PREOPERATIVE MANAGEMENT
To determine the extent of injury, a meticulous preoperative assessment is crucial. A detailed history of the traumatic event should be taken. One must consider the presence of an intraocular foreign body. If a perforating injury is suspected, a rigid protective shield should be used to avoid any inadvertent pressure on the globe that may result in extrusion of intraocular contents.1 Radiologic testing is obtained as necessary, again stressing to all of those involved the importance of avoiding external pressure on the eye during the study. Orders are written to give the patient nothing by mouth in anticipation of surgical intervention. Antiemetics and analgesics are administered to avoid vomiting and agitation. Appropriate informed consent for the surgical procedure(s) anticipated should be obtained before administering any pain medications or sedatives. Immediate family members should be notified when possible.
The proper operating room staff should be assembled and surgery should be performed as soon as possible. Administration of broad-spectrum intravenous antibiotics is begun immediately. A combination of a cephalosporin (e.g., cefazolin) or vancomycin for gram-positive bacteria and an aminoglycoside (e.g., gentamicin) for gram-negative prophylaxis may be appropriate. Vancomycin may be preferable to a cephalosporin because of better coverage of Bacillus species and resistant Staphylococcus epidermidis.2 Clindamycin is added to this regimen if an intraocular foreign body is present, especially if the wound has been contaminated with vegetable matter, because such patients are prone to devastating Bacillus infections.2 Fluoroquinolone agents (e.g., levofloxacin), which have a broad spectrum of activity against gram-positive, gram-negative, and atypical bacteria, may be used as well. Subconjunctival antibiotic injections are avoided preoperatively because they may cause eyelid squeezing and could potentially deliver concentrated drug into the open globe. Similarly, topical antibiotics are avoided unless the wound is self-sealing. Although the risk of tetanus from an anterior segment injury is low, tetanus prophylaxis should be administered in accordance with the recommendations of the Centers for Disease Control and Prevention.3
During repair of corneoscleral lacerations, cultures should be taken from the wound margins, prolapsed and devitalized tissues, and intraocular foreign bodies if there is a high index of suspicion for infection. Specimens are inoculated directly on blood and chocolate agar plates, Sabouraud's dextrose medium for fungus, and fluid thioglycolate or cooked meat broth for anaerobes.
CONJUNCTIVAL LACERATIONS
With any conjunctival laceration, one should always consider the possibility of an occult globe rupture, laceration, or retained intraocular foreign body. A topical anesthetic agent should be instilled and the laceration carefully examined with particular attention to the underlying sclera. Conjunctival lacerations of less than 1 cm generally do not require surgical repair. Smaller wounds that are gaped as the result of tissue avulsion, or those with poor appositional closure, should be sutured closed. For larger conjunctival lacerations, closure may be obtained by good wound apposition and suturing with either absorbable or nylon sutures. Care is taken to avoid incarcerating Tenon's capsule. Lacerations involving the plica, semilunar fold, or caruncle warrant restoration of normal anatomic relationships to prevent scarring and poor cosmesis.
CORNEAL LACERATIONS
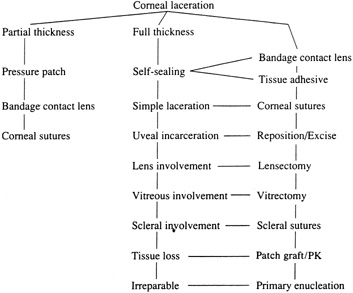
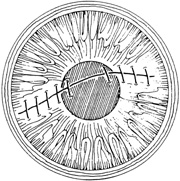
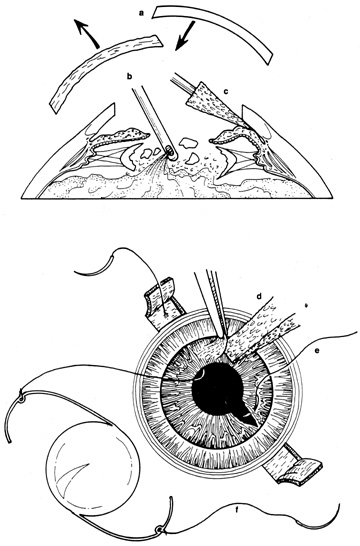
With surgical repair of corneoscleral lacerations, the primary goal is to achieve a watertight globe and maintain structural integrity. Secondary goals include removing any disrupted lens fragments and vitreous, repositioning any uveal tissue, relieving vitreous incarceration, removing any intraocular foreign bodies, and restoring normal anatomic relationships. A methodic surgical strategy is essential for injury repair. Such a stepwise schema affords the surgeon a standard and reproducible approach to the protean manifestations of penetrating anterior segment trauma (Fig. 1).
|
Partial-Thickness Corneal Lacerations
Corneal lacerations that appear to be nonperforating must be examined carefully to rule out any rupture of Descemet's membrane. Seidel testing with 2% fluorescein should be performed to check for microscopic leaks. Gentle digital pressure may reveal a self-sealing wound. Such self-sealing wounds may be a result of stromal swelling when tears and aqueous come into contact with raw stroma. If the injury is indeed full thickness, treatment strategy is notably affected whereas partial thickness injuries may be treated on an outpatient basis, full-thickness lacerations are at risk for endophthalmitis and hospitalization with intravenous antibiotic usage may be considered.
