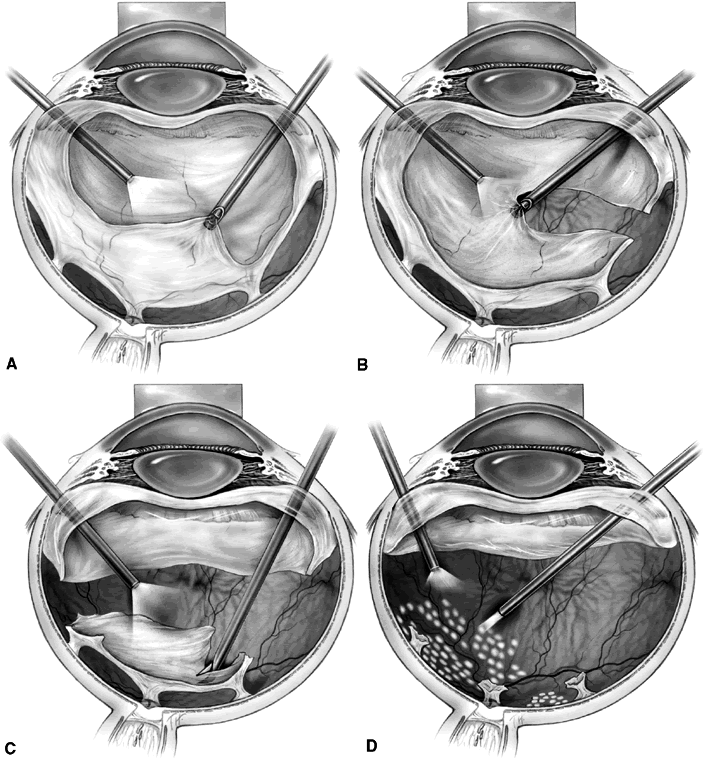
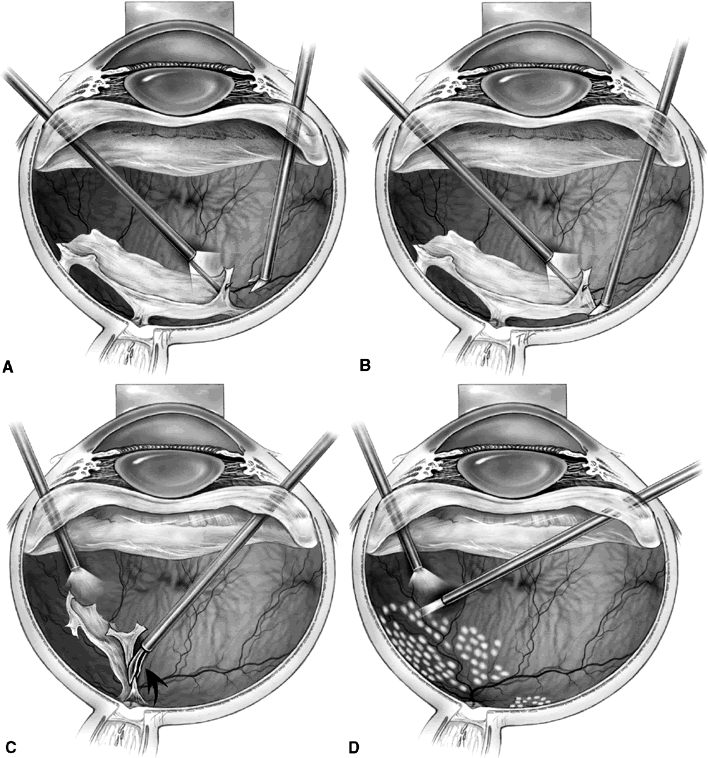
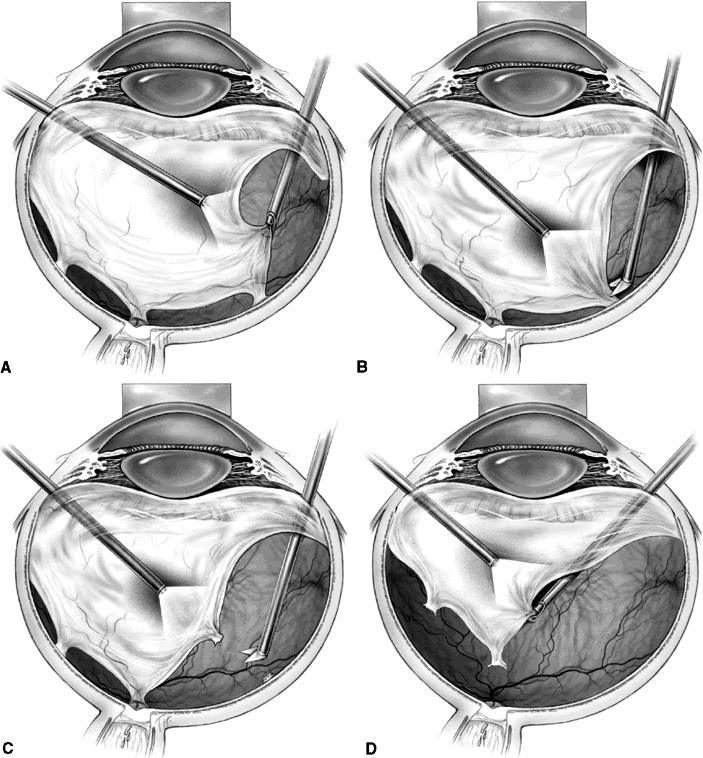
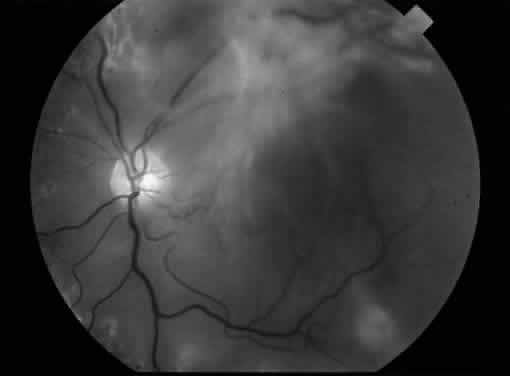
1. Klein R, Klein BEK: Vision disorders in diabetes. In National Diabetes
Data Group: Diabetes in America: Diabetes Data Compiled 1984, (DHHS publ. no. [NIH] 85–1468). Bethesda, MD, U.S. Department
of Health and Human Services, 1985 2. Diabetic Retinopathy Study Research Group: Preliminary report on effects
of photocoagulation therapy. Am J Ophthalmol 81:383, 1976 3. Diabetic Retinopathy Study Research Group: Photocoagulation treatment of
PDR: Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS
report no. 8. Ophthalmology 88:583, 1981 4. Early Treatment Diabetic Retinopathy Study Research Group: Photocoagulation
for diabetic macular edema, ETDRS report no. 1. Arch Ophthalmol 103:1796, 1985 5. Early Treatment Diabetic Retinopathy Study Research Group: Pars plana vitrectomy
in the early treatment diabetic retinopathy study, ETDRS report
no. 17. Ophthalmology 99:1351, 1992 6. Machemer R, Buettner H, Norton EWD, Parel JM: Vitrectomy: A pars plana approach. Trans Am Acad Ophthalmol Otolaryngol 72:410, 1968 7. Machemer R, Parel JM, Buettner H: A new concept for vitreous surgery. I. Instrumentation. Am J Ophthalmol 74: 1, 1972 8. Machemer R: Vitrectomy in diabetic retinopathy removal of preretinal proliferations. Trans
Am Acad Ophthalmol Otolaryngol 79:OP-394, 1975 9. Fox GM, Blumenkranz MS, Iverson DA et al: Long-Acting Gas Intraocular Tamponade
in Diabetic Vitrectomy. American Academy of Ophthalmology Annual
Meeting, Dallas, November 1992 10. The Silicone Study Group: Vitrectomy with silicone oil or sulfur hexafluoride
gas in eyes with severe proliferative vitreoretinopathy: Results
of a randomized clinical trial. Arch Ophthalmol 110:770, 1992 11. The Silicone Study Group: Vitrectomy with silicone oil or perfluoropropane
gas in eyes with severe proliferative vitreoretinopathy: Results of
a randomized clinical trial. Arch Ophthalmol 110:780, 1992 12. Mathis A, Pagot V, David JL: The use of perfluorodecalin in diabetic vitrectomy. Fortschr Ophthlmol 88:148, 1991 13. Williams DF, Williams GA, Hartz A et al: Results of vitrectomy for diabetic traction retinal detachments using the
en bloc excision technique. Ophthalmology 99:1351, 1992 14. Pendergast SD, Martin DF, Proia AD, Jaffe GJ et al: Removal of optic disc stalks during diabetic vitrectomy. Retina 15:25, 1995 15. Diabetic Retinopathy Vitrectomy Study Group: Early vitrectomy for severe
vitreous hemorrhage in diabetic retinopathy. Arch Ophthalmol 108:958, 1990 16. Diabetic Retinopathy Vitrectomy Study Group: Early vitrectomy for severe
