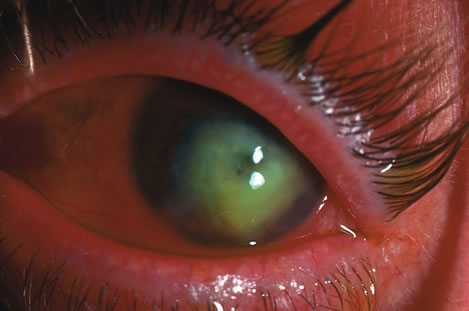
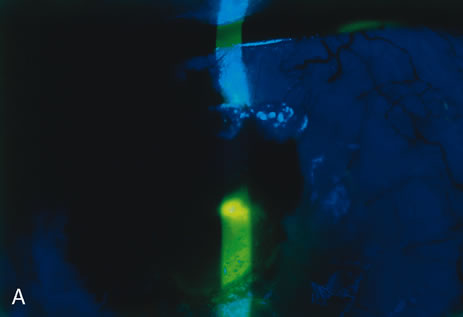
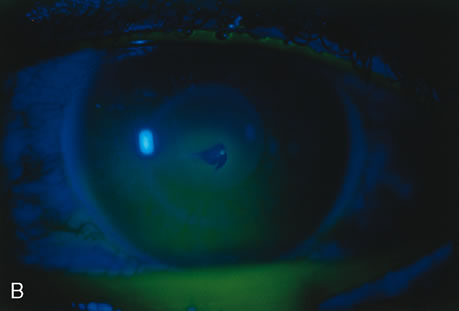
| Treatment of corneal perforation has two goals. One is to re-establish
the integrity of the globe, and the other is to treat the underlying
problem so that ulceration and perforation will not recur. With infectious processes, appropriate antimicrobial therapy must be initiated. Often, 24 hours
of appropriate antibiotic treatment is carried
out in bacterial perforations before definitively treating the perforation. In
situations in which eyelid deformity has led to the problem, the
eyelid abnormalities can be addressed concurrent with the treatment
of the perforation. The same is true of patients with dry eyes in
whom tear replacement, punctal occlusion, and other measures can be carried
out at the same time as treatment of the perforation. With decreased
corneal sensation, tarsorrhaphy can be performed after treatment
of the perforation. In inflammatory conditions, immunosuppressant treatment
may be necessary coincident with definitive repair and systemically
or topically after repair.4 Definitive, that is, reparative, treatment of corneal perforation depends
on the cause, location, size, and status of treatment of the underlying
condition. Accompanying problems such as endophthalmitis and cataract
may also play a role in the decision on definitive treatment. Self-sealing perforations may need no reparative treatment. These
perforations may occur with shelved lacerations or with small puncture
wounds in which tissue swelling occludes the perforation tract. Wounds
that are sealed by tissue prolapse usually require repair. Self-sealed
wounds with tissue displacement may also be best repaired
to reduce the likelihood of induction of irregular astigmatism. Very small perforations with no associated tissue displacement may be amenable
to the use of patching or soft contact lens placement. This is
most likely to be effective in small leaks evident only with pressure
or in descemetoceles in which tissue stabilization is essential to allow
healing and in which the inciting process is under control.5 Small dry eye perforations are perhaps the most responsive to this approach. When
soft contact lenses are used, frequent follow-up is
essential, and more aggressive techniques may be necessary if this fails. The use of tissue adhesives is an appealing approach to treatment of small
perforations or descemetoceles with impending perforation. The advantage
is that the adhesive may be applied under topical anesthesia at
the slit lamp or in a minor procedure room. The disadvantages are that
the glue may induce significant inflammation, may be uncomfortable for
the patient, may adhere inadequately, may serve to harbor organisms
once polymerized,6 and is often effective only in small perforations that can be readily
dried. The tissue adhesives most commonly used in the United States are
cyanoacrylates, usually isobutyl or higher alkyl compounds. None of
these are approved for ophthalmic use by the Food and Drug Administration. 2-Octyl
cyanoacrylate (Dermabond, Ethicon, Somerville, NJ) is
approved by the U.S. Food and Drug Administration (FDA) for
use on skin and has been effective in sealing corneal perforations. These
substances polymerize rapidly when in contact with water. Various
techniques have been described for the use of tissue adhesives. All
require débridement of necrotic tissue and epithelium
surrounding the perforation, drying of the area to which the glue is
to be applied, and application of the least amount of glue that can
cover the defect. Drying of the defect can be carried out with cellulose
sponges, and air or viscoelastic may be placed behind the perforation
to separate tissue and reduce the fluid present.8 The glue itself may be applied to a small plastic disk and then placed