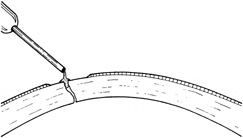
The goals in treating partial thickness corneal lacerations include preventing infection and promoting re-epithelialization and stromal healing. Every effort is made to minimize scarring and surface irregularity. If the wound edges are in good apposition with no wound gape, pressure patching with the use of prophylactic topical antibiotics is sufficient. Re-epithelialization is usually rapid. If the wound is unstable, a bandage soft contact lens may be used to support the wound and encourage re-epithelialization by minimizing the traumatic effect of lid movement (Fig. 2). Topical antibiotic prophylaxis, as well as cycloplegia for comfort, is used while the lens is in place. The lens should remain in place until wound healing is stable and complete re-epithelialization has occurred.
Full-Thickness Corneal Lacerations
BANDAGE SOFT CONTACT LENS.
For small, self-sealing corneal perforations, a bandage contact lens may be sufficient to protect and support the wound as it heals. Such lacerations include nondisplaced, beveled, self-sealing wounds. If aqueous leakage persists for more than 24 hours or there is progressive shallowing of the anterior chamber, more definitive treatment should be undertaken. Treatment with a bandage contact lens in children should be done only with extreme caution and close follow-up care.
In cases that respond satisfactorily, the contact lens should be kept in place until the wound has stabilized (usually 3–6 weeks). A protective shield should be worn at all times. Topical antibiotic prophylaxis and cycloplegia are recommended with the lens in place.
TISSUE ADHESIVE.
Cyanoacrylate tissue adhesive has been successfully used by ophthalmologists for many years despite not being approved by the U.S. Food and Drug Administration. Tissue adhesive is particularly useful for puncture wounds with small amounts of central tissue loss and selected small lacerations.4
Tissue adhesive should be applied to a dry, deepithelialized bed. A capillary microapplicator or fine-gauge disposable needle is used to apply a very thin film of adhesive over the site (Fig. 3). Alternatively, adhesive may be applied using a sterile 2- to 4-mm polyethylene or silicone disc affixed to the end of an applicator stick with sterile ophthalmic ointment. The disc may be left in place or removed with care. Three to five minutes are required for polymerization of the adhesive. A bandage soft contact lens is applied for comfort and to prevent the glue from dislodging. While the contact lens is in place, a prophylactic antibiotic drop is administered. Over time, the tissue adhesive dislodges spontaneously as the wound surface reepithelializes. Alternatively, it may be gently removed with forceps after adequate stromal healing has occurred.
SUTURE REPAIR OF SIMPLE CORNEAL LACERATIONS.
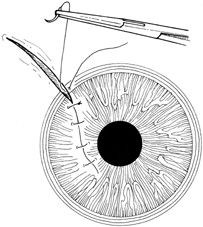
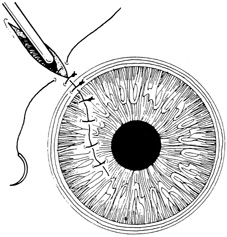
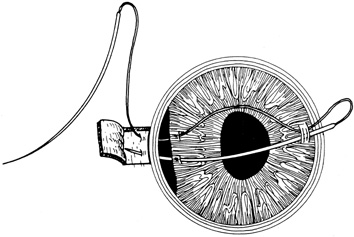
Most lacerating anterior segment injuries require the placement of corneal sutures. The primary goal of corneal suturing is to achieve a watertight wound. Secondary goals include minimizing scarring, restoring normal anatomic relationships, and reconstructing the normal corneal topographic contours. Local anesthesia may be used in selected cases. General anesthesia is used for more severe injuries or with an uncooperative patient, unless medically contraindicated. The affected eye is aseptically prepared and draped with caution to avoid any unnecessary pressure on the globe. To stabilize or manipulate the globe, 6-0 silk stay sutures may be placed at the limbus, 90 degrees away from the wound. Rectus bridle sutures may be passed as well, again taking care to avoid any pressure on the globe.
