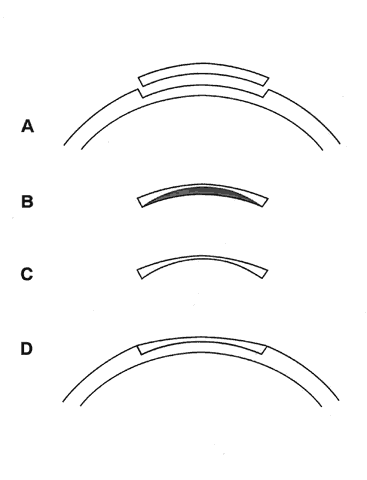
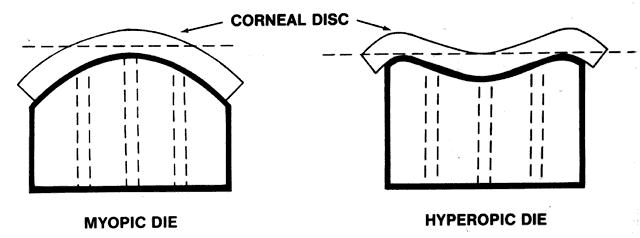
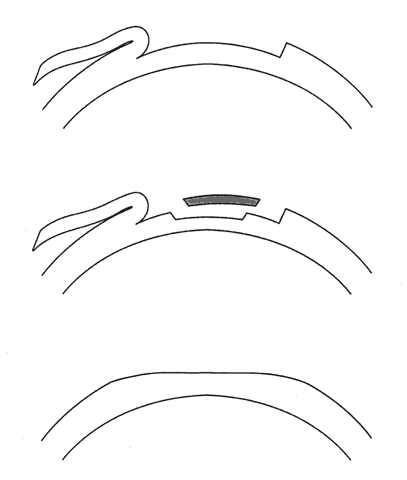
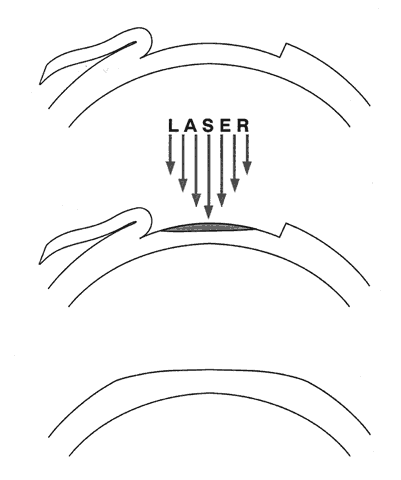
1. Ainslie D: The surgical correction of refractive errors by keratomileusis and keratophakia. Am J Ophthalmol 8:349, 1976 2. Arenas-Archila E, Sanchez-Thorin JC, Naranjo-Uribe JP et al: Myopic keratomileusis in situ: A preliminary report. J Cataract Refract Surg 17:424, 1991 3. Barker BA, Swinger CA: Keratophakia and keratomileusis. Int Ophthalmol Clin 28:126, 1988 4. Barraquer C, Gutierrez AM, Espinosa A: Myopic keratomileusis: Short-term results. Refract Corneal Surg 5:307, 1989 5. Barraquer JI: Queratomileusis para la correccion de la myopia. Arch Soc Am Oftalmol Optom 5:25, 1964 6. Barraquer JI: Keratomileusis for myopia and aphakia. Ophthalmology 88:701, 1981 7. Barraquer JI: Keratomileusis for the correction of myopia. Arch Soc Am Oftalmol Optom 16:221, 1982 8. Barraquer JI: Long term results of myopic keratomileusis--1982. Arch Soc Am Oftalmol Optom 19:137, 1983 9. Barraquer JI, Viteri E: Results of myopic keratomileusis. J Refractive Surg 3:98, 1987 10. Bas AM, Nano HD Jr: In situ myopic keratomileusis results in 30 eyes at 15 months. Refract Corneal Surg 7:223, 1991 11. Bosc JM, Montard M, Delbosc B et al: Non-freeze myopic keratomileusis. Retrospective study of 27 consecutive
operations. J Fr Ophthalmol 13:10, 1990 12. Buratto L, Ferrari M, Genisi C: Myopic keratomileusis with the excimer laser: One-year follow up. Refract Corneal Surg 9:12, 1993 13. Casebeer JC, Slade SG, Dybbs A et al: Intraoperative pachometry during automated lamellar keratoplasty: A preliminary
report. Refract Corneal Surg 10:41, 1994 14. Dossi F, Bosio P: Myopic keratomileusis: Results with a follow-up over one year. J Cataract Refract Surg 13:417, 1987 15. Durand L, Burillon C, Resal R: Refractive surgery and the non-freeze BKS set. Reliability of our Lyon
methods. Bull Soc Ophtalmol Fr 90:441, 1990 16. Ganem S, Aron-Rosa D, Gross M et al: Myopic keratomileusis by excimer laser on a lathe. J Refract Corneal Surg 10:575, 1994 17. Gomes M: Keratomileusis-in-situ using manual dissection of corneal flap for high
myopia. J Refract Corneal Surg 10(suppl):S255, 1994 18. Hoffman RF, Bechara SJ: An independent evaluation of second generation suction microkeratomes. Refract Corneal Surg 8:348, 1992 19. Kornmehl EW, Swinger CA, Pugh W et al: Corneascope evaluation of myopic keratomileusis (ARVO abstracts). Invest Ophthalmol Vis Sci 26(suppl):283, 1985 20. Krawicz T: Lamellar corneal stromectomy. Am J Ophthalmol 57:828, 1964 21. Krumeich JH: Indications, techniques, and complications of myopic keratomileusis. Int Ophthalmol Clin 23:75, 1983 22. Maxwell WA: Myopic keratomileusis: Initial results and myopic keratomileusis combined