over the perforation (Fig. 3), applied directly from the tip of a fine needle attached to a tuberculin
syringe,9 or applied with a specially made plastic applicator (Squeez-ett, Ellman
Manufacturing Co, Hewlett, NY).10,11 The glue may come off and need to be reapplied, at times repeatedly. Usually, because
of the rough surface of the polymerized glue, a bandage
soft contact lens is placed on the eye after the glue has polymerized. The
glue may be left in place until there is obvious healing of the
perforation, until the glue spontaneously loosens because epithelium
has grown beneath it, or until a more definitive procedure such as keratoplasty
is carried out. Fibrin adhesives (e.g., Tisseel, Immuno
Canada, Ltd) may be used in a similar fashion but have less inherent
strength than the cyanoacrylates and have been used less frequently
in the United States.12 Photopolymerized sealants are also being studied. These have the advantage
of more controlled polymerization and hardening through laser irradiation
after adhesive placement rather than the uncontrolled, very rapid
polymerization seen with the cyanoacrylates.13 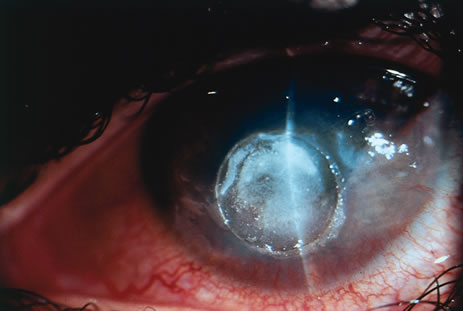 Fig. 3. This is a cornea with a perforation glued with cyanoacrylate applied using
a plastic disk. Fig. 3. This is a cornea with a perforation glued with cyanoacrylate applied using
a plastic disk.
|
Conjunctival flaps play a less important role in the treatment of perforations
than they do in the prevention of progression of corneal melting. Nonetheless, in
some leaking descemetoceles and small perforations, conjunctival
flaps may serve as a temporizing measure before keratoplasty.14 With the use of tissue adhesives and patch grafting, however, the use
of conjunctival flaps for perforation has become almost obsolete. Partial-thickness scleral flaps may be dissected with a base at
the limbus and then reflected onto the cornea and sutured in place to
treat small peripheral corneal perforations. To be most effective, the
epithelium and the necrotic material surrounding the leak must be removed, and
dissection of a small lamellar bed is helpful in suturing the
sclera to the cornea. This technique is cosmetically less acceptable
than the use of corneal material, but it may be of value in emergency
situations. Another technique using autologous cornea has been described
in which a small trephine (2 mm) was used to dissect a half-thickness
peripheral corneal button, which was then sutured
in place over a perforation in the cornea of the same eye.15 The donor site healed without complication, and the perforation was repaired
as well. An intralamellar flap of cornea folded over the perforation
site and then covered with a donor lamellar graft has also been
described.16 Amniotic membrane has recently reappeared as a surgical tool and has been
used to treat corneal ulceration and perforation. It may be used over
fibrin glue to seal perforations17 but is more frequently used alone by filling the corneal defect with multilayered
membrane, which is sutured in place and then covered with
a larger piece of amniotic membrane with the epithelial surface anterior.18,19 The most frequently used techniques for definitive repair of perforations
involve some form of keratoplasty using donor material. The choice
between lamellar and full-thickness penetrating keratoplasty depends
on a number of factors, including location and size of the perforation, donor
tissue availability, and associated ocular findings. My
preference is to choose lamellar grafting when the perforation is small
and peripheral. Also, when there is marked anterior segment inflammation
and a formed chamber, lamellar “patch grafting” may avoid
instrumentation of the anterior chamber and the risk of fibrin outpouring, chamber
flattening, and formation of synechiae.