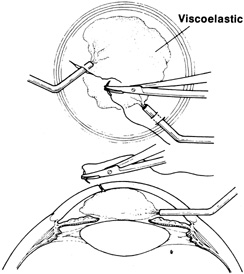
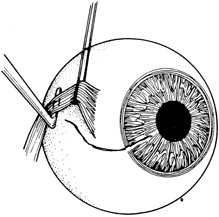
If the anterior chamber is formed and the wound is watertight, sutures may be placed directly without entering the anterior chamber with instrumentation. For a wound that is less stable, a viscoelastic may be irrigated into the anterior chamber either directly through the wound itself or through a separate limbal paracentesis incision (Fig. 4). This will aid in re-forming the anterior chamber, tamponade intraocular contents, and protect the corneal endothelium, iris, and lens. Balanced salt solution or air may also be used to re-form the anterior chamber. In most cases, a limbal paracentesis is preferred because it will minimize disruption of the wound edges and permit better access as the case proceeds.5 A 15-degree sharp microsurgical knife or MVR blade may be used to create the paracentesis, placing the incision 90 degrees away from the wound. If a very shallow or flat anterior chamber is present, extreme care should be taken to avoid damage to the iris or lens when constructing the paracentesis site.
|
Temporary sutures may be used if the initial placement of deep definitive sutures would cause loss/flattening of the anterior chamber. These temporary sutures should be placed without distorting the wound. Accurate placement of the final definitive sutures can be achieved subsequently. The number of temporary sutures should be minimized, however, to prevent undue trauma to the wound margins and surrounding stroma.
For corneal suturing, 10-0 monofilament nylon on a fine spatula-design microsurgical needle is used. This suture causes little tissue reaction, keeping vascularization and scarring at a minimum. Even finer 11-0 nylon suture may be used for lacerations near or involving the visual axis. Computed corneal topography analysis of corneal suturing techniques has suggested strategies designed to ensure adequate wound closure, minimize corneal scarring, and restore the native corneal contour.6,7
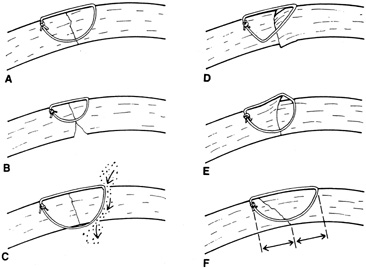
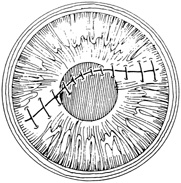
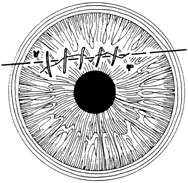
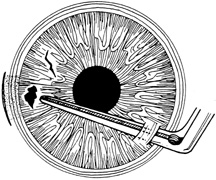
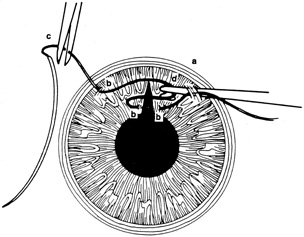
A number of options are available for suturing a corneal laceration. Tissue margins should appose as precisely as possible while suturing. The simplest method is to progressively halve the wound with simple interrupted sutures. Corneal sutures should be approximately 90% to 95% depth through the stroma, 1.5 mm in length, and of equal depth on each side of the wound. Shallow sutures create internal wound gape, whereas sutures of unequal length and depth on each side of the wound result in wound override. Sutures should generally not be passed through 100% thickness because the tract may theoretically act as a conduit allowing microorganisms from the external surface to enter the eye. For shelved lacerations, sutures should be placed equidistant with respect to the internal aspect of the wound and tied with minimal tension to achieve good tissue apposition, avoiding wound slippage with consequent tissue override (Fig. 5). Wounds with edematous or irregular margins generally require longer sutures for closure.
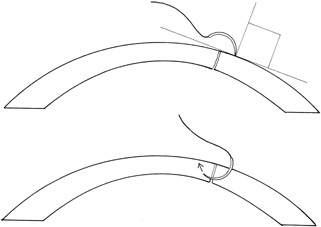
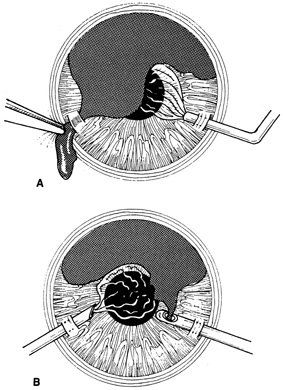
Placement of sutures through the visual axis should be avoided. If it is necessary to pass sutures near the visual axis, they may be placed on each side of, but not directly through, the axis itself. In addition, the bite closest to the visual axis may be made shorter, thus avoiding this area (Fig. 6). A “no-touch” technique also may be used to minimize trauma at the visual axis. With this technique, the eye is secured by grasping the globe with forceps away from the cornea. The suture needle end is then placed perpendicular to the corneal surface, and the needle is rotated through the corneal tissue following the needle's curvature (Fig. 7).8
|
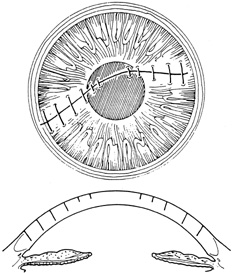
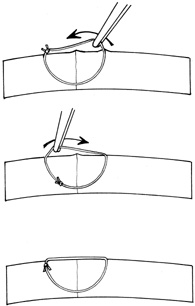
With more complex lacerations, good wound apposition with minimal distortion is the goal. An irregular wound is first subdivided into straight segments with interrupted sutures. Perpendicular areas of the laceration should be sutured before beveled ones to achieve watertight closure with the fewest number of sutures. As suggested by Rowsey and Hays,7 long, deep, and relatively tight peripheral wound sutures in combination with shorter, shallower, appositional sutures near the visual axis may help restore the normal corneal contour by restoring the relatively steep central corneal dome (Fig. 8). For irregular or curvilinear lacerations, all interrupted sutures should be placed perpendicular to the wound, thus avoiding transverse shifting of the wound margins. Although a running shoestring suturing technique may be used with straight lacerations to minimize astigmatism and facilitate subsequent suture removal, a running suture may tend to distort an irregular or curvilinear laceration because it will tend to form a straight line. Thus, if used, running suture bites should be placed perpendicular to and equidistant from an imaginary “regression” line through the wound rather than with respect to the wound itself (Fig. 9).
