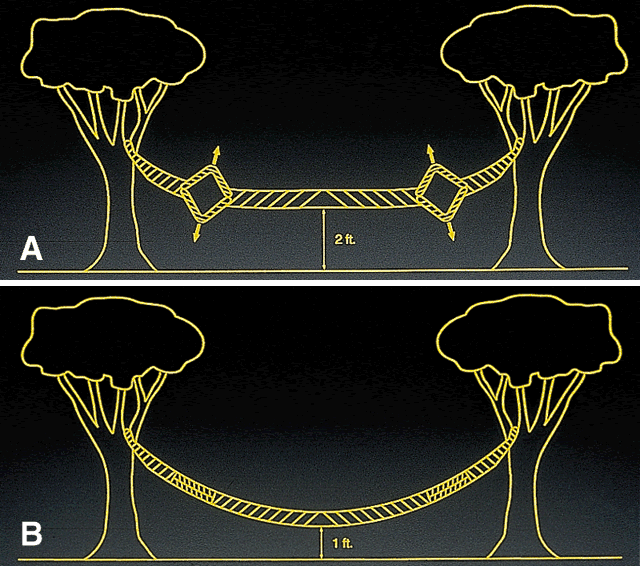
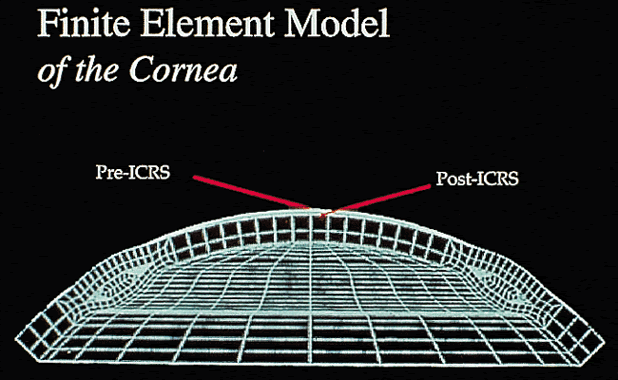
Early theoretical and animal work speculated that this ring would alter the corneal curvature through expansion or constriction of the device's diameter, causing a flattening or steeping for myopia and hyperopia, respectively.4 Further refinement of a model for the refractive effect came from Silvestrin's group5 postulating an arc shortening effect of the ring. Their model assumed that there are corneal lamellae that stretch from limbus to limbus. In that model, a spacer element such as the ICR separates corneal lamellae, resulting in a shortening of the arc length that is correlated with the device thickness (Fig. 1). Eye bank studies by T.E. Burris and coworkers then demonstrated the effect of varying implant thickness.6 By increasing the thickness of the ICR, an increased change in spherical equivalent was documented in a nearly linear fashion. Pinsky and colleagues7 then refined the model further by using finite element modeling (Fig. 2). A 360-degree ICR was used in initial studies in nonfunctional and myopic eyes in the Brazil and the United States.8 All eyes tolerated the implants well without evidence of extrusion or corneal thinning. Studies in nonfunctional eyes demonstrated the safety of the ICR (KeraVision, Fremont, CA).9 Based on these results, the US Food and Drug Administration (FDA) phase II studies of the intrastromal ring were started.
|
|
The original ICR was a 360-degree PMMA ring with an external diameter of 8.1 mm, internal diameter of 6.8 mm, and thicknesses of 0.25 to 0.45 mm. Uncorrected visual acuity of 20/40 or better was obtained in 85% of 66 patients observed on a followup basis for 1 year.10 The ring was well tolerated; explants were performed in ten eyes. In all cases, eyes after explantation returned to within 1 diopter (D) of their preoperative refraction, indicating the potential removability of the ICR.11
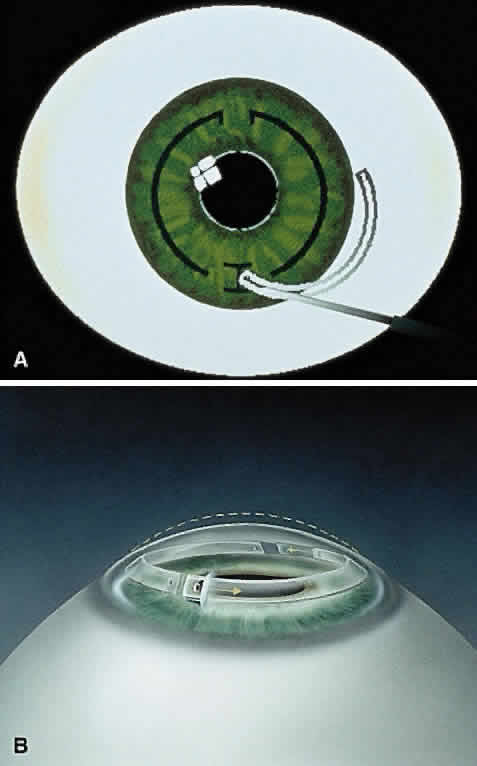
In the phase II study, wound healing problems were observed with the PMMA ring directly beneath the incision.12 To address this issue, the ring was then redesigned into two 150-degree arc segments, which simplified insertion technique and separated the implant material away from the radial incision. The ring segments were then placed through a 1.8 mm superior incision at two thirds the corneal depth. Studies showed that the ICR segments were similar in refractive effect to those of the ICR.13,14
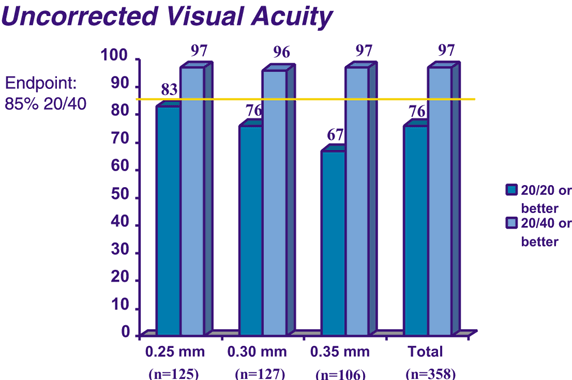
A phase III study has been completed to show the effects of the ICR segments for mild to moderate degrees of myopia with two 150-degree arc PMMA segments.15 Three segment thicknesses have been evaluated: 0.25, 0.30, and 0.35 mm. The objectives of the study were to rule out major safety risks, to ensure an acceptable outcome in uncorrected visual acuity, to ensure predictability of refractive effect, and to ensure stability of the refractive effect.16