Lamellar keratoplasty depends on the same principles as the use of tissue
adhesive, that is, débridement of necrotic material and removal of
surrounding epithelium. Additionally, however, a clean edge for suture
placement is necessary and a dry bed is not necessary. The surgery may
be done with the use of local or general anesthesia. The use of general
anesthesia avoids the increase in orbital and intraocular pressure of
a local anesthetic injection and decreases the risk of increasing the
fluid leak or causing loss of a formed chamber. Local, especially low-volume
peribulbar, anesthesia may work as well in situations in which general
anesthesia must be avoided because of systemic illness in the patient.
A recent study has shown lack of anesthetic related complications in 140
patients undergoing repair of open globe injuries under local anesthetic
with intravenous sedation.20 The technique
of surgery is to use a trephine to surround the involved area and, if
possible, to cut into the tissue to sufficient depth to create a sharply
marginated bed (Figs. 4,
5, and 6).
More often, the eye is soft, so the trephine is painted with methylene
blue and used to mark the area to be dissected. A sharp blade is then
used to further deepen the edges of the bed. The margin of the tissue
to be removed is then grasped, and a lamellar dissection of the tissue
is carried out toward the center of the perforation. This is usually done
with sharp dissection using a lamellar dissecting blade, such as a no.
66 Beaver blade or a crescent-type knife (Fig.
7). The central portion is removed last because the chamber, if not
already lost, may flatten at this point (Fig.
8). Once the bed is dissected, the donor is prepared by lamellar splitting
from a whole donor eye, dissection from a donor corneoscleral button held
in a clamp or artificial anterior chamber, or full-thickness punching
from a corneoscleral button. When a whole donor eye is used, the eye is
grasped in a gauze pad and a sharp blade is used to incise the cornea
at the limbus to the depth needed (Fig.
9). For a 50% lamellar bed, the donor should be dissected to 50%
thickness or less. The cornea is then split with a spatula or dissecting
blade (Fig. 10) and trephined
from the anterior surface (Figs.
11 and 12). Our tendency
is to oversize the donor diameter by 0.5 mm for small grafts and 1 mm
for larger grafts. For a very deeply dissected recipient bed, a full-thickness
donor may be used and punched from the endothelial side.
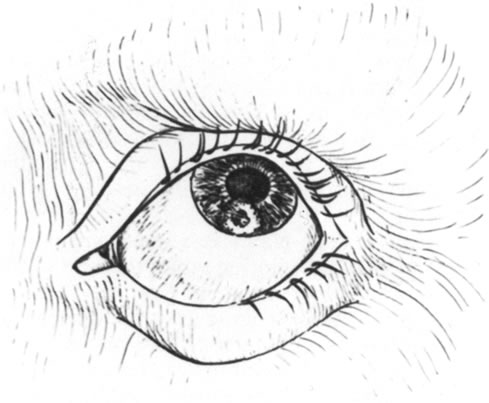 Fig. 4. Drawing of a perforated cornea. Fig. 4. Drawing of a perforated cornea.
|
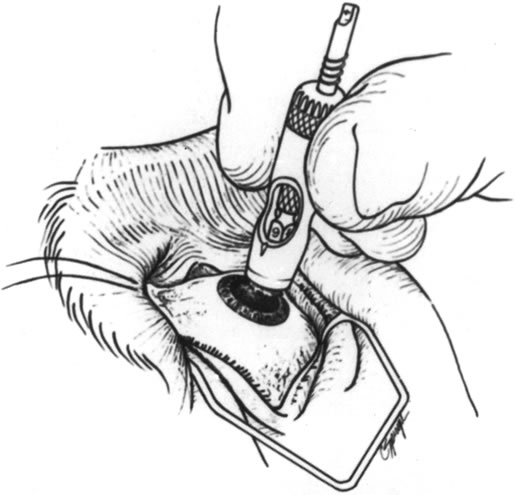 Fig. 5. Using a trephine to surround the area of corneal perforation and necrosis. Fig. 5. Using a trephine to surround the area of corneal perforation and necrosis.
