HISTORY
The ability of light energy to create a hole was demonstrated by Boerhave in the 18th century. He used the thermal effect of light energy focused through a magnifying glass to burn a hole in a piece of paper.1 In 1956, Meyer-Schwickerath reported the use of the light energy of the xenon arc photocoagulator to create iridotomies.2 This technique unfortunately yielded a high incidence of corneal and lenticular opacities. Subsequently, the ruby laser was used to create iridotomies in rabbits and humans.3–5 Zweng and colleagues, L'Esperance and Kelly, and Patz,6,7 in their pioneering work with continuous-wave argon laser energy, adapted it to be delivered through a slit-lamp system, enabling precise focusing as well as the possibility of treating the iris plane.
The word laser is an acronym for light amplification by stimulated emission of radiation. The argon laser has a wavelength of 454 to 514 nm and is an intense coherent light beam of pure blue-green color. It is located in the part of the spectrum that is optimal for effective interaction with both hemoglobin and pigment. These properties, and the ability to focus the energy precisely on the plane of the iris, initially made continuous-wave argon laser energy the ideal method of creating holes in the iris. The terms iridectomy and iridotomy are used interchangeably.
Several investigators report series of patients in whom argon laser energy was used to produce iridectomies.8–11 Difficulties in achieving patent iridectomies in eyes with dark brown irides or light blue irides were overcome by modifying treatment parameters and using a special contact lens developed by Abraham.
From these early reports beginning in 1973, laser iridectomy has been performed on hundreds of thousands of patients. The relative safety of the procedure and ease of performance combined with few complications accounted for its overwhelming acceptance by ophthalmologists. By the early 1980s, argon laser iridectomy had replaced the traditional surgical iridectomy as the procedure of choice for creating a hole in the iris.
It was in 1984 that the first reports of the successful performance of an iridectomy using the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser were reported.12,13 Moster and associates in 1986 reported a controlled study comparing argon and Nd:YAG iridectomies.14 The mean number of applications to produce iris penetration was 6 with the Nd:YAG laser and 73 with the argon laser. Microhyphema was more prevalent in the Nd:YAG iridectomy group, but there was less inflammation.
In 1987, Tomey and colleagues reported on 271 consecutive patients (373 eyes) in whom Nd:YAG laser iridectomy had been performed.15 They believed that the Nd:YAG laser offered the following advantages: lower energy level; lower incidence of spontaneous closure of the iridotomy; less inflammation; fewer required applications; absence of thermal injury to the cornea, lens, and retina; efficacy with opaque corneas; and effectiveness independent of iris color.
The Nd:YAG laser creates iridotomies by a different mechanical principal than the argon laser. The laser medium contains yttrium, aluminum, and garnet crystal with suspended neodymium atoms. It has a wavelength of 1064 nm, which is invisible to the naked eye and is in the infrared range, but it is paired with a low-powered continuous-wave helium neon aiming beam that produces a red light, which is used for focusing. Most Nd:YAG lasers are of the fundamental q-switched type and work by producing photodisruption of tissue. The photodisruption process releases shock waves that mechanically cause tissue disruption. Iris color and the presence of melanin pigment, which is important in argon laser iridectomy technique, is not a significant factor with the Nd:YAG laser. Some Nd:YAG lasers are capable of producing thermal effects using continuous-wave or pulsed action.
During the late 1980s the Nd:YAG laser became the laser of initial choice for performing iridectomies. This is because of the relative ease of performance, the reduced rate of closure of the iridectomies, and less postoperative inflammation.
INDICATIONS
Early attempts at argon laser iridectomy were not consistently successful; this was discouraging and limited its use. However, with improvements in laser design and the development of the Abraham iridectomy lens, now any patient requiring an iridectomy is considered a laser candidate. An iridectomy is indicated to relieve angle-closure glaucoma caused by a pupillary block mechanism. In eyes with angle closure caused by uveitis (without posterior synechiae), neovascular glaucoma, or the iridocorneal endothelial syndrome, an iridectomy is not helpful or indicated because the angle-closure glaucoma is not secondary to a pupillary block mechanism. Laser iridectomy is the procedure of choice in all forms of angle-closure glaucoma in which the pupillary block mechanism exists.
The indications for laser iridectomy have replaced most of the indications for a surgical iridectomy (Table 1). Currently, most residents do not get the opportunity to see or perform a surgical iridectomy, and this is a tribute to the development of laser technology and the improved techniques. A surgical iridectomy is done only if a laser iridectomy cannot be performed. This may occur in eyes with uncontrolled pressure and significant corneal edema that cannot be cleared by medical treatment. Eyes with flat anterior chambers or eyes with corneas that are so scarred that iris details cannot be seen also may not be able to have a laser iridectomy performed.
