In infants less than 3 or 4 months of age, it is usually possible to perform tonometry using either a hand-held Goldmann applanation tonometer or an electronic tonometer, the Tono-Pen (Mentor Ophthalmics, Norwell, MA). The Schiøtz tonometer is relatively inaccurate when used to measure the intraocular pressure (IOP) of an infant eye and should be used only to gauge a general range of IOP. The young infant should be swaddled and held in its mother's arms while the eyelids are gently pried apart to perform tonometry. The Tono-Pen device, which has a very small (about 2 mm) measuring surface, is well suited to the small intrapalpebral fissures of an infant5 and can measure IOP without placing undue pressure on the lids; the use of a lid speculum can often be avoided. The observer's head does not need to be right next to the patient's, which can alarm some children. The Tono-Pen has been found to be accurate compared with both cannulated cadaver eyes and Goldmann tonometry,6,7 but no studies exist to assess its accuracy in infant eyes.
The office measurement of an IOP much greater than 20 mmHg in a calm, resting infant is suspicious for glaucoma when other signs and symptoms suggest the disease,8 as is an asymmetry of more than 5 mmHg in suspected unilateral or asymmetric cases. IOP measurements taken when the child is crying usually cannot be interpreted, because a nonglaucomatous infant can have an IOP in the forties or fifties when crying vigorously and squeezing the eyes shut.
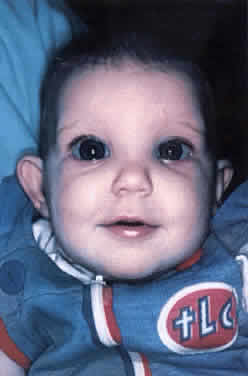
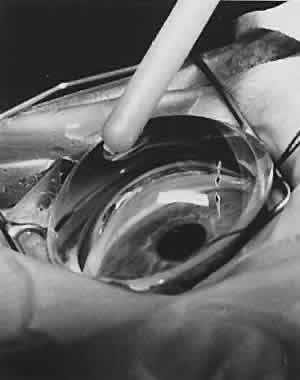
Funduscopy is at the heart of the diagnosis of congenital glaucoma. It can be greatly facilitated by using a Richardson or Koeppe (Ocular Instruments, Bellevue, WA) diagnostic infant gonioscopy lens that has no central depression. These lenses permit good visualization of the disc, even through a small pupil.
When the diagnosis of glaucoma is strongly suspected on the basis of the office evaluation, an examination under anesthesia should be recommended to the child's parents. Once the diagnosis is firmly established, the parents will be in a state of shock, unwilling to believe that their young child has a potentially blinding eye disease. The ophthalmologist should devote a significant amount of time to the counseling and education of the child's parents regarding the diagnostic and surgical plan that will be recommended. It must be emphasized to the parents at the outset that although the surgery is usually highly successful, a decade or more of follow-up will be necessary. Frequent examinations under anesthesia, amblyopia therapy, and other interventions are part of this long follow-up care, particularly in early childhood. Because some of the infantile and pediatric glaucomas represent genetic diseases,9–11 with subsequent siblings at increased risk for the disease, appropriate counseling by a clinical geneticist familiar with ophthalmic disease should be arranged.
Congenital and pediatric glaucomas are ophthalmic emergencies, and in most cases an examination under anesthesia and probable surgery should be scheduled within 24 to 72 hours of the initial diagnosis. When for logistical or medical reasons this proves impossible, the IOP can be lowered somewhat using aqueous suppressant medications. Topical β-adrenergic antagonists can often be safely used on an every-12-hour dosage schedule, but because the potential for systemic side effects is increased in a small infant, the lowest dose should be used initially, and the infant must be carefully monitored.12–14 Carbonic anhydrase inhibitors can also be used. Acetazolamide can be administered parenterally or orally (5 to 10 mg/kg every 6 hours). For outpatient use, the pharmacist will usually find it easiest to compound an elixir based on the parenteral powder; as much as 500 mg can be made up in 5 ml of strongly flavored elixir, but 250 mg/5 ml is more palatable. The elixir is stable for about 1 week at room temperature; refrigeration improves the taste but does not lengthen stability. In adults, the topical carbonic anhydrase inhibitors dorzolamide (Trusopt) and brinzolamide (Azopt) achieve IOP-lowering comparable to the systemic use of acetazolamide and additive to β-blockers; there are no published reports regarding their efficacy in infants, however.
The α2-agonist brimonidine (Alphagan) should be avoided in infants. After several reports of near-fatal apnea in young infants after dosing with brimonidine, the US Food and Drug Administration ordered relabeling of the drug to indicate its contraindication in small children. Although similar problems have not been reported with apraclonidine (Iopidine), because it is in the same class of medications it too should probably not be used in young children.
