foveal involvement in the event of CNV recurrence and allowed a wider margin of safety for laser photocoagulation.10
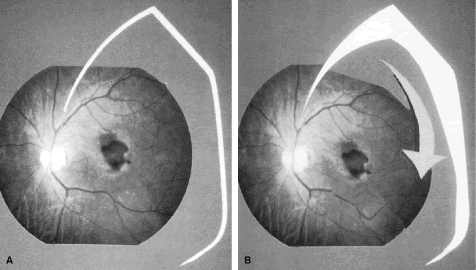
Ninomiya and colleagues have used partial retinotomies in a small series of patients. After detachment of the posterior hyaloid and an extensive vitrectomy, the retina was detached via an external approach involving an infusing 27-gauge needle guided into the subretinal space. Endodiathermy marked the area to be excised, which began at the first bifurcation of the superotemporal arcade extending radially then following an arc from the superotemporal quadrant to the inferotemporal quadrant. Intraocular scissors are then used to cut the diathermized retina. The CNV is removed using forceps after the retinal flap is reflected. An air-fluid exchange is performed and the retina is unfolded then rotated clockwise with the aid of a soft-tipped infusion cannula (Fig. 3A and B). As the fovea is inferiorly displaced one disc diameter, a bare area of RPE is exposed superiorly. Endolaser is then applied along the edges of the retinotomy and silicone oil is infused.
In this series, two patients experienced an improvement in their vision, whereas one patient did not. Epiretinal membranes, retinal detachment, and neovascular glaucoma were among the complications noted.11
The other major category of macular translocation involves only punctate retinotomies. The chief mechanisms by which the neurosensory retina is displaced involves creating a redundancy of retina relative to the eyewall. Stretching of the neurosensory retina with subretinal fluid also plays a role. By not performing large retinotomies, incidence of proliferative vitreoretinopathy (PVR) is decreased.
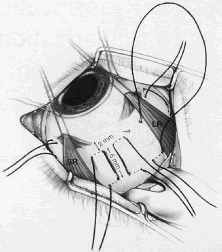
Limited macular translocation is the method of displacing the neurosensory retina without curvilinear retinotomies; instead the imbrication of the sclera causes a relative redundancy of retina. de Juan and colleagues 12 have led the way in developing this method of macular translocation. Lewis and coworkers employ a similar technique. The initial steps involve placing five to six scleral mattress sutures in the superotemporal quadrant (Fig. 4). These sutures are placed 2 mm behind the recti insertions with the scleral bites separated by 6 mm. One additional suture is placed medial to the superior rectus; another is placed inferior to the lateral rectus. These sutures are left untied. A standard three-port pars plana vitrectomy is performed with removal of the posterior hyaloid. A 39- to 41-gauge flexible cannula is then used to infuse BSS into three to six separate sites within the temporal arcades to create blister-like limited retinal detachments involving the macula and extending to the ora serrata from the superotemporal to the inferonasal retinal periphery (Fig. 5). An air-fluid exchange and the retinal manipulator are sometimes needed to facilitate the coalescence of the multiple retinal detachments. The imbrication process is then completed by tightening the scleral sutures after lowering the intraocular pressure. This causes a redundancy in the retina relative to the underlying sclera. A partial (70%) air-fluid exchange is then performed, leaving the retina detached (Fig. 6). After upright positioning for 24 hours, the subretinal fluid resolves.13 Following macular translocation, a fluorescein angiogram may then be performed, guiding the laser photocoagulation treatment of the now extrafoveal CNV.14
The combined effect of stretching the retina with injected subretinal fluid, relative retinal excess because of scleral imbrication, partial air-fluid exchange, and gravity displaces the fovea inferiorly an average of 1200 microns. Of the 10 eyes in the study, 6 experienced postoperative decrease in their vision. The best visual outcome was 20/80. However, this was noted in only 2 eyes.13
With limited macular translocation, superior translocation of the fovea is more difficult because of positioning issues. Also, there is a measure of unpredictability to the amount of macular translocation inherent to the process of spontaneous absorption of subretinal fluid. Complications include insufficient foveal displacement, macular folds, retinal detachments, and inexplicable decrease in postoperative vision.13
Lewis described the technique of using radial and circumferential chorioscleral outfolding with titanium clips in limited macular translocation. The main advantage of this technique lies in greatly reducing the internal surface area of the sclera relative to the retina. When this technique is combined with intraoperative retinal reattachment, the degree of macular translocation is both large and predictable, especially with radial chorioscleral outfolding.
A standard three-port pars plana vitrectomy with removal of the posterior hyaloid is first performed. Limited retinal detachments are then performed via the subretinal infusion of BSS via a 39-gauge cannula. The intraocular pressure is then lowered and attention is turned to performing the radial outfolding. The superotemporal quadrant is exposed, and a 10-mm partial thickness incision is made in the sclera close to the superior rectus starting 2 mm behind the muscle insertion. Forceps are used to create an outpouching of sclera that is then secured with placement of six 4-mm titanium clips (Fig. 7A and B). An air-fluid exchange is performed while the subretinal fluid is removed via a drainage retinotomy created with a 30-gauge cannula posterior to the equator. A soft-tipped cannula may be used to facilitate manipulation of the retina. A retinal fold along the equator is created and the fovea is effectively displaced. At the conclusion of the procedure, a 70% air bubble is left and the patient is positioned upright until the air is resorbed. After a fluorescein angiogram confirms displacement of the CNV to an extrafoveal location, laser photocoagulation is performed. The fovea was displaced by a median of 1977 μm (range 1644 to 3200 μm) when using radial chorioscleral outfolding, exceeding published results of limited macular translocation with scleral infolding.15
However, even without scleral infolding or outfolding, macular translocation is possible. de Juan and Vander16 reported a case of foveal displacement by 500 μm in a patient who underwent three-port pars plana vitrectomy, removal of the posterior hyaloid, limited retinal detachment, partial air-fluid exchange, and positioning. The amount of macular translocation allowed for laser photocoagulation of the postoperative extrafoveal CNV. This patient's baseline visual acuity was preserved at 20/70. This case emphasizes the effect of retinal stretching that can be achieved with subretinal infusion and gravitational forces.