vitreous hemorrhage in diabetic retinopathy. Arch Ophthalmol 108:958, 1990 17. Thompson JT, DeBustros S, Michels RG, Rice TA: Results and prognostic factors in vitrectomy for diabetic vitreous hemorrhage. Arch Ophthalmol 105:191, 1987 18. Charles S, Flinn CE: The natural history of diabetic extramacular traction retinal detachment. Arch Ophthalmol 99:66, 1981 19. Blankenship GW: Preoperative prognostic factors in diabetic pars plana vitrectomy. Ophthalmology 89:1246, 1982 20. Thompson JT, DeBustros S, Michels RG, Rice TA: Results and prognostic factors in vitrectomy for diabetic traction retinal
detachment of the macula. Arch Ophthalmol 105: 497, 1987 21. Thompson JT, DeBustros S, Michels RG, Rice TA: Results and prognostic factors in vitrectomy for diabetic traction-rhegmatogenous
retinal detachment. Arch Ophthalmol 105: 503, 1987 22. Davis MD: Vitreous contraction in PDR. Arch Ophthalmol 74:741, 1965 23. Diabetic Retinopathy Vitrectomy Research Group: Two year course of visual
acuity in severe PDR with conventional management. Ophthalmology 92:492, 1985 24. Lewis H, Abrams GW, Blumenkranz MS, Campo RV: Vitrectomy for diabetic macular traction and edema associated with posterior
hyaloidal traction. Ophthalmology 99:753, 1992 25. Harbour JW, Smiddy WE, Flynn HW, Rubsamen PE: Vitrectomy for diabetic macular edema associated with a thickened and taut
posterior hyaloid membrane. Am J Ophthalmol 121:405, 1996 26. Tachi N, Ogino N: Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol 122: 258, 1996 27. Keates RH, McGowan KA: The effect of topical indomethacin ophthalmic solution in maintaining mydriasis
during cataract surgery. Ann Ophthalmol 16:1116, 1984 28. Allaire C, Lablache CM, Trinquand C, Bazin R: Comparative efficacy of 0. 1% indomethacin eye drops, 0.03% flurbiprofen
eye drops and placebo for maintaining peroperative mydriasis. J Fr Ophthalmol 17:103, 1994 29. Haimann MH, Abrams GW: Prevention of lens opacification during diabetic vitrectomy. Ophthalmology 91:116, 1984 30. Oyakawa RT, Schachat AP, Michels RG, Rice TA: Complications of vitreous surgery for diabetic retinopathy. I. Intraoperative
complications. Ophthalmology 90:517, 1983 31. DeBustros S, Glaser BM, Johnson MA: Thrombin infusion for the control of intraocular bleeding during vitreous
surgery. Arch Ophthalmol 103:837, 1985 32. Oyakawa RT, Schachat AP, Michels RG, Rice TA: Complications of vitreous surgery for diabetic retinopathy. Ophthalmology 90:517, 1983 33. Kreiger AE: Wound complications in pars plana vitrectomy. Retina 13:335, 1993 34. Cleary PE, Ryan SJ: Method of production and natural history of experimental posterior penetrating
eye injury in the rhesus monkey. Am J Ophthalmol 88:212, 1979 35. Campochiaro PA, Jerlan JA, Glaser BM: Serum contains chemoattractants for human pigment epithelial cells. Arch Ophthalmol 102:1830, 1984 36. Tardif YM, Schepens CL: Closed vitreous surgery. XV. Fibrovascular ingrowth from the pars plana