|
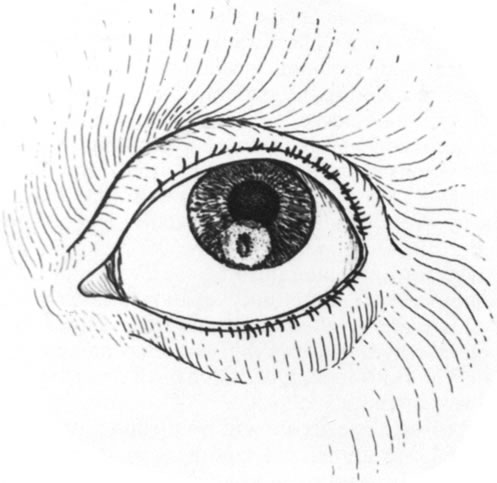 Fig. 6. The area of perforation and necrosis has been surrounded by the trephine
mark. Fig. 6. The area of perforation and necrosis has been surrounded by the trephine
mark.
|
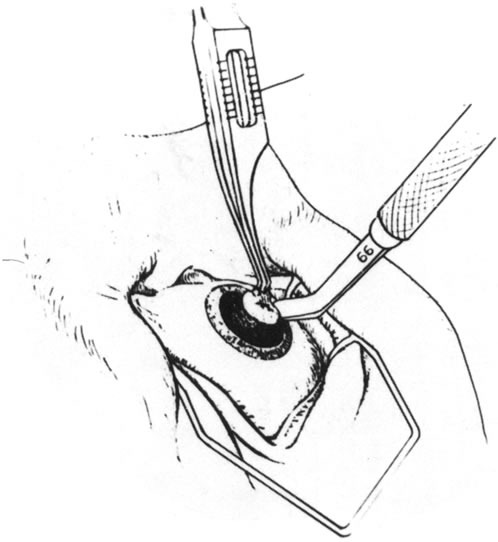 Fig. 7. The lamellar dissection is being carried out. Fig. 7. The lamellar dissection is being carried out.
|
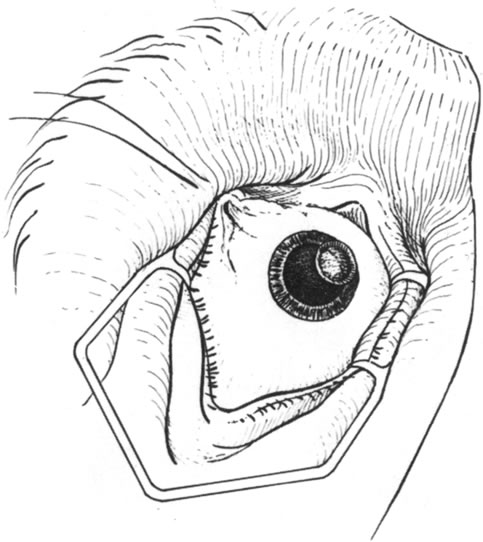 Fig. 8. The dissected lamellar bed. Note the clean margins to which the donor material
can be sutured. Fig. 8. The dissected lamellar bed. Note the clean margins to which the donor material
can be sutured.
|
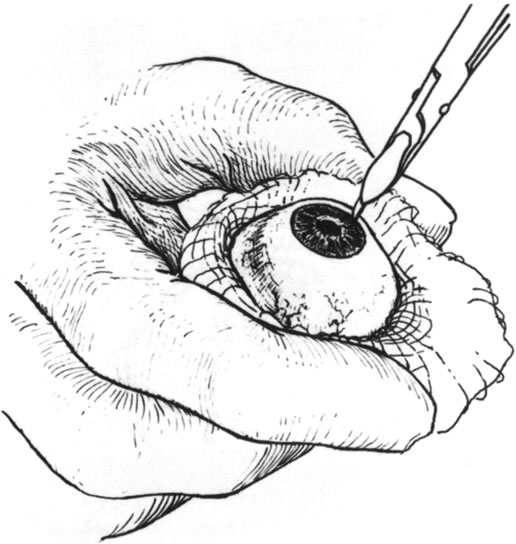 Fig. 9. A sharp blade is used to incise the donor cornea to partial thickness. Fig. 9. A sharp blade is used to incise the donor cornea to partial thickness.