There are advantages and disadvantages to each of the myriad of approaches to macular translocation. The unique clinical scenario for each case will determine the method of macular translocation most likely to benefit the patient. The size and location of the underlying CNV dictates the particular technique that most effectively achieves the necessary degree of macular translocation. For example, a patient with CNV requiring 200 to 500 μm may be an ideal candidate for limited macular translocation with or without scleral infolding, whereas a patient with CNV that requires foveal displacement of at least 1500 μm may benefit from limited macular translocation with scleral outfolding or 360-degree retinotomy.
Macular translocation is an innovative surgical approach to the treatment of subfoveal disease. Limited macular translocation with scleral outfolding or infolding offers the best chances of visual improvement in 44% to 48% of patients.15 However, further randomized clinical studies are required to establish its role in the treatment of patients with subfoveal CNV.
Direct surgical extraction of subfoveal CNV without macular translocation is under investigation. There have been variable reports of visual improvement after submacular surgery. CNV has been classified into different types. The kind of CNV may play a role in determining postoperative visual acuity. Type 1 CNV is seen in AMD and occurs underneath or enmeshed with RPE. The defects in Bruch's membrane and areas of attenuated RPE are diffuse. Removal of CNV of this type is, therefore, more difficult and often results in damage to the supportive cellular structures underlying the fovea. This damage is associated with poor visual recovery.17
Type 2 CNV grows from a small focal defect in Bruch's membrane. Because this type of CNV grows over the RPE, it may be possible to remove subretinal CNV with little damage to RPE. Conditions such as POHS, high myopia, and idiopathic etiology predispose individuals to this type of CNV.17
In general, surgical extraction of subfoveal CNV resulting from POHS and idiopathic etiology has been beneficial. However, a similar benefit has not been noted in patients who underwent extraction of subfoveal CNV resulting from AMD.17
The submacular surgery trial (SST) pilot study is currently investigating the value of extracting subfoveal CNV secondary to AMD, POHS, and idiopathic etiology. One of its chief objectives was to determine via several pilot studies whether or not larger multicenter trials are worthy of pursuit. One pilot study has demonstrated no advantage of submacular surgery over laser treatment of recurrent subfoveal CNV.18
In another arm of the SST, the value of submacular surgery versus observation for patients with idiopathic CNV or CNV secondary to POHS has been approved and is under investigation in a full-phase trial. Two hundred fifty patients will be enrolled and followed for 4 years. Other arms of the SST pilot study focus on CNV secondary to AMD. One branch is investigating the effect of submacular surgery versus observation in patients with new, large CNV between 3.5 to 9.0 MPS disc areas. Another study focuses on submacular surgery for CNV and associated hemorrhage. Patients enrolled have a subfoveal lesion measuring greater than 3.5 MPS disc areas with at least 50% hemorrhage comprising the visible lesion.19
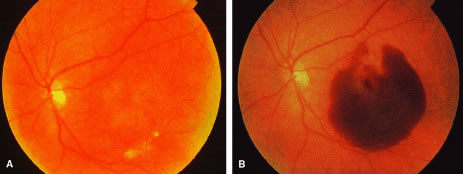
The clinical importance of submacular hemorrhages cannot be overemphasized. Submacular hemorrhages may occur from a variety of etiologies. The mechanisms of injury leading to visual loss have been discussed earlier. Visual recovery is often related to the etiology of the hemorrhage. Patients with healthy RPE and photoreceptors such as those with idiopathic CNV, trauma, retinal arterial macroaneurysms, and idiopathic polypoidal vasculopathy have a better visual prognosis than those with AMD who have diseased RPE and photoreceptors.20 Nonetheless, because permanent visual loss ensues with the development of a disciform scar especially in patients with AMD, the removal or displacement of submacular hemorrhages is critical. The goal of treatment is to move the hemorrhage away from the macula. Adequate displacement of submacular hemorrhage may lead to improvement of central visual acuity and allows management of any underlying CNV.
Submacular hemorrhages may be managed with vitrectomy, pneumatic displacement, or observation. In the surgical management of submacular hemorrhage, a standard three-port vitrectomy is performed with removal of the posterior hyaloid face. Then a retinotomy is created temporal to the fovea to allow for subretinal forceps to remove both the hemorrhage and the CNV. Among patients with AMD, the visual prognosis is poor because RPE and photoreceptors are damaged with the removal of blood and CNV.21
Tissue plasminogen activator (t-PA) may be used to help dissolve the subretinal blood. t-PA, a 70-kD serine protease, converts plasminogen to plasmin thereby allowing it to dissolve fibrin.22 A 20- to 36-gauge cannula may be used to preoperatively or intraoperatively inject t-PA into the subretinal space.4,23–27 The submacular hemorrhage is then extruded via a retinotomy site. PFC may also be used to “steamroll” the dissolved blood clot through the retinotomy site. This helps minimize subretinal manipulation, which avoids surgically induced photoreceptor and RPE damage.26,28