sclerotomy. Arch Ophthalmol 95:235, 1977 37. Blumenkranz MS: Lens-sparing treatment of recurrent diabetic vitreous hemorrhage
associated with anterior neovascularization by silicone oil
injection, cryopexy, and laser photocoagulation. In Stirpe M (ed): Anterior
and Posterior Segment Surgery: Mutual Problems and Common Interests. Acta
of the Fifth International Congress on Vitreoretinal Surgery, pp 60, 176. New
York, Ophthalmic Communication Society, 1998 38. Michels RG: Vitreoretinal and anterior segment surgery through the pars plana. Part
I. Ann Ophthalmol 8:1353, 1976 39. Michels RG, Stark WJ: Vitrectomy technique in anterior segment surgery. Trans Am Acad Ophthalmol Otolaryngol 81:382, 1976 40. The Silicone Study Group: Vitrectomy with silicone oil or perfluoropropane
gas in eyes with severe proliferative vitreoretinopathy: Results of
a randomized clinical trial. Arch Ophthalmol 110:780, 1992 41. Williams GA, Lambrou FH, Jaffe GA et al: Treatment of post-vitrectomy fibrin formation with intraocular tissue plasminogen
activator. Am J Ophthalmol 106:1055, 1988 42. Brownlee M, Vlassara H, Cerami A: Inhibition of heparin-catalyzed human antithrombin III activity by non-enzymatic
glycosylation: Possible role in fibrin deposition in diabetes. Diabetes 33:532, 1984 43. Sowers JR, Tuck ML, Sowers DK: Plasma antithrombin III and thrombin generation time: Correlation with
hemoglobin A1 and fasting serum glucose in young diabetic women. Diabetes Care 3:665, 1980 44. Aaberg TM, Flynn HW Jr, Schiffman J, Newton J: Nosocomial acute-onset postoperative endophthalmitis survey: A 10-year
review of incidence and outcomes. Ophthalmology 105:1004, 1998 45. Cohen SM, Flynn HW Jr, Murray TG et al: Endophthalmitis after pars plana vitrectomy. Ophthalmology 102:705, 1995 46. Meredith TA: Antimicrobial pharmacokinetics in endophthalmitis treatment: Studies of
ceftazidime. Trans Am Ophthalmol Soc 91:653, 1993 47. Endophthalmitis Vitrectomy Study Group: Results of the endophthalmitis
vitrectomy study: A randomized trial of immediate vitrectomy and of intravenous
antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch
Ophthalmol 113:1479, 1995 48. Rice TA, Michels RG, Maguire MG, Rice EF: The effect of lensectomy on the incidence of iris neovascularization and
neovascular glaucoma after vitrectomy for diabetic retinopathy. Am J Ophthalmol 95:1, 1983 49. Kokame GT, Flynn HW, Blankenship GW: Posterior chamber intraocular lens implantation during diabetic pars plana
vitrectomy. Ophthalmology 96:603, 1989 50. Blankenship GW: Posterior chamber intraocular lens implantation during pars plana lensectomy
and vitrectomy for diabetic complications. Graefes Arch Clin Exp Ophthalmol 227:136, 1989 51. Blankenship GW, Flynn HW, Kokame GT: Posterior chamber intraocular lens insertion during pars plana lensectomy
