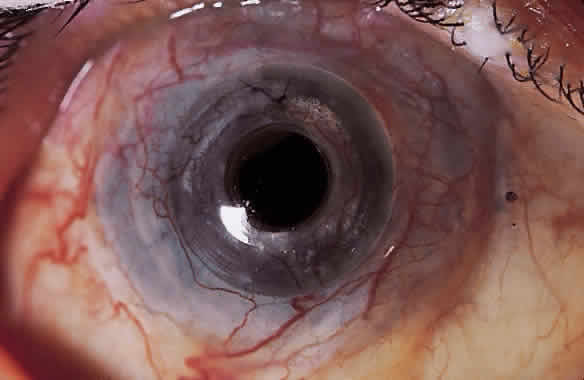
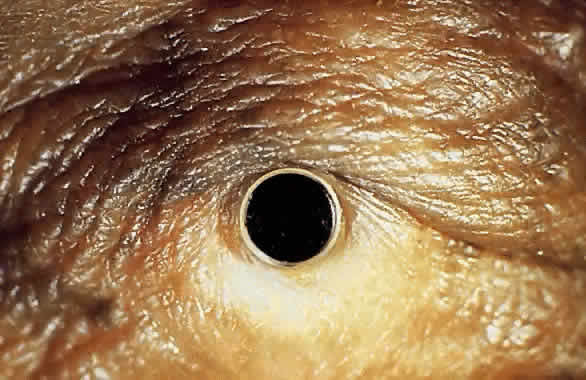
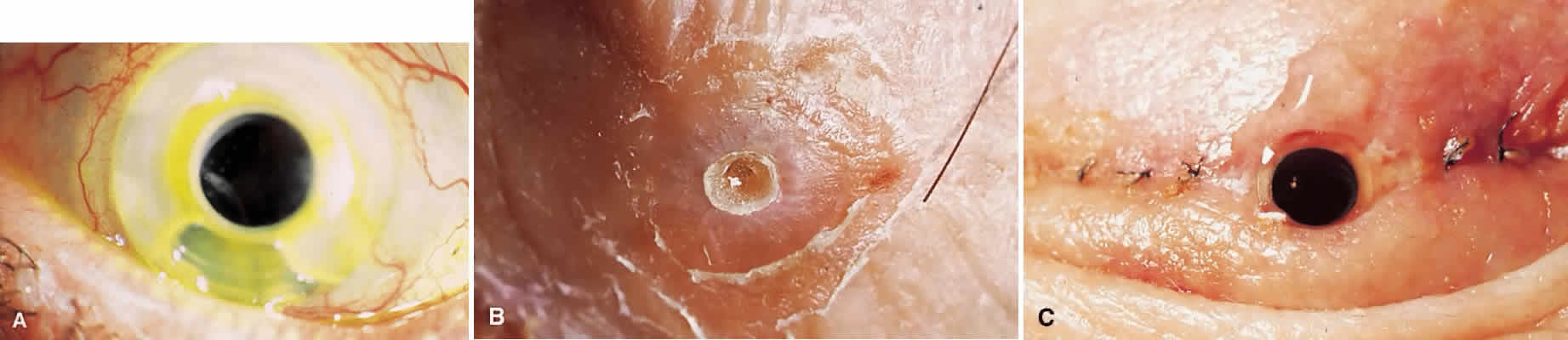
The material most frequently used for the central optical portion of the keratoprosthesis has been medical-grade PMMA. PMMA is completely transparent and biologically inert, and there is now extensive experience with its use in intraocular lenses. However, many different types of material have been used for the skirts or plates used to secure the optical stem in the cornea, such as perforated grids of PMMA, Teflon, nylon, and ceramics. More recently, attention has been directed to porous designs using materials such as Proplast,14 polytetrafluorethylene,15 hydrogels,16,17 collagen,18 and various copolymers.19,20 These materials have been manufactured and developed with the idea that colonization with natural tissue elements would subsequently hold and anchor the device more securely in place, thus decreasing the likelihood of future extrusion. In the 1960s, Strampelli21 introduced a novel idea of inserting the PMMA stem into a slice of dental bone from the patient's jaw, with the hope that the bone would subsequently heal better into the patient's cornea. This is, however, a formidable multistage procedure. DeVoe and Cardona22 made an important observation that in an extremely dry eye, if the prosthesis is allowed to protrude through the skin rather than between the lids, safety is enhanced and the extrusion rate is subsequently reduced. Considerable modifications to various keratoprosthesis designs have occurred over the years, but whether an ideal keratoprosthesis can ever be designed that will integrate into the human cornea without the risk of extrusion or necrosis has yet to be determined.
