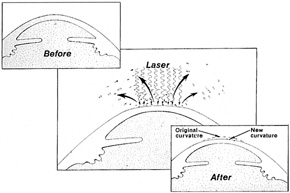
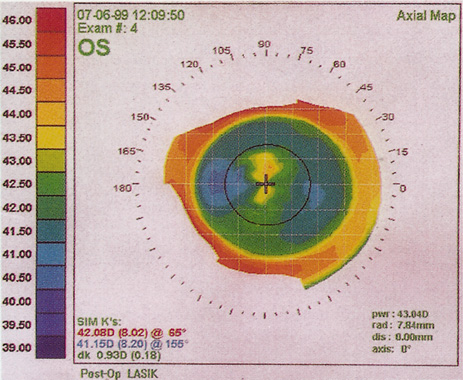
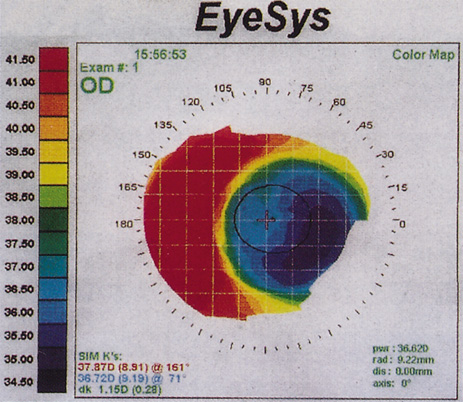
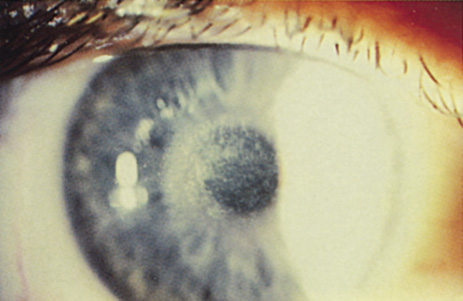
1. Salz JJ: Radial keratotomy versus photorefractive keratectomy. In: Thompson FB, McDonnell PJ (eds). Color Atlas/Text of Excimer Laser Surgery: The Cornea. New York: Igako-Shoin 1993:63–75 2. Thompson KP: Photorefractive keratectomy. Contemporary refractive surgery. Ophthalmol Clin North Am 5:745–751, 1992 3. Brancato R, Tavola A, Carones , et al: Excimer laser photorefractive keratectomy for myopia: results in 1165 eyes. Refract Corneal Surg 9:S95–S104, 1993 4. Trokel SL, Srinivasan R, Braren B: Excimer laser surgery of the cornea. Am J Ophthalmol 96:710–715, 1983 5. Srinivasan R: Ablation of polymers and biological tissue by ultraviolet lasers. Science 234:559–565, 1983 6. Puliafito CA, Wong K, Steinert RF: Quantitative and ultrastructural studies of excimer laser ablation of the
cornea at 193 and 248 nanometers. Lasers Surg Med 7:155–159, 1987 7. Ren QS, Gailitis R, Thompson KP, Lin JT: Ablation of the cornea and synthetic polymers with a UV (213 nm) solid
state laser. IEEE J Quant Electron (Special Issue Lasers Biol Med) 26:2284–2288, 1990 8. Srinivasan R, Sutcliffe E: Dynamics of the ultraviolet laser ablation of corneal tissue. Am J Ophthalmol 103:470–471, 1987 9. Tenner A, Neuhann T, Schroeder E: Excimer laser radial keratotomy in the living human eye. A preliminary
report. J Refract Surg 4:5–8, 1988 10. Cotliar AM, Schubert HD, Mandel ER, et al: Excimer laser radial keratotomy. Ophthalmology 92:206, 1985 11. Marshall I, Trokel SL, Rothery S, Schubert H: An ultrastructural study of corneal incisions induced by an excimer laser
at 193 nm. Ophthalmology 92:749–758, 1985 12. McDonald MB, Frantz JM, Klyce SD, et al: Central photorefractive keratectomy for myopia: The blind eye study. Arch Ophthalmol 108:799–808, 1990 13. Seiler T, Kahle G, Kriegerowski M: Excimer laser (193 nm) myopic keratomileusis in sighted and blind
human eyes. Refract Corneal Surg 6:165–173, 1990 14. McDonald MB, Liu JC, Andrade H, et al: Clinical results of 193 nm excimer laser central photorefractive keratectomy
for myopia: The partially sighted and sighted eye studies. Invest Ophthalmol Vis Sci 31:S245, 1990 15. Taylor DM, L'Esperance RA, Del Pero RA, el al.: Human excimer laser lamellar keratectomy: A clinical study. Ophthalmology 96:654–664, 1989 16. Liu JC, McDonald MB, Vernell R, et al: Myopic excimer laser photorefractive keratectomy: An analysis of clinical
correlations. Refract Corneal Surg 6:321–328, 1990 17. Fantes FE, Hanna KD, Waring GO, et al: Wound healing after excimer laser keratomileusis (photorefractive
keratectomy) in monkeys. Arch Ophthalmol 108:665–675, 1990 18. Malley DS, Steinert RF, Puliafito CA, Dobi ET: Immunofluorescence study of corneal wound healing after excimer laser anterior
keratectomy in the monkey eye. Arch Ophthalmol 108:1316–1322, 1990 19. Hanna KD, Pouliquen YM, Waring GO, et al: Corneal wound healing in monkeys after repeated excimer laser photorefractive
keratectomy. Arch Ophthalmol 109:1291–1296, 1992 20. Hanna KD, Pouliquen YM, Salvodelli M, et al: Corneal wound healing in monkeys 18 months after excimer laser photorefractive
keratectomy. Refract Corneal Surg 6:340–345, 1990 21. Serdarevic ON: Excimer laser therapy for experimental candida keratitis. Am J Ophthalmol 99:534, 1985 22. SunderRaj N, Geiss MJ, Fantes F, et al: Healing of excimer laser ablated monkey corneas: An immunohistochemical