Latanoprost (Xalatan), a prostaglandin F2α analog, has proved extremely useful in the management of glaucoma in adults; in children, however, it appears less useful. Enyedi and coworkers15 found a clinically relevant IOP response in only a small portion of their patients, an experience mirrored in my practice. Altuna and colleagues16 found similar disappointing results in patients with glaucoma associated with Sturge-Weber syndrome.
Medical therapy of congenital glaucoma is not recommended for routine use and should be used only if an examination under anesthesia and surgery must be deferred. It is far better to proceed promptly with the definitive evaluation and treatment.
EXAMINATION UNDER ANESTHESIA
General anesthetics are known to have a potent effect on IOP in both normal and glaucomatous persons. The anesthesiologist responsible for administering the general anesthetic in these cases should be familiar with the issues involved and willing to work with the surgeon to obtain the most useful information possible during the crucial first few minutes of anesthesia.
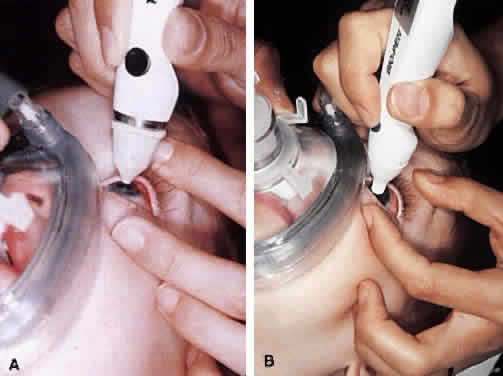
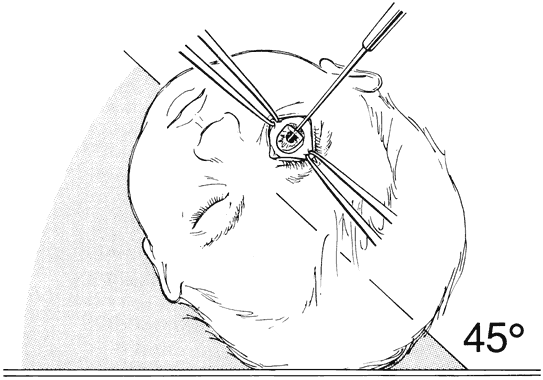
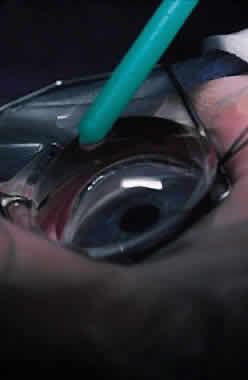
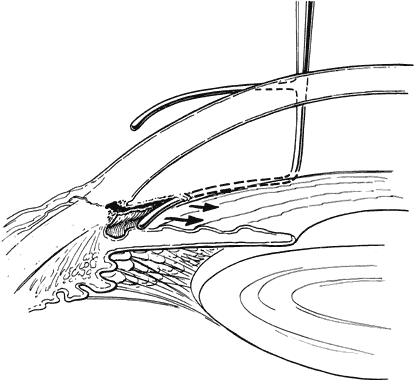
Virtually all of the inhalational anesthetics are known to depress the IOP within minutes of administration.17 Ketamine HCl slowly elevates the IOP as deeper anesthesia is attained.18,19 The benzodiazepines do not appear to have a significant effect on IOP when used in preoperative doses. Midazolam HCl (Versed) is often used as a preoperative sedative in children, and in many cases the child is sufficiently sedated with this medication to perform a quick IOP measurement before the administration of an inhalational general anesthetic. Such a measurement, with the child resting comfortably but not under the influence of the potent inhalational anesthetics, is probably the most accurate. If such a situation cannot be achieved, as in the case of a highly agitated infant or toddler, the anesthesiologist should be aware that it is preferable for the surgeon to measure the IOP as early as possible during the induction of general anesthesia (Fig. 2). At this point in the anesthetic induction, however, airway management is of paramount importance, and the surgeon must defer to the judgment of the anesthesiologist as to when measurements can safely be taken. In most cases, endotracheal intubation is appropriate.
Table 1 lists the information that should be obtained during an initial or follow-up examination under anesthesia. Once the initial IOP measurements are made, the surgeon should tell the anesthesiologist what will remain to be done during the rest of the examination if surgery is not planned. Portions of the examination that require a clear media, such as ophthalmoscopy, retinoscopy, and fundus photography, should be performed first, followed by axial length measurement, gonioscopy, and corneal diameter measurements.