|
To facilitate subsequent suture removal from the cornea, a locked 2-1-1 knot or a 1-1-1 slipknot may be used. All suture knot ends should be trimmed short and then buried superficially on the side away from the visual axis. This minimizes scarring, inflammation, neovascularization, and buildup of mucus. The knot should be buried by directing the ends away from the surface to ease future removal (Fig. 10).
Once the laceration is repaired, the anterior chamber should be deepened, preferably through the paracentesis port, if not through the wound itself. The wound is then checked for leaks using dry cellulose sponges or 2% fluorescein sodium. The paracentesis port is usually self-sealing; however, if it is leaking, stromal hydration using a canula or closure with a single interrupted 10-0 nylon suture may be necessary.
STELLATE CORNEAL LACERATIONS.
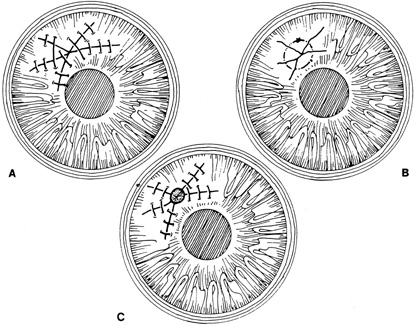
Stellate lacerations and partial tissue avulsions are often difficult to repair because it is difficult to obtain watertight closure. Good tissue apposition is often difficult to obtain. Several techniques may be used for stellate lacerations. These include multiple interrupted sutures, bridging sutures, pursestring sutures, and star-shaped sutures9. If closing a stellate laceration is difficult, and leaking persists, tissue adhesive or a corneal patch graft may be used (Fig. 11).
|
If an avulsed tissue pedicle is seen, it should be carefully replaced into position and sutured into place. Such avulsed tissue is best pulled into place by angling sutures toward the apex of the wound.6
CORNEAL LACERATIONS WITH UVEAL PROLAPSE.
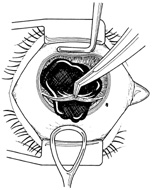
Iris incarceration and prolapse are frequently noted conditions that accompany corneal lacerations. A peaked pupil signals tissue incarceration. If prolapsed tissue is present, it must first be carefully examined. Usually, macerated, feathery, devitalized, or depigmented iris should be excised. The prolapsed tissue should be evaluated for any signs of surface epithelialization. In this case, it should be excised to prevent any epithelial cells from proliferating in the anterior chamber. In general, tissue that has been prolapsed for longer than 24 hours should be excised to avoid infection; however, if the tissue appears healthy, it may be replaced with caution. Any tissue to be excised should be gently grasped with fine forceps without applying undue traction. The tissue is then cut flush with the cornea. When excising any tissue, every effort is made to preserve as much as possible to maintain a normal iris diaphragm and conserve tissue for secondary reconstructive efforts.8
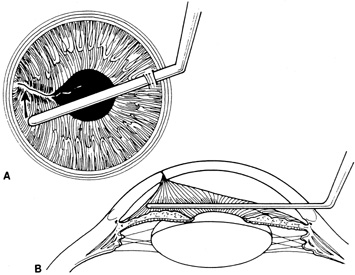
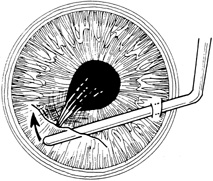
Incarcerated tissue will usually require mechanical reposition. Selected cases of mild incarceration may be treated with pharmacologic agents. Agents such as intraocular acetylcholine or carbachol cause pupil constriction, thus pulling iris from the wound. If incarcerated iris is located in the central cornea, mydriatic agents (e.g., intraocular epinephrine 1:10,000) may be used. If mechanical reposition is required, simply deepening the anterior chamber may release incarcerated tissue. Viscoelastic agents may be irrigated through the paracentesis port or the wound adjacent to the involved iris tissue in an attempt to draw the incarcerated iris from the wound (Fig. 12). If unsuccessful, a cyclodialysis spatula or irrigating canula may be passed through the paracentesis site and used to directly sweep incarcerated tissue free (Fig. 13). Care is always taken to avoid trauma to the corneal endothelium, iris, and lens. Any excised tissue should be sent to the pathology department for examination.
|
|
Once incarcerated or prolapsed tissue is excised or repositioned, the corneal laceration is sutured as described earlier. Careful inspection is warranted once closure is complete to ensure that no tissue incarceration persists. The anterior chamber should be filled through the paracentesis site, and wound integrity should be ascertained.