evaluation. Arch Ophthalmol 108:1604–1610, 1990 23. Hanna KD, Chastang JC, Asfar L, et al: Scanning slit delivery system. J Cataract Refract Surg 15:390–396, 1989 24. Caro RG, Muller DF: A medical excimer laser system for corneal surgery and laser angioplasty. Lasers Med SPIE 712:95–98, 1986 25. Yoder PR, Telfair WB, Warner JW, Martin CA: Application of the excimer laser to area recontouring of the cornea. Excimer Lasers Appl SPIE 1023:260–267, 1988 26. Munnerlyn CR, Koons SJ, Marshall L: Photorefractive keratectomy: a technique for laser refractive surgery. J Cataract Refract Surg 14:46–52, 1988 27. McDonald MB, Kaufman HE, Frantz JM, et al: Excimer laser ablation in a human eye. Arch Ophthalmol 107:641–642, 1989 28. Duffey RJ, Leaming D: Trends in refractive surgery in the United States. J Cataract Refract Surg 30:1781–1785, 2004 29. Seiler T, Wollensak J: Complications of laser keratomileusis with the excimer laser (193nm). Klin Monatsbl Augenheilkd 200:648–653, 1992 30. Seiler T, Holschbach A, Derse M, et al: Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology 101:153–160, 1994 31. Cua IY, Pepose JS: Late corneal scarring after photorefractive keratectomy concurrent with
development of systemic lupus erythematosis. J Refract Surg 18:750–752, 2002 32. Ambrosio R Jr, Klyce SD, Wilson SE: Corneal topographic and pachymetric screening of keratorefractive patients. J Refract Surg 19:24–29, 2003 33. Sun R, Gimbel HV, Kaye GB: Photorefractive keratectomy in keratoconus suspects. J Cataract Refract Surg 25:1461–1466, 1999 34. Terrell J, Bechara SJ, Nesburn A, et al: The effect of globe fixation on ablation zone centration in photorefractive
keratectomy. Am J Ophthalmol 119:612–619, 1995 35. Campos M, Hertzog L, Wand XW, et al: Corneal surface after deepithelialization using a sharp and a dull instrument. Ophthalmic Surg 23:618–621, 1992 36. Pallikaris IG, Karoutis AD, Lydataki SE, Siganos DS: Rotating brush for fast removal of corneal epithelium. J Refract Corneal Surg 10:439–442, 1994 37. Shah S, Doyle SJ, Chatterjee A, et al: Comparison of 18% ethanol and mechanical debridement for epithelial
removal before photorefractive keratectomy. J Refract Surg 14:S212– S214, 1998 38. Carones F, Fiore T, Brancato R: Mechanical vs alcohol epithelial removal during photorefractive keratectomy. J Refract Surg 15:556–562, 1999 39. Abad JC, An B, Power WJ, et al: A prospective evaluation of alcohol-assisted versus mechanical epithelial
removal before photorefractive keratectomy. Ophthalmology 104:1566–1574, 1997 40. Kapadia MS, Meisler DM, Wilson SE: Epithelial removal with the excimer laser (laser-scrape) in photorefractive
keratectomy retreatment. Ophthalmology 106:29–34, 1999 41. Dougherty PJ, Wellish KL, Maloney RK: Excimer laser ablation rate and corneal hydration. Am J Ophthalmol 118:169–176, 1994 42. Camellin M: Laser epithelial keratomileusis for myopia. J Refract Surg 19:666–667, 2003 43. Taneri S, Zieske JD, Azar DT: Evolution, techniques, clinical outcomes, and pathophysiology of LASEK: a
review of the literature. Surv Ophthalmol 49:576–560, 2004 44. Jozato H, Guyton DL: Centering corneal surgical procedures. Am J Ophthalmol 103:263–275, 1987 45. Cavanaugh TB, Durrie DS, Riedel SM, et al: Topographical analysis of the centration of excimer laser photorefractive
keratectomy. J Cataract Refract Surg 19:136–143, 1993 46. Chalita MR, Roth AS, Krueger RR: Wavefront guided surface ablation with prophylactic use of mitomycin C
after a buttonhole laser in situ keratomileusis flap. J Refract Surg 20:176–178, 2004 47. Sher NA, Frantz JM, Talley A, et al: Topical diclofenac in the treatment of ocular pain after excimer photorefractive