and vitrectomy for complications of PDR. Am J Ophthalmol 108:1, 1989 52. Benson WE, Brown GC, Tasman W, McNamara JA: Extracapsular cataract extraction, posterior chamber lens insertion, and
pars plana vitrectomy in one operation. Ophthalmology 97:918, 1990 53. Slusher MM, Seaton AD: Loss of visibility caused by moisture condensation on the posterior surface
of a silicone intraocular lens during fluid/gas exchange after posterior
vitrectomy. Am J Ophthalmol 118:667, 1994 54. Eaton AM, Jaffe GJ, McCuen BW II, Mincey GJ: Condensation on the posterior surface of silicone intraocular lenses during
fluid-air exchange. Ophthalmology 102:733, 1995 55. Hainsworth DP, Chen SN, Cox TA, Jaffe GJ: Condensation on polymethylmethacrylate, acrylic polymer, and silicone intraocular
lenses after fluid-air exchange in rabbits. Ophthalmology 103:1410, 1996 56. Kusaka S, Kodama T, Ohashi Y: Condensation of silicone oil on the posterior surface of a silicone intraocular
lens during vitrectomy. Am J Ophthalmol 121:574, 1996 57. Khawly JA, Lambert RJ, Jaffe GJ: Intraocular lens changes after short- and long-term exposure to intraocular
silicone oil: An in vivo study. Ophthalmology 105:1227, 1998 58. Lucke KH, Foerster MH, Laqua H: Lon-term results of vitrectomy and silicone oil in 500 cases of complicated
retinal detachments. Am J Ophthalmol 104:624, 1987 59. DeJuan E Jr, Hardy M, Hatchell DL, Hatchell MC: The effect of intraocular silicone oil on anterior chamber oxygen pressure
in cats. Arch Ophthalmol 104:1063, 1984 60. Yeo JH, Glaser BM, Michels RG: Silicone oil in the treatment of complicated retinal detachments. Ophthalmology 94:1109, 1987 61. Lean JS, Leaver PK, Cooling RJ, McLeod D: Management of complex retinal detachments by vitrectomy and fluid/silicone
exchange. Trans Ophthalmol Soc UK 102:203, 1982 62. Mcleod D: Silicone-oil injection during closed microsurgery for diabetic retinal
detachment. Graefes Arch Clin Exp Ophthalmol 224:55, 1986 63. Rinkoff JS, De Juan E Jr, McCuen BW II: Silicone oil for retinal detachment with advanced proliferative vitreoretinopathy
following failed vitrectomy for PDR. Am J Ophthalmol 101:181, 1986 64. DeCorral LR, Peyman GA: Pars plana vitrectomy and intravitreal silicone oil injection in eyes with
rubeosis iridis. Can J Ophthalmol 21:10, 1986 65. McCuen BW II, Rinkoff JS: Silicone oil for progressive anterior ocular neovascularization after failed
diabetic vitrectomy. Arch Ophthalmol 107:677, 1989 66. Heimann K, Dahl B, Dimopoulos S, Lemmen KD: Graefes Arch Clin Exp Ophthalmol 227:152, 1989 67. Gonvers M: Temporary silicone oil tamponade in the treatment of complicated diabetic