CORNEAL LACERATIONS WITH LENS OR VITREOUS INVOLVEMENT.
Perforating injuries may also have lens and vitreous involvement. Direct and indirect forces may damage the lens capsule, causing cataract formation. Penetration or rupture of the lens capsule often leads to lens opacification. Blunt trauma may result in globe distortion with equatorial expansion, which can cause capsular rupture or result in dehiscence of zonules with consequent lens subluxation or dislocation. Vitreous may find its way around the lens into the anterior chamber when loss of zonules occurs. Careful preoperative and intraoperative assessment will dictate whether lens removal is required at the time of primary repair. Removal of a cataractous lens rarely needs to be done in the emergency setting. In particular, there are some situations in which lens removal in the acute setting is not recommended. For instance, a fibrinous anterior chamber reaction and pupillary membrane may occasionally masquerade as a flocculent cataract10 or poor visualization secondary to an edematous cornea may make intraocular surgery hazardous. However, if surgical visualization is good, it is preferable to complete all surgical interventions at one setting.11 If any doubt regarding lens clarity exists, the lens should be left in place and, if necessary, removed at a later date. There are other situations in which primary removal of the lens is indicated. A lens with a disrupted capsule and flocculent cortical material liberated into the anterior chamber should be removed at the primary setting in an effort to prevent phacogenic postoperative inflammation. In cases in which vitreous is involved with lens remnants, this may be best addressed in the initial surgery. In addition, when it is clear that a lens is cataractous and surgical visualization is good, the lens may be removed in the primary operation.12,13 Lens removal should take place only under controlled circumstances with proper instrumentation. A cataractous lens with an intact capsule and no liberated cortical material may be removed at a later date.
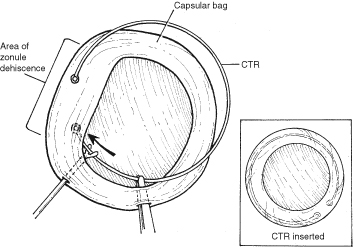
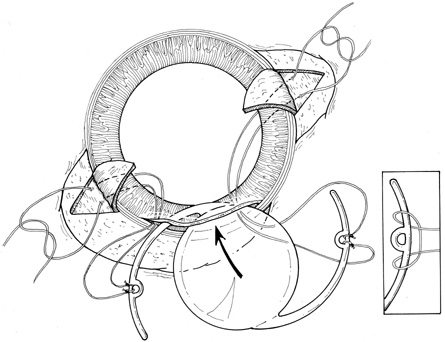
With lens removal, attention should first be paid to any prolapsed or incarcerated tissue. This should be repositioned or excised, and the corneal laceration should be sutured and the anterior chamber re-formed as described. The nature of the injury will suggest the surgical technique used for lens removal. If the lens capsule remains intact, standard phacoemulsification or extracapsular cataract extraction may be performed, making every effort to preserve the integrity of the posterior capsule. In children and young adults, a soft cataractous lens may be removed through a small incision using manual or automated aspiration. When extracting the nucleus in the patient post-trauma, one should be aware of the possibility of poor zonular support. Should zonular weakness, dialysis, or loss be present, a capsular tension ring (CTR) may be used. Implanting a CTR may decrease intraoperative zonule dehiscence, subluxation of the capsular bag, and posterior capsular rupture. A CTR inserted before phacoemulsification will permit support of the capsular bag, reestablishing its contour and protecting it from getting aspirated.14 The CTR (Morcher; distributed by FCI Ophthalmics, Inc., Marshfield Hills, MA) comes in three sizes and is usually inserted after capsulorrhexis and hydrodissection. For insertion, it is best to enter the bag in the area of greatest zonular weakness, thus placing stress on the stronger zonules, 180 degrees away. The ring is dialed into the bag using a second instrument (e.g., Y-hook) (Fig. 14). Alternatively, a CTR injector may be used.
|
If the lens capsule is ruptured, vitreous is involved, or the lens is subluxated or dislocated, microvitrectomy instrumentation should be used, because caution must be taken not to exert vitreous traction during lens removal. This may be performed through either a limbal or pars plana approach, depending on the surgeon's preference. It is important to remove all lens fragments from the anterior chamber, using caution to prevent any remnants from receding into the vitreous chamber. Any vitreous remaining in the anterior chamber should be removed to prevent incarceration into the wound.