keratectomy. Refract Corneal Surg 9:425–436, 1993 48. Arshinoff S, D'Addario D, Sadler C, et al: Use of nonsteroidal anti-inflammatory drugs in excimer laser photorefractive
keratectomy. J Cataract Refract Surg 20:S216– S222, 1994 49. Tutton MK, Cherry PM, Raj PS, Fsadni MG: Efficacy and safety of topical diclofenac in reducing ocular pain after
excimer photorefractive keratectomy. J Cataract Refract Surg 22:536–541, 1996 50. Assouline M, Renard G, Arne JL, et al: A prospective randomized trial of topical soluble 0.1% indomethacin
versus 0.1% diclofenac versus placebo for the control of pain
following excimer laser photorefractive keratectomy. Ophthalmic Surg Lasers 29:365–374, 1998 51. Forster W, Ratkay I, Krueger R, Busse H: Topical diclofenac sodium after excimer laser phototherapeutic keratectomy. J Refract Surg 13:311–313, 1997 52. Verma S, Marshall J: Control of pain after photorefractive keratectomy. J Refract Surg 12:358–364, 1996 53. Cherry PM: The treatment of pain following excimer laser photorefractive keratectomy: Additive
effect of local anesthetic drops, topical diclofenac, and
bandage soft contact. Ophthalmic Surg Lasers 27:S477– S480, 1996 54. Shahinian L Jr, Jain S, Jager RD, et al: Dilute topical proparacaine for pain relief after photorefractive keratectomy. Ophthalmology 104:1327–1332, 1997 55. Ar Ozdamar A, Aktunc R, Ercikan C: The effects of topical steroids on refractive outcome and corneal haze, thickness, and
curvature after photorefractive keratectomy with a 6.0-mm
ablation diameter. Ophthalmic Surg Lasers 29:621–627, 1998 56. Gartry DS, Kerr-Muir MG, Marshall J: The effect of topical corticosteroids on refraction and corneal haze following
excimer laser treatment of myopia: an update. A prospective, randomized, double-masked
study. Eye 7:584–590, 1993 57. O'Brart DP, Lohmann CP, Klonos G, et al: The effects of topical corticosteroids and plasmin inhibitors on refractive
outcome, haze, and visual performance after photorefractive keratectomy. A
prospective, randomized, observer-masked study. Ophthalmology 101:1565–1574, 1994 58. Corbett MC, O'Brart DP, Marshall J: Do topical corticosteroids have a role following excimer laser photorefractive
keratectomy? J Refract Surg 11:380–387, 1995 59. Baek SH, Chang JH, Choi SY, Ki Lee JH: The effect of topical corticosteroids on refractive outcome and corneal
haze after photorefractive keratectomy. J Refract Surg 13:644–652, 1997 60. Choi SY, Kim HY, Kim JY, et al: Two-year follow-up of eyes without topical corticosteroid treatment after
photorefractive keratectomy (PRK). Korean J Ophthalmol 12:25–29, 1998 61. Chalita MR, Roth AS, Krueger RR: Wavefront guided surface ablation with prophylactic use of mitomycin C
after a buttonhole laser in situ keratomileusis flap. J Refract Surg 20:176–178, 2004 62. Hersh PS, Stulting RD, Steinert RF, et al: Results of phase III excimer laser photorefractive keratectomy for myopia. Ophthalmology 104:1535–1553, 1997 63. Seiler T, McDonnell PJ: Excimer laser photorefractive keratectomy. Surv Ophthamol 40:89–118, 1995 64. Dutt S, Steinert RF, Raizman MB, et al: One-year results of excimer laser photorefractive keratectomy for low to
moderate myopia. Arch Ophthalmol 112:1427–1436, 1994 65. McCarty C, Alfred G, Taylor H, et al: Comparison of results of excimer laser correction of all degrees of myopia