TABLE 1. Indications for Laser Iridectomy
Nonperforate surgical iridectomy
Acute angle-closure glaucoma
Fellow eye of a patient with acute angle-closure glaucoma
Chronic angle-closure glaucoma
Positive provocative test result
Aphakic or pseudophakic pupillary block
Uveitis with 360° posterior synechiae
Before a trabeculoplasty to open the angle approach and facilitate treatment
Differentiating a pupillary block in aphakia or pseudophakia from ciliovitreal
block
With the advent of the Nd:YAG laser and the development of the Wise lens with a + 103 D button, it is possible to achieve a patent iridectomy by using laser-directed energy in virtually 100% of patients.15
PREOPERATIVE PREPARATION
The important aspects of preparation involve the maintenance of the clearest cornea possible, a constricted pupil, and control of inflammation and intraocular pressure (IOP). If an eye has experienced an acute attack that is being treated medically but still is inflamed or has an edematous cornea that precludes safely performing an iridectomy, it may be more prudent to control the inflammation, let the corneal edema clear, and perform the iridectomy later. The patient should be placed on a miotic during this interval plus instructed to frequently use topical steroids and any other glaucoma medications necessary to control IOP. During this treatment interval, the fellow eye should have a laser iridectomy performed to eliminate the possibility of it having an acute attack. However, if it is feasible to perform the iridectomy in the acute eye on the day of presentation, it should be done, thus eliminating the possibility of a subsequent attack of angle closure.
If the patient has noncongestive narrow-angle glaucoma and is on a miotic, the miotic should be instilled 1 hour before laser surgery. If the patient has not been on a miotic, pilocarpine 2% should be instilled 1 hour before laser surgery. The miotic constricts the pupil and promotes penetration of the laser energy by placing the iris on stretch and making it thinner. It also reduces the contraction of the iris and pupil peaking, which sometimes is seen as the iris is treated with argon laser energy. A small pupil also reduces the possibility of laser energy inadvertently going through the pupil and striking the retina. The eye undergoing laser is routinely pretreated with topical apraclonidine. It constricts blood vessels and reduces the chance of a pressure spike. This assumes that a miotic and beta blocker, if not contraindicated, have already been used. It is recommended that any procedure adversely affecting corneal clarity not be performed on the day of laser treatment.
Laser Contact Lens
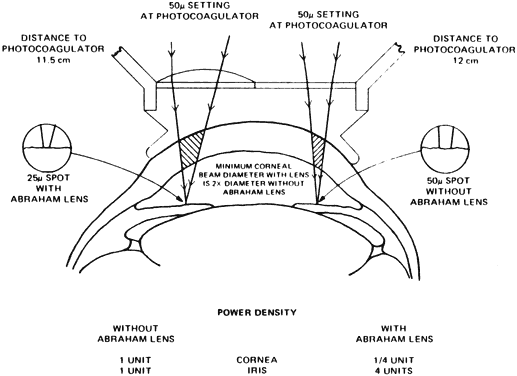
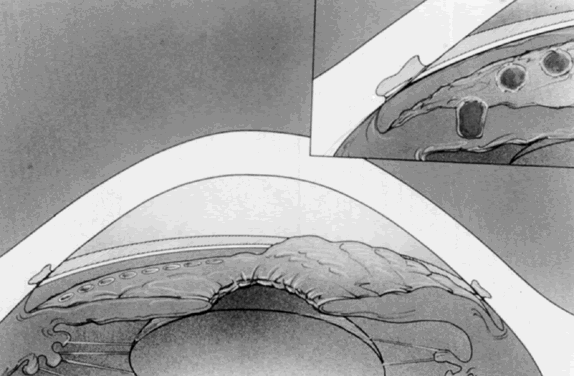
The use of an antireflection-coated lens with a strong plus button greatly facilitates the ability to penetrate the iris and has improved the success rate in achieving iridectomies. It is so important to the success of the procedure that its use is considered mandatory. The Abraham lens consists of a modified Goldmann-type fundus lens with an 8-mm hole trephined into its periphery (Fig. 1) and a 66-D plano convex button bonded into the trephine hole. The Wise lens has a 103-D button. All front surfaces are covered with an antireflective coating. The convex lens enlarges the laser beam at the corneal surface, thus reducing the chances of epithelial corneal changes and decreasing the laser beam diameter at the iris surface, which increases the power density (Fig. 2). The power density at the cornea is one fourth as great with the Abraham lens but is increased to four times as great at the iris surface, which facilitates penetration. The lens also holds the lids apart and