Intraocular lens implantation in the primary trauma setting is a controversial area; however, generally it is associated with a more favorable visual outcome.12,13 The patient's age, the visual and refractive status of the fellow eye, and the ability to use an aphakic contact lens postoperatively are factors in the decision. If the posterior capsule is intact, and the patient's age is appropriate, a posterior chamber intraocular lens may be implanted. Currently, intraocular lenses are used at a much younger age. In cases in which there is inadequate capsular support, posterior chamber lenses may be supported by transscleral or iris sutures (Fig. 15). 15,16 An anterior chamber lens may also be placed in the absence of capsular support after all vitreous and lens remnants are removed. These should likely be reserved for older patients; however, intraocular lens implantation should not be performed if visualization is poor or the eye is severely inflamed and may be deferred if secondary anterior segment reconstructive surgery is anticipated. Moreover, although intraocular lens placement may be preferable in some patients, contact lens correction may afford a better optical result in those patients with irregular astigmatism from a corneal scar.17
In corneal lacerations with lens involvement, posterior capsular rupture with vitreous involvement is not infrequent. The anterior chamber should be carefully visualized to detect any incarcerated vitreous strands that, if present, should be removed from the wound. This helps to prevent complications of chronic inflammation, cystoid macular edema, vitreous fibrosis and retinal detachment, and infection mediated by a vitreous wick to the external environment.
Automated vitrectomy (preferably bimanual) is performed. Access may be through the limbus, pars plana, or both depending on the surgical situation and surgeon's preference. An irrigation sleeve may also be used, allowing vitrectomy through a single limbal site or through the wound itself.
Once vitrectomy has been performed, cellulose sponges should be used to touch the wound, ensuring that no vitreous remains. If any vitreous is present, Vannas scissors are used to cut below the cellulose sponge, flush with the wound. A vitreous sweep or cyclodialysis spatula may also be used through the paracentesis site to mechanically sweep away any incarcerated vitreous (Fig. 16). Any peaking or movement of the pupil indicates overriding vitreous strands. At the conclusion of vitreous removal the pupil should be round and the wound should be cleared of vitreous incarceration.
|
CORNEOSCLERAL LACERATIONS
Corneal lacerations that extend past the limbus need careful examination to determine the full extent of the wound. Rectus bridle sutures of 4-0 silk or limbal fixation sutures of 6-0 silk may be used with caution if the globe is unstable. However, for large lacerations with structural deformation, sutures should be placed to restore wound integrity before rigorous exploration of the globe. Extreme care must be emphasized throughout the surgery to avoid iatrogenic damage.
Initially, the limbus should be reapproximated with 8-0 or 9-0 nonabsorbable nylon or silk sutures to restore normal anatomic relationships. Any prolapsed or incarcerated iris should be repositioned, after which the corneal portion of the laceration is repaired as described earlier. The scleral laceration is then explored to ascertain the extent of damage. For smaller lacerations, a localized conjunctival peritomy is made to explore the wound. A 360-degree peritomy is made for larger lacerations to explore all quadrants. During exploration, care is always taken to avoid iatrogenic avulsion of intraocular contents. The use of loupes rather than the surgical microscope may facilitate the broader three-dimensional view required for repair of scleral laceration.
As in the anterior segment, it is important to clear the wound of any prolapsed or incarcerated vitreous. Any prolapsed vitreous should be secured with dry cellulose sponges and cut flush with the sclera, avoiding traction on the vitreous. With good visualization, vitrectomy instrumentation may be used to remove vitreous at the wound. Should the wound extend through the pars plana, the vitrector may be placed intraocularly as long as visualization is good. If visualization is poor, or the lesion extends beyond the pars plana posteriorly, the vitrector should not be passed into the eye because this may create further damage to intraocular structures.
There are many options in selecting suture material for scleral closure. Although some surgeons prefer nonabsorbable sutures (i.e., 8-0 nylon or silk), others may use absorbable materials (i.e., 7-0 polyglactin 910). For larger defects, nonabsorbable sutures should be used. When suturing gaping wounds, it may be helpful to advance the needle completely through one wound margin and then reload the needle holder before making a second pass, thus avoiding globe distortion (Fig. 17). For closing sclera over prolapsed uvea, the wound is often most easily closed from the anterior (limbal) end with interrupted sutures, which are placed successively, proceeding posteriorly. This is called the “zippering” or “close-as-you-go” technique. With this technique, sutures are placed in close proximity to one another in an attempt to achieve oversewing of the uveal tissue with the sclera. The surgical assistant may use a spatula to gently depress the uvea into the eye during suture tightening to avoid tissue incarceration during scleral closure (Fig. 18). Another suturing strategy is to successively bisect the wound while having the assistant use a spatula to press any prolapsed tissue back into the eye as sutures are tightened.
|
|
If the scleral laceration is gaped, uveal tissue is often prolapsed. Because excision of this tissue may be accompanied by severe bleeding and the possibility of cutting retinal tissue, every effort should be made to reposit this tissue. When prolapsed tissue needs to be excised for wound closure, it should be identified histologically. If retinal tissue is identified, this places the eye in a very poor prognosis category.