at 12 months postoperatively. Am J Ophthalmol 121:372–383, 1996 66. Tuunanen TH, Tervo TT: Results of photorefractive keratectomy for low, moderate, and high myopia. J Refract Surg 14:437–446, 1998 67. Rajan MS, Jaycock P, O'Brart D, et al: A long-term study of photorefractive keratectomy 12-year follow-up. Ophthalmology 111:1799–1800, 2004 68. Hashemi H, Taheri SM, Fotouhi A, Kheiltash A: Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation
after photorefractive keratectomy in high myopia: A prospective clinical
study. BMC Ophthalmol 4:12, 2004 69. Cennamo G, Rosa N, Breve MA, di Grazia M: Technical improvements in photorefractive keratectomy for the correction
of high myopia. J Refract Surg 19:438–442, 2003 70. Steinert RF, Hersh PS: Spherical and aspherical photorefractive keratectomy and laser in-situ
keratomileusis for moderate to high myopia: two prospective, randomized
clinical trials. Summit Technology PRK-LASIK Study Group. Trans Am Ophthalmol Soc 96:197–221, 1998 71. Schipper I, Senn P, Wienecke L, Oyo-Szerenyi KD: Photoastigmatic refractive keratectomy for primary treatment and revision
of myopic astigmatism. J Cataract Refract Surg 23:1465–1471, 1997 72. Goggin MJ, Kenna PF, Lavery FL: Photoastigmatic refractive keratectomy for compound myopic astigmatism
with a Nidek laser. J Refract Surg 13:162–166, 1997 73. Zad Haviv D, Vishnevskia-Dai V, Morad Y, et al: Excimer laser photoastigmatic refractive keratectomy: eighteen-month follow-up. Ophthalmology 105:620–623, 1998 74. Febbraro JL, Aron-Rosa D, Gross M, et al: One year clinical results of photoastigmatic refractive keratectomy for
compound myopic astigmatism. J Cataract Refract Surg 25:911–920, 1999 75. Dausch D, Dausch S, Schroder E: Wavefront-supported photorefractive keratectomy: 12 month follow-up. J Refract Surg 19:405–411, 2003 76. Anshutz T: Laser correction of hyperopia and presbyopia. Int Ophthalmol Clin 34:107–137, 1994 77. Dausch D, Klein R, Schroder E: Excimer laser photorefractive keratectomy for hyperopia. J Refract Corneal Surg 9:20–28, 1993 78. Jackson WB, Casson E, Hodge WG, et al: Laser vision correction for low hyperopia. An 18-month assessment of safety
and efficacy. Ophthalmology 105:1727–1738, 1998 79. Sener B, Ozdamar A, Aras C, Yanyali A: Photorefractive keratotectomy for hyperopia and aphakia with a scanning
spot excimer laser. J Refract Surg 13:620–623, 1997 80. Vinciguerra P, Epstein D, Radice P, Azzolini M: Long-term results of photorefractive keratectomy for hyperopia and hyperopic
astigmatism. J Refract Surg 14:S183– S185, 1998 81. Pirouzian A, Thornton JA, Ngo S: A randomized prosective clinical trial comparing laser subepithelial keratomileusis
and photorefractive keratectomy. Arch Ophthalmol 122:11–16, 2004 82. Hashemi H, Fotouhi A, Foudazi H, et al: prospective, randomized, paired comparison of laser epithelial keratomileusis
and photorefractive keratectomy for myopia less than –6.50 diopters. J Refract Surg 20:217–212, 2004 83. Kaya V, Oncel B, Sivrikaya H, Yilmaz OF: Prosepective, paired comparison of laser in situ keratomileusis and laser
pithelial keratomileusis for myopia less than –6.00 diopters. J Refract Surg 20:223–228, 2004 84. Buzzonetti L, Iarossi G, Valente P, et al: Comparison of wavefront aberration changes in the anterior corneal surface
after laser-assisted subepithelial keratectomy and laser in situ keratomileusis: A
preliminary study. J Cataract Refract Surg 30:1929–1923, 2004 85. Kim JK, Kim SS, Lee HK, et al: Laser in situ keratomileusis versus laser-assisted subepithelial keratectomy
for the correction of high myopia. J Cataract Refract Surg 30:1405–1411, 2004 86. McGhee CNJ, Bryce IG: Natural history of central topographic islands following excimer laser