|
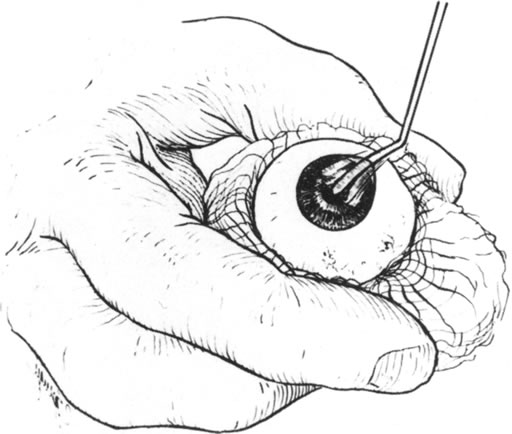 Fig. 10. A cyclodialysis spatula is used to lamellarly dissect the donor cornea. Fig. 10. A cyclodialysis spatula is used to lamellarly dissect the donor cornea.
|
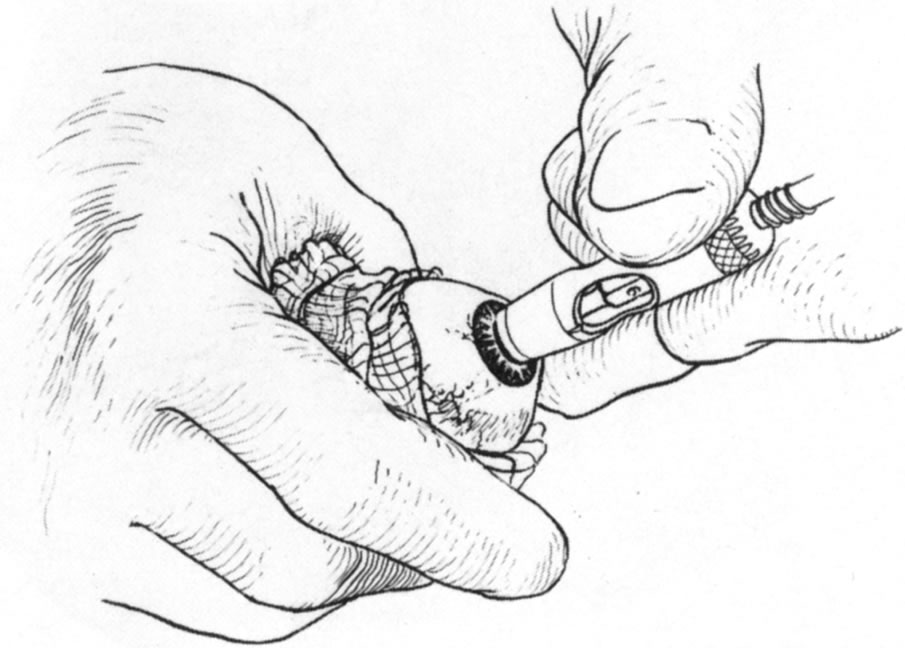 Fig. 11. Trephination of the donor cornea. Fig. 11. Trephination of the donor cornea.
|
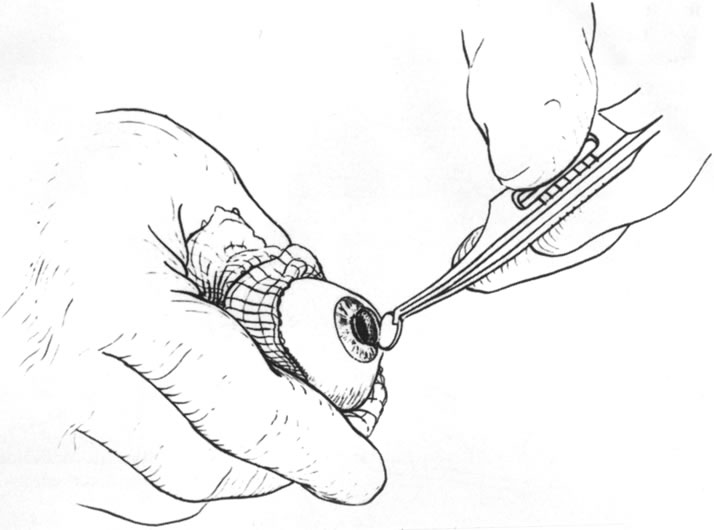 Fig. 12. Removal of the anterior dissected lamellar corneal tissue. Fig. 12. Removal of the anterior dissected lamellar corneal tissue.
|
Endothelium and Descemet's membrane can be readily removed before
use of the tissue. For a peripheral deep bed and a small graft, full-thickness
donor tissue may be obtained from the center of the donor
cornea, making it thinner than the recipient bed. Donor tissue that
is too thick may ride anterior to the recipient cornea and become disrupted
by patient blinking. Once the donor tissue is prepared, it is
sutured into the recipient bed with interrupted or continuous sutures (Figs. 13 and 14). Materials other than cornea may be used, such as sclera or periosteum, although
they are more difficult to work with than cornea. 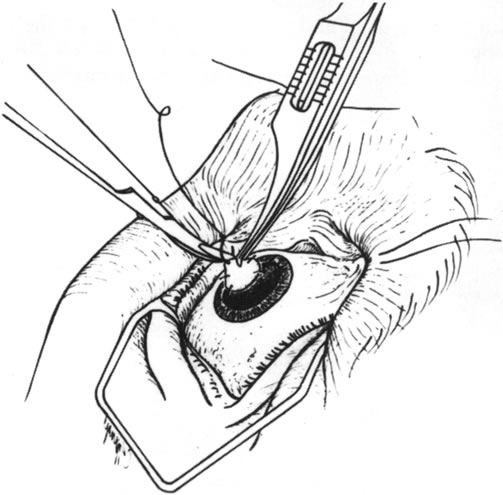 Fig. 13. Suturing the lamellar donor tissue into the dissected bed. Fig. 13. Suturing the lamellar donor tissue into the dissected bed.
|
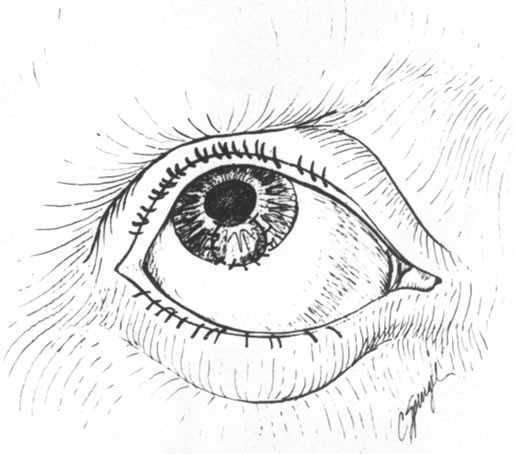 Fig. 14. Postoperative appearance of lamellar corneal patch graft. Fig. 14. Postoperative appearance of lamellar corneal patch graft.
|
Penetrating keratoplasty for corneal perforation is the most aggressive
approach but may also be mandated by the circumstances present. Large
perforations that are too large to seal with tissue adhesives or lamellar
patch grafting, and smaller perforations surrounded by large areas
of tissue necrosis may warrant penetrating grafts. The technique is
that of standard penetrating keratoplasty with modifications because of
the softness of the eye. With smaller perforations, tissue adhesives
may be used to temporarily plug the leak so that trephination may be
performed. Viscoelastics may be used to help form the anterior chamber
by injection through the perforation site. Either way, a trephine large
enough to surround all the necrotic tissue should be used. In a very
soft eye, it may be painted with methylene blue and used to make a mark
for later scissors cutting. In this circumstance, the scissors blade
is inserted through the perforation site and used to cut out to the
trephine mark and then to excise the tissue delimited by the mark. Once
the diseased cornea has been excised, the chamber is formed with viscoelastic
material, avoiding if possible excess iris manipulation because
of the profuse fibrin production that may be induced. A donor cornea
that is 0.25 to 0.50 mm larger is then sutured in place. Outcomes in penetrating keratoplasty for perforation depend in part on
the underlying disorder. Grafts in uninflamed eyes may do well, but outcomes
in inflamed eyes are less favorable. Keratoplasty “à chaud,” or
while hot, in eyes with microbial keratitis certainly
has a less favorable result than in eyes where infection is no longer
active. Kirkness and colleagues21 showed a 90% 5-year graft survival rate in eyes with noninfected
perforations or scarring subsequent to microbial keratitis, compared
with 51% in eyes with acute microbial keratitis with or
without perforation. Nobe and associates22 also showed a much higher failure rate in grafts done acutely. Nevertheless, in
these difficult circumstances, penetrating grafts may be the
only appropriate means of restoring ocular integrity. |