In wounds that are posterior, it is more difficult to visualize and mechanically remove incarcerated or prolapsed vitreous. In these cases, the primary surgical goal is simply to secure a watertight closure of the globe because posterior vitreous prolapse and incarceration are usually accompanied by severe retinal damage that will require subsequent vitreous surgery.18 At times, scleral lacerations may extend far posteriorly, and may not be accessible. In these situations, it is preferable to leave the most posterior portion of the wound unsutured rather than to cause excessive globe distortion and prolapse of intraocular contents through extensive manipulation during suturing. If there is loss of scleral tissue, a patch graft using donor sclera may be sutured in place using interrupted nonabsorbably sutures.
After the visible scleral laceration is repaired, the adjacent sclera as well as other quadrants should be explored to exclude additional injuries. Adjacent quadrants are cleared of overlying Tenon's capsule, and the sclera is exposed with the use of retractors. To facilitate manipulation and exploration of the globe, sutures of 4-0 silk or 2-0 cotton may be used to sling the rectus muscles for retraction. The sclera is thinnest behind the muscle insertions; thus, careful exploration of these areas is crucial. If the scleral laceration extends behind the muscle, it may be sutured while an assistant retracts the muscle (Fig. 19). If this is difficult, or if the full extent of the laceration is not viewed, the muscle may be disinserted after being secured with a double-armed 6-0 polyglactin 910 suture, which subsequently is used to reattach it at its original insertion.
|
Posterior Scleral Lacerations
Scleral lacerations may occur as an isolated event without any corneal involvement. Although most blunt ruptures are found at or anterior to the equator, 10% to 20% are located posteriorly.19 Many of these occur posterior to the rectus muscle insertions where the sclera is thinnest and intrinsically weak.20 Posterior scleral lacerations may also arise from direct lacerating trauma or double perforating projectile injuries. Signs of a posterior rupture include poor visual acuity, a shallow or very deep anterior chamber, hyphema, low intraocular pressure, pupillary irregularity, bullous subconjunctival hemorrhage and chemosis, and a brownish subconjunctival hue caused by prolapsed uvea.19
In repairing posterior scleral lacerations, a 360-degree peritomy is performed and all quadrants of the globe are explored. Any scleral laceration that is encountered may be repaired as it is uncovered to avoid progressive prolapse of intraocular contents created by globe distortion during further exploration. Should a laceration be found, complete exploration should still be performed to look for any additional lacerations.
Corneoscleral Lacerations with Tissue Loss
Corneoscleral lacerations with tissue loss are difficult to manage. Puncture wounds and small avulsive injuries may be closed with tight sutures; however, this creates tissue distortion and results in marked postoperative scarring and astigmatism. Tissue adhesive may be used in conjunction with sutures for these cases. Caution must be used because cyanoacrylate adhesive has a strong affinity to nylon sutures; therefore as the adhesive loosens, the suture may come with it. With larger avulsive injuries, tissue replacement is achieved with lamellar or full-thickness patch grafts using either fresh or preserved corneal and/or scleral tissue. Patch grafting allows restoration of structural integrity while maintaining normal ocular architecture.
Lamellar patch grafts are useful where irregular wound margins and/or stromal necrosis make full-thickness dissection and suturing difficult. Full-thickness grafts require a healthy recipient tissue. Both lamellar and full-thickness patch grafts are indicated for perforating corneal injuries with loss of tissue.
Penetrating keratoplasty is rarely indicated during primary repair after trauma. Keratoplasty is usually delayed until the eye is uninflamed and free of infection, thus improving graft prognosis. In primary repair, it is better to use conservative measures such as multiple sutures or patch grafting to restore the globe. In cases in which there is extensive loss of corneal tissue and anterior segment disruption, however, penetrating keratoplasty and anterior segment reconstruction may be undertaken as a primary procedure.
Irreparable Anterior Segment Injury
In all trauma cases, repair of the globe should be attempted. There are some severe corneoscleral lacerations, however, that are beyond repair. These usually involve loss of retinal tissue and/or marked destruction of the globe. Should primary enucleation be considered, appropriate informed consent must be obtained
ANTERIOR SEGMENT FOREIGN BODIES
The eye trauma surgeon should always suspect an intraocular foreign body. Foreign bodies are most common after activities involving striking metal on metal.21,22 However, vegetable matter, glass, and other materials may be involved.23
Typically, the foreign body is small and the eye may not show obvious signs of trauma. Entry wounds through the cornea may be small and self-sealing, and those through the sclera may be covered by subconjunctival hemorrhage or edema. The entry site and intraocular site of damage may help delineate the trajectory and resting place of an intraocular foreign body.