photorefractive keratectomy. J Cataract Refract Surg 22:1151–1158, 1996 87. Krueger RR, Saedy NF, McDonnell PJ: Clinical analysis of steep central islands after excimer laser photorefractive
keratectomy. Arch Ophthalmol 114:377–381, 1996 88. Levin S, Carson CA, Garrett SK, Taylor HR: Prevalence of central islands after excimer laser refractive surgery. J Cataract Refract Surg 21:21–26, 1995 89. Abbas UL, Hersh PS: Early corneal topography patterns after excimer laser photorefractive keratectomy
for myopia. J Refract Surg 15:124–31, 1999 90. Bor Z, Hopp B, Racz B, et al: Plume emission, shock wave and surface wave formation during excimer laser
ablation of the cornea. Refract Corneal Surg 9:S111–S115, 1993 91. Kreuger RR, Krasinski JS, Radzewicz C, et al: Photography of shock waves during excimer laser ablation of the cornea: Effect
of helium gas on propagation velocity. Cornea 12:330–334, 1993 92. Puliafito CA, Stern D, Krueger RR, Mandel ER: High-speed photography of excimer laser ablation of the cornea. Arch Ophthalmol 105:1255–1259, 1987 93. Campos M, Cuevas K, Garbus J, et al: Corneal wound healing after excimer laser ablation: Effects of nitrogen
gas blower. Ophthalmology 99:893–897, 1992 94. Itoi M, Ryan R, Depaolis M, Aquavella JV: Clinical effect of blowing nitrogen gas across the cornea during photorefractive
keratectomy. J Refract Surg 13:69–73, 1997 95. Noack J, Tonnies R, Hohla K, et al: Influence of ablation plume dynamics on the formation of central islands
in excimer laser photorefractive keratectomy. Ophthalmology 104:823–830, 1997 96. Oshika T, Klyce SD, Smolek MK, McDonald MB: Corneal hydration and central islands after excimer laser photorefractive
keratectomy. J Cataract Refract Surg 24:1575–1580, 1998 97. Lin DT: Corneal topographic analysis after excimer photorefractive keratectomy. Ophthalmology 101:1432–1439, 1994 98. Ambrosio G, Cennamo G, De Marco R, et al: Visual function before and after photorefractive keratectomy for myopia. J Refract Corneal Surg 10:129–136, 1994 99. O'Brart DP, Lohmann CP, Fitzke FW, et al: Night vision after excimer laser photorefractive keratectomy: Haze and
halos. Eur J Ophthalmol 4:43–51, 1994 100. Verdon W, Bullimore M, Maloney RK: Visual performance after photorefractive keratectomy. A prospective study. Arch Ophthalmol 114:1465–1472, 1996 101. O'Brart DP, Corbett MC, Lohmann CP, et al: The effects of ablation diameter on the outcome of excimer laser photorefractive
keratectomy. A prospective, randomized, double-blind study. Arch Ophthalmol 113:438–443, 1995 102. O'Brart DP, Corbett MC, Verma S, et al: Effects of ablation diameter, depth, and edge contour on the outcome of
photorefractive keratectomy. J Refract Surg 12:50–60, 1996 103. O'Brart DP, Gartry DS, Lohmann CP, et al: Excimer laser photorefractive keratectomy for myopia: Comparison of 4.00- and 5.00-millimeter
ablation zones. J Refract Corneal Surg 10:87–94, 1994 104. Seiler T, Holschbach A, Derse M, et al: Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology 101:153–160, 1994 105. Niesen U, Businger U, Hartmann P, et al: Glare sensitivity and visual acuity after excimer laser photorefractive
keratectomy for myopia. Br J Ophthalmol 81:136–140, 1997 106. Schwartz-Goldstein BH, Hersh PS: Corneal topography of phase III excimer laser photorefractive keratectomy: Optical
zone centration analysis. Ophthalmology 102:951–962, 1995 107. Cantera E, Cantera I, Olivieri L: Corneal topographic analysis of photorefractive keratectomy in 175 myopic