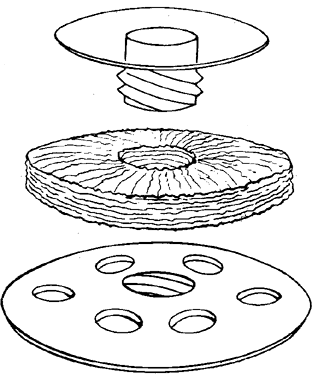
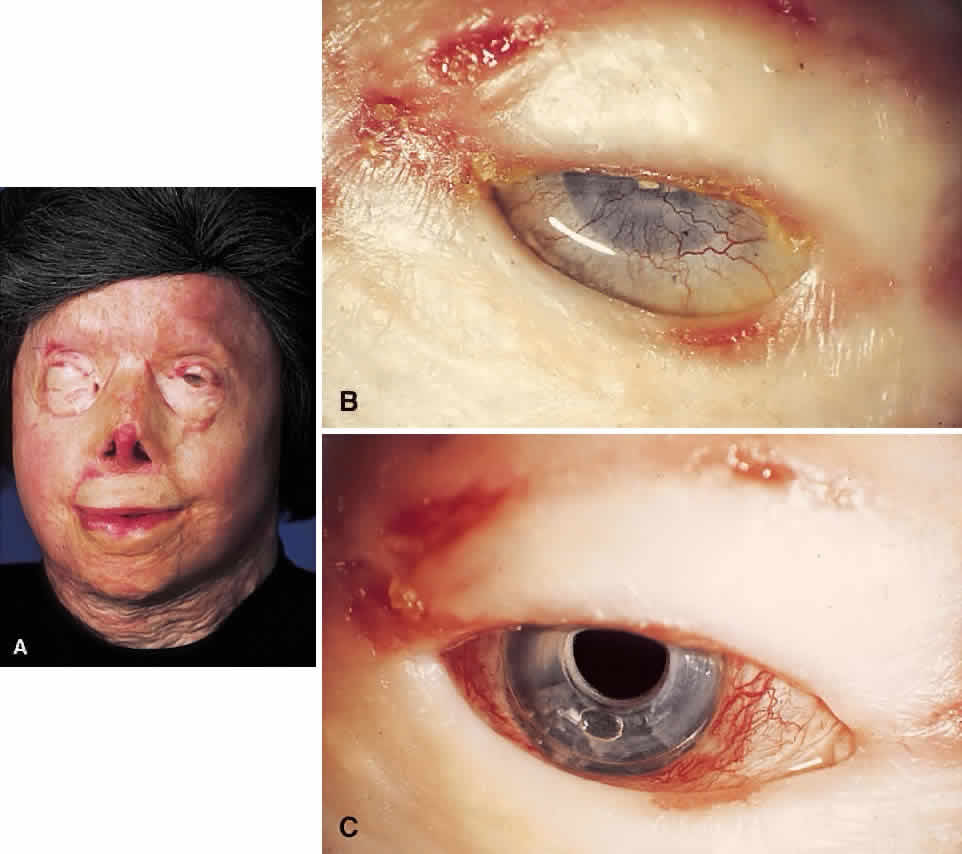
My practice is to use an all-PMMA device like that shown in Figure 1 (Dohlman-Doane Keratoprosthesis types I and II). The standard type I device is used in all but extremely dry eyes, where a “through-the-lid” approach with a type II device is preferred. In the latter case, the anterior nub is designed to protrude through an opening in the lid skin. The dimensions described for the two devices allow satisfactory visual fields. Thus, the type I permits a peripheral field of approximately 60 degrees; type II, with a longer stem, can give a visual field of approximately 40 degrees. The manufacture of these devices has previously been described.23 The Strampelli and Cardona devices are the other most frequently used models.