In the search for small perforating injuries, Seidel's testing with 2% fluorescein may be used. Gentle digital pressure may disclose a self-sealing wound. Foreign bodies frequently lodge in the anterior chamber angle and may display overlying focal corneal edema. Thus, gonioscopy may be useful in detecting the foreign body, using minimal pressure on the globe during examination. Foreign bodies may also embed themselves in the lens and may create a focal cataract. Iris transillumination defects may signal an entry site.
Plain roentgenograms, computed tomography, magnetic resonance imaging, and B-scan ultrasonography all may help detect an intraocular foreign body. Magnetic resonance imaging is to be avoided if the foreign body is metallic, because the magnetic field may move the object, resulting in tissue damage.24,25 Ultrasound biomicroscopy (UMB) and B-scan ultrasound examinations are also very useful for localizing an intraocular foreign body.26
The composition of the foreign body involved determines the urgency of its removal.27 Whereas some materials (iron, copper, and other metals) may be toxic and stimulate inflammation, others (vegetable matter) may pose a risk for infection. These items should be removed promptly. Inert substances such as plastic and glass may be well tolerated and left in place in certain situations.
The location of the intraocular foreign body and the status of surrounding ocular structures dictate which surgical technique should be used. Foreign bodies located in the anterior chamber angle, iris, or lens may be removed through a limbal incision either directly over the object or across the anterior chamber (Fig. 20). The use of fine foreign body forceps may minimize incision size and intraocular manipulation. With metallic foreign bodies there are some instances in which a rare earth magnet may be used to facilitate extraction.
|
A viscoelastic agent is useful for maintaining the anterior chamber and protecting the intraocular structures during manipulation of an intraocular foreign body. A viscoelastic agent may also be used to hydraulically move a foreign body across the anterior chamber. Intracameral miotic agents (acetylcholine or carbachol) may also be used to constrict the pupil and protect the lens. In cases in which the lens is disrupted, the foreign body may be removed along with the lens. The nature and extent of injury will dictate the appropriate surgical method.
POSTOPERATIVE MANAGEMENT
Once primary surgical repair of anterior segment penetrating trauma has been achieved, medical therapy should be instituted to (1) control infection, (2) suppress inflammation, and (3) stabilize the ocular surface.
On conclusion of surgery, subconjunctival antibiotics are administered (e.g., cefazolin or vancomycin and gentamicin). Patients admitted to the hospital should receive a few days of treatment with intravenous antibiotics, usually a cephalosporin or vancomycin and an aminoglycoside. Most cases of bacterial endophthalmitis present by the fifth postoperative day. Clindamycin should be considered in cases involving vegetable matter to cover Bacillus species.2 Topical fortified antibiotics (e.g., cefazolin, 100 mg/mL, and tobramycin, 14 mg/mL) or fourth generation fluoroquinolones are given every 2 hours for 4 days, and then the dose is tapered. Once culture results are obtained, therapy is adjusted accordingly. Patients who are discharged may be given oral antibiotics (e.g., levofloxacin) along with topical fortified antibiotics or fluoroquinolone.
To minimize scarring and new vessel ingrowth, topical corticosteroids may be given. One must weigh the anti-inflammatory advantages against the risk of infection with corticosteroid use. Intensive corticosteroid use in early wound healing may also diminish the rate of stromal healing as well as the tensile strength of the wound.28 Therefore, unless the eye is markedly inflamed, topical corticosteroid use should be kept at a minimum in the early postoperative period. Once the potential for infection diminishes, and if scarring or neovascularization is noted, moderate-dose corticosteroids may be added. Oral corticosteroids may be used with caution in severely inflamed eyes. Topical β-blockers and carbonic anhydrase inhibitors are used as necessary for intraocular pressure control. To stabilize the ocular surface, lubricants, bandage contact lenses, patching, and tarsorrhaphy may be used as necessary.
Sutures should be removed from a corneal laceration under a number of circumstances (Table 1). Adequate wound healing is demonstrated by a gray fibrosis of the laceration edges and can best be seen by oblique slit lamp illumination or retroillumination. Corneal vascularization is also generally indicative of adequate wound healing. Corneal lacerations near the limbus heal more quickly than central wounds. Sutures that loosen spontaneously because of wound contraction are providing no further tensile strength to the cornea and should be removed. Exposed sutures that may provide a nidus for infection or inflammation should be removed if the wound is stable. In addition, those sutures to which new blood vessels are attracted should be observed carefully and removed if necessary. Keratometry, Placido's disc imagery, and computed corneal topographic analysis are valuable for observing sutures that are producing excessive tissue compression and, therefore, corneal astigmatism. If corneal integrity allows, the compressing sutures can be selectively removed to correct this induced astigmatism and thus speed visual recovery.
TABLE 1. Indications for Suture Removal after Corneal Laceration Repair
| Loose suture |
| Exposed suture |
| New blood vessels to suture |
| Mucus accumulation |
| Suture infection or abscess |
| Healed wound |
| Grayish wound fibrosis |
| Earlier healing |
| Near limbus |
| Pediatric population |
| Astigmatism/topography management |