eyes. Refract Corneal Surg 9:S19–S22, 1993 108. Schwartz-Goldstein BH, Hersh PS: Corneal topography of phase III excimer laser photorefractive keratectomy: Optical
zone centration analysis. Ophthalmology 102:951–962, 1995 109. Balestrazzi E, De Mofetta V, Spadea L, et al: Histological, immunohistochemical, and ultrastructural findings in human
corneas after photorefractive keratectomy. J Refract Surg 11:181–187, 1995 110. Lohmann CP, MacRobert I, Patmore A, et al: A histopathological study of photorefractive keratectomy. Lasers Light Ophthalmol 6:149–158, 1994 111. Fagerholm P, Hamberg-Nystrom H, Tengroth B: Wound healing and myopic regression following photorefractive keratectomy. Acta Ophthalmol 72:229–223, 1994 112. L'Esperance FA, Taylor DM, Warner JW: Human excimer laser keratectomy. Short-term histopathology. J Refract Surg 4:118–112, 1988 113. Lohmann C, Gartry D, Kerr Muir M, et al: ‘Haze’ in photorefractive keratectomy: Its origins and consequences. Lasers Light Ophthalmol 4:15–13, 1991 114. Corbett MC, Prydall JI, Verma S, et al: An in vivo investigation of the structures responsible for corneal haze
after photorefractive keratectomy and their effect on visual function. Ophthalmology 103:1366–1368, 1996 115. Moller-Pedersen T, Vogel M, Li HF, et al: Quantification of stromal thinning, epithelial thickness, and corneal haze
after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology 104:360–336, 1997 116. Corbett MC, Prydall JI, Verma S, et al: An in vivo investigation of the structures responsible for corneal haze
after photorefractive keratectomy and their effect on visual function. Ophthalmology 103:1366–1368, 1996 117. Tengroth B, Epstein D, Fagerholm P, et al: Excimer laser photorefractive keratectomy for myopia: Clinical results
in sighted eyes. Ophthalmology 100:739–745, 1993 118. Salz JI, Maguen E, Nesburn AB, et al: A two-year experience with excimer laser photorefractive keratectomy for
myopia. Ophthalmology 100:873–882, 1993 119. Gartry DS, Ker Muir MG, Marshall J: Photorefractive keratectomy with an argon fluoride excimer laser: A clinical
study. Refract Corneal Surg 7:420–343, 1991 120. Caubet E: Cause of subepithelial corneal haze over 18 months after photorefractive
keratectomy for myopia. Refract Corneal Surg 9:S65–S67, 1993 121. Taylor HR, McCarty CA, Aldred GR: Predictability of excimer laser treatment of myopia. Arch Ophthalmol 114:248–225, 1996 122. Seiler T, Holschbach A, Derse M, et al: Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology 101:153–160, 1994 123. Braunstein RE, Jain S, McCally RL, et al: Objective measurement of corneal light scattering after excimer laser keratectomy. Ophthalmology 103:439–444, 1996 124. Corbett MC, Verma S, O'Brart DPS, et al: The effect of ablation profile on wound healing and visual performance
one year after excimer laser PRK. Br J Ophthalmol 80:224–223, 1996 125. Nagy ZZ, Hiscott P, Seitz B, et al: Ultraviolet-B enhances corneal stromal response to 193-nm excimer laser
treatment. Ophthalmology 104:375–338, 1997 126. Meyer JC, Stulting RD, Thompson KP, Durrie DS: Late onset of corneal scar after excimer laser photorefractive keratectomy. Am J Ophthalmol 121:529–523, 1996 127. Lipshitz I, Loewenstein A, Varssano D, Lazar M: Late onset corneal haze after photorefractive keratectomy for moderate
and high myopia. Ophthalmology 104:369–367, 1997 128. Kuo IC, Lee SM, Hwang DG: Late-onset corneal haze and myopic regression after photorefractive keratectomy (PRK). Cornea 23:350–355, 2004 129. Marques EF, Leite EB, Cunha-Vaz JG: Corticosteroids for reversal of myopic regression after photorefractive