retinal detachments. Graefes Arch Clin Exp Ophthalmol 228:415, 1990 68. Bourman ND, Blumenkranz MS, Cox MS, Trese MT: Silicone oil for the treatment of severe PDR. Ophthalmology 96:759, 1989 69. Gnad H, Skorpik C, Paroussis P et al: Temporary silicone oil implantation following vitrectomy in PDR. Klin Monatsbl Augenheilkd 189:388, 1986 70. Hoerauf H, Roider J, Bopp S et al: Endotamponade with silicon oil in severe proliferative retinopathy with
attached retina. Ophthalmologe 92:657, 1995 71. Gabel VP, Beck P: Does silicone oil improve the prognosis of severe proliferative diabetic
retinopathy? Klin Monatsbl Augenheilkd 197:112, 1990 72. Glaser BM, D'Amore PA, Michels RG et al: Demonstration of vasoproliferative activity from mammalian retina. J Cell Biol 84:298, 1980 73. Madreperla SA, McCuen BW II: Inferior peripheral iridectomy in patients receiving silicone oil: Rates
of postoperative closure and effect on oil position. Retina 15:87, 1995 74. Jaffe GJ, Lewis H, Han DP et al: Treatment of postvitrectomy fibrin pupillary block with tissue plasminogen
activator. Am J Ophthalmol 108:170, 1989 75. Jaffe GJ, Abrams GW, Williams GA, Han DP: Tissue plasminogen activator for postvitrectomy fibrin formation. Ophthalmology 97:184, 1990 76. Johnson RN, Olsen KR, Hernandez E: Intravitreal tissue plasminogen activator treatment of experimental vitreous
hemorrhage. Arch Ophthalmol 107:891, 1989 77. Verstraeten TC, Chapman C, Hartzer M et al: Pharmacologic induction of posterior vitreous detachment in the rabbit. Arch Ophthalmol 111:849, 1993 78. Blumenkranz MS, Ophir A, Claflin AJ, Hajek A: Fluorouracil for the treatment of massive periretinal proliferation. Am J Ophthalmol 94:458, 1982 79. Borhani H, Peyman GA, Thompson R, Thompson H: Suppression of experimental proliferative vitreoretinopathy by sustained
intraocular delivery of 5-FU. Int Ophthalmol 19:43, 1995 80. Blankenship GW: Evaluation of a single intravitreal injection of 5-fluorouracil in vitrectomy
cases. Graefes Arch Clin Exp Ophthalmol 227:565, 1989 81. Iverson DA, Katsura H, Hartzer MK, Blumenkranz MS: Inhibition of intraocular fibrin formation following infusion of low-molecular-weight
heparin during vitrectomy. Arch Ophthalmol 109:405, 1991 82. Johnson RN, Balyeat E, Stern WH: Heparin prophylaxis for intraocular fibrin. Ophthalmology 94:597, 1987 83. Johnson RN, Blankenship G: A prospective randomized clinical trial of heparin therapy for postoperative
intraocular fibrin. Ophthalmology 95:312, 1988 84. Williams RG, Chang S, Comaratta MR, Simoni G: Does the presence of heparin and dexamethasone in the vitrectomy infusate
reduce reproliferation in proliferative vitreoretinopathy? Graefes Arch Clin Exp Ophthalmol 234:496, 1996 85. Thompson JT, Glaser BM, Michels RG, DeBustros S: The use of intravitreal thrombin to control hemorrhage during vitrectomy. Ophthalmology 93:279, 1986 86. DeBustros S, Glaser BM, Johnson MA: Thrombin infusion for the control of intraocular bleeding during vitreous
surgery. Arch Ophthalmol 103:837, 1985 |