with other procedures. J Cataract Refract Surg 13:518, 1987 23. Maxwell WA, Nordan LT: Myopic keratomileusis: Early experience. J Refract Surg 1:124, 1986 24. Neumann AC, McCarty G, Sanders DR: Delayed regression of effect in myopic epikeratophakia vs myopic keratomileusis
for high myopia. Refract Corneal Surg 5:161, 1989 25. Nordan LT, Fallor MK: Myopic keratomileusis: 74 consecutive non-amblyopic cases with one year
follow-up. J Refract Surg 2:124, 1986 26. Pallikaris IG, Papatzanaki ME, Siganos DS et al: A corneal flap technique for laser in situ keratomileusis. Human studies. Arch Ophthalmol 109:1699, 1991 27. Pallikaris IG, Siganos DS: Excimer laser in situ keratomileusis and photorefractive keratectomy for
correction of high myopia. J Refract Corneal Surg 10:498, 1994 28. Peyman GA: A method for modifying corneal curvature. U.S Patent 4,840,175, June 20, 1989 29. Polit F, Ibrahim O, El-Maghraby A et al: Cryolathe keratomileusis for correction of myopia of 4.00 to 8.00 diopters. Refract Corneal Surg 9:259, 1993 30. Ruiz LA, Rowsey JJ: A new refractive surgical approach: In situ keratomileusis for myopia and
lamellar keratoplasty for hyperopia. Ophthalmology 95(suppl):145, 1988 31. Stonecipher KG, Parmeley VC, Rowsey JJ et al: Refractive corneal surgery with the Draeger rotary microkeratome in human
cadaver eyes. J Refract Corneal Surg 10:49, 1994 32. Swinger CA, Barker BA: Prospective evaluation of myopic keratomileusis. Ophthalmology 91:785, 1984 33. Swinger CA, Barker BA: Myopic keratomileusis following radial keratotomy. J Refract Surg 1:53, 1985 34. Swinger CA, Barraquer JI: Keratophakia and keratomileusis: Clinical results. Ophthalmology 88:709, 1981 35. Swinger CA, Krumeich J, Cassiday D: Planar lamellar refractive keratoplasty. J Refract Surg 2:17, 1986 36. Swinger CA, Lai ST: Solid state photoablative decomposition--The Novatec
laser. In Salz JJ, McDonnell PJ, McDonald MB (eds): Corneal Laser Surgery, p 269. St. Louis, CV Mosby, 1995 37. Swinger CA, Villasenor RA: Homoplastic keratomileusis for the correction of myopia. J Refractive Surg 1:219, 1985 38. Troutman RC, Swinger CA: Refractive keratoplasty--Keratophakia and keratomileusis. Trans Am Ophthalmol Soc 76:329, 1978 39. Tucker DN, Barraquer JI: Refractive keratoplasty: Clinical results in sixty-seven cases. Am J Ophthalmol 5:335, 1973 40. Zavala EY, Krumeich J, Binder PS: Laboratory evaluation of freeze vs nonfreeze lamellar refractive keratoplasty. Arch Ophthalmol 105:1125, 1987 41. Swinger C, Cassidy D: Method and Apparatus for Modifying Corneal Buttons. U.S. patent 4,660,556, April 28, 1987 |