keratectomy. J Refract Surg 11:S30, 1995 130. Tanabe T, Miyata K, Samejima T, et al: Influence of wavefront aberration and corneal subepithelial haze on low-contrast
visual acuity after photorefractive keratectomy. Am J Ophthalmol 138:620–624, 2004 131. Dutt S, Steinert RF, Raizman MB, et al: One-year results of excimer laser photorefractive keratectomy for low to
moderate myopia. Arch Ophthalmol 112:1427–1436, 1994 132. Hersh PS, Schein OD, Steinert R: Characteristics influencing outcomes of excimer laser photorefractive keratectomy. Summit
Photorefractive Keratectomy Phase III Study Group. Ophthalmology 103:1962–1969, 1996 133. Loewenstein A, Lipshitz I, Levanon D, et al: Influence of patient age on photorefractive keratectomy for myopia. J Refract Surg 13:23–22, 1997 134. Durrie DS, Lesher MP, Hunkeler JD: Treatment of overcorrection after myopic photorefractive keratectomy. J Refract Corneal Surg 10:S29, 1994 135. Cherry PM: Removal of epithelium and scraping the underlying stroma as treatment for
photorefractive keratectomy overcorrection or undercorrection of myopia. Ophthalmic Surg Lasers 27:S487–S4879, 1996 136. Kim JH, Sah WJ, Park CK, et al: Myopic regression after photorefractive keratectomy. Ophthalmic Surg Lasers 27:S435–S439, 1996 137. Corbett MC, O'Brart DP, Warburton FG, Marshall J: Biologic and environmental risk factors for regression after photorefractive
keratectomy. Ophthalmology 103:1381–1391, 1996 138. Taylor HR, McCarty CA, Aldred GF: Predictability of excimer laser treatment of myopia. Arch Ophthalmol 114:248–251, 1996 139. O'Brart DP, Corbett MC, Lohmann CP, et al: The effects of ablation diameter on the outcome of excimer laser photorefractive
keratectomy. A prospective, randomized, double-blind study. Arch Ophthalmol 113:438–443, 1995 140. O'Brart DP, Corbett MC, Verma S, et al: Effects of ablation diameter, depth, and edge contour on the outcome of
photorefractive keratectomy. J Refract Surg 12:50–60, 1996 141. O'Brart DP, Gartry DS, Lohmann CP, et al: Excimer laser photorefractive keratectomy for myopia: Comparison of 4.00- and 5.00-millimeter
ablation zones. J Refract Corneal Surg 10:87–94, 1994 142. Seiler T, Holschbach A, Derse M, et al: Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology 101:153–160, 1994 143. Shah SI, Hersh PS: Photorefractive keratectomy for myopia with a 6-mm beam diameter. J Refract Surg 12:341–346, 1996 144. Maguire LJ, Bechara S: Epithelial distortions at the ablation zone margin after excimer laser
photorefractive keratectomy for myopia. Am J Ophthalmol 117:809–810, 1994 145. Epstein D, Tengroth B, Fagerholm P, Hamberg-Nystrom H: Excimer retreatment of regression after photorefractive keratectomy. Am J Ophthalmol 117:456–461, 1994 146. Sutton G, Kalski RS, Lawless MA, Rogers C: Excimer retreatment for scarring and regression after photorefractive keratectomy
for myopia. Br J Ophthalmol 79:756–759, 1995 147. Snibson GR, McCarty CA, Aldred GR, et al: Retreatment after excimer laser photorefractive keratectomy. Am J Ophthalmol 121:250–257, 1996 148. Matta CA, Piebenga LW, Deitz MR, Tauber J: Excimer retreatment for myopic photorefractive keratectomy failures. Six
to 18-month follow-up. Ophthalmology 103:444–451, 1996 149. Hefetz L, Krakowski D, Haviv D, et al: Results of a repeated excimer laser photorefractive keratectomy for myopia. Ophthalmic Surg Lasers 28:8–13, 1997 150. Loewenstein A, Lipshitz I, Varssano D, Lazar M: Excimer laser reablation. Ophthalmic Surg Lasers 28:282–287, 1997 151. Pop M, Aras M: Photorefractive keratectomy retreatments for regression. One year follow