TABLE 1. Essential Information for the Examination Under Anesthesia
Intraocular pressure
Optic nerve examination (drawing, cup-to-disc estimation, photography if
possible)
Fundus examination
Cycloplegic retinoscopy
Axial length determination
Corneal diameter (horizontal and vertical)
Gonioscopy
Preoperative dilation should be avoided if the iris and anterior chamber angle appearance will be important factors in making the initial diagnosis, or if corneal opacities prevent any view of the fundus. However, ophthalmoscopy, retinoscopy, and fundus photography are important parts of the initial and subsequent evaluations of a child with glaucoma, and preoperative dilation with a short-acting mydriatic should be performed before entering the operating room to avoid prolonging anesthesia while waiting for dilation. If necessary, intracameral acetylcholine can be instilled during surgery to constrict the pupil to allow safe intraocular maneuvers.
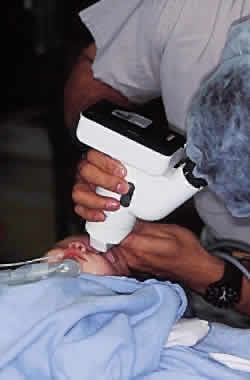
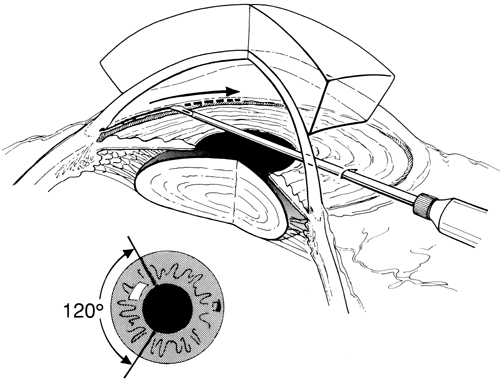
Funduscopy is essential, with both a direct and an indirect ophthalmoscope. The Richardson or Koeppe diagnostic gonioscopy lenses can be of great assistance (Fig. 3). The lens is placed, and then direct ophthalmoscopy is performed through the lens. After this, the periphery should be carefully evaluated with an indirect ophthalmoscope.
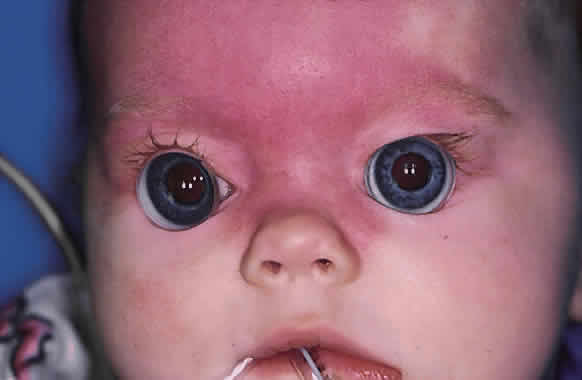
Fundus photography of the optic nerve allows the surgeon to document the extent of progression of the disease (Fig. 4). It is well known that the optic nerve cupping seen in congenital and juvenile glaucoma can to some extent reverse itself,20–22 and this can be used in some cases to gauge the success of therapy. Similarly, axial length measurements by ultrasonic biometry can help establish the degree of disparity between the two eyes, particularly in unilateral cases.23–25 In many cases the disparity decreases after successful therapy,23 and thus a constant or increasing disparity between the two eyes may signal a worsening clinical situation. The Bio-Pen hand-held ultrasonic biometric ruler (see Fig. 2) is a portable device that can be brought to the operating room for intraoperative biometry; because it is similar in size, operation, and appearance to the Tono-Pen, it can also be used in the office setting in young children who have become accustomed to IOP measurements being taken with that device.
Gonioscopy is a mandatory part of the examination under anesthesia. It allows the surgeon to identify the underlying congenital or juvenile glaucoma diagnosis and permits appropriate surgical planning. The Koeppe gonioscopic lens, introduced in 1919,26 has significant advantages over the Zeiss four-mirror or Goldmann three-mirror gonioprism. Chief among these is the ability to compare simultaneously the gonioscopic appearance of two eyes in the same patient (see Fig. 3). The surgeon should be familiar with the gonioscopic findings in primary congenital glaucoma as well as the various secondary glaucomas seen in childhood. Gonioscopic photography can help document the appearance of the anterior chamber angle before and after treatment.
The cornea should be measured both horizontally and vertically, and the presence or absence of Haab's striae or other corneal abnormalities should be noted. Enlargement of the corneal diameter over time is worrisome for progressive buphthalmos, and some clinicians believe these measurements to be more useful than axial length determinations.27 In many cases, however, these measurements are difficult to make accurately or consistently because of uncertain landmarks at the corneoscleral limbus.
Cycloplegic retinoscopy during follow-up examinations (both under anesthesia and in the office) are used to monitor for progressive axial myopia28 and to provide the spectacle correction to be used in conjunction with patching or other amblyopia therapy.