up. Ophthalmology 103:1979–1984, 1996 152. Rozsival P, Feuermannova A: Retreatment after photorefractive keratectomy for low myopia. Ophthalmology 105:1189–1193, 1998 153. Chatterjee A, Shah S, Bessant DA, Doyle SJ: Results of excimer laser retreatment of residual myopia after previous
photorefractive keratectomy. Ophthalmology 104:1321–1326, 1997 154. Gartry DS, Larkin FP, Hill AR, et al: Retreatment for significant regression after excimer laser photorefractive
keratectomy. Ophthalmology 105:131–141, 1998 155. Pop M, Aras M: Photorefractive keratectomy retreatments for regression. One year follow
up. Ophthalmology 103:1979–1984, 1996 156. Gartry DS, Larkin FP, Hill AR, et al: Retreatment for significant regression after excimer laser photorefractive
keratectomy. Ophthalmology 105:131–141, 1998 157. Teal P, Breslin C, Arshinoff S, Edmison D: Corneal subepithelial infiltrates following excimer laser photorefractive
keratectomy. J Cataract Refract Surg 21:516–518, 1995 158. Sher NA, Frantz JM, Talley A, et al: Topical diclofenac in the treatment of ocular pain after excimer photorefractive
keratectomy. Refract Corneal Surg 9: 425–436, 1993 159. Sampath R, Ridgway AE, Leatherbarrow B: Bacterial keratitis following excimer laser photorefractive keratectomy: A
case report. Eye 8:481–482, 1994 160. Gartry DS, Ker Muir MG, Marshall J: Photorefractive keratectomy with an argon fluoride excimer laser: A clinical
study. Refract Corneal Surg 7:420–443, 1991 161. Brancato R, Tavola A, Carones F, et al: Excimer laser photorefractive keratectomy for myopia: Results in 1165 eyes. Refract Corneal Surg 9:95–104, 1993 162. Gartry DS, Kerr-Muir MG, Marshall J: The effect of topical corticosteroids on refraction and corneal haze following
excimer laser treatment of myopia: An update. A prospective, randomized, double-masked
study. Eye 7:584–590, 1993 163. Chatterjee A, Shah S, Bessant DA, et al: Reduction in intraocular pressure after excimer laser photorefractive keratectomy. Correlation
with pretreatment myopia. Ophthalmology 104:355–359, 1997 164. Abbasoglu OE, Bowman RW, Cavanagh HD, McCulley JP: Reliability of intraocular pressure measurements after myopic excimer photorefractive
keratectomy. Ophthalmology 105:2193–2196, 1998 165. Mardelli PG, Piebenga LW, Matta CS, et al: Corneal endothelial status 12 to 55 months after excimer laser photorefractive
keratectomy. Ophthalmology 102:544–549, 1995 166. Carones F, Brancato R, Venturi E, Morico A: The corneal endothelium after myopic excimer laser photorefractive keratectomy. Arch Ophthalmol 112:920–924, 1994 167. Stulting RD, Thompson KP, Waring GO III, Lynn M: The effect of photorefractive keratectomy on the corneal endothelium. Ophthalmology 103:1357–1365, 1996 168. Trocme SD, Mack KA, Gill KS, et al: Central and peripheral endothelial cell changes after excimer laser photorefractive
keratectomy for myopia. Arch Ophthalmol 114:925–928, 1996 169. Cennamo G, Rosa N, Del Prete A, et al: The corneal endothelium 12 months after photorefractive keratectomy in
high myopia. Acta Ophthalmol Scand 75:128–130, 1997 170. Kent DG, Solomon KD, Peng Q, et al: Effect of surface photorefractive keratectomy and laser in situ keratomileusis
on the corneal endothelium. J Cataract Refract Surg 23:386–397, 1997 171. Stulting RD, Thompson KP, Waring GO III, Lynn M: The effect of photorefractive keratectomy on the corneal endothelium. Ophthalmology 103:1357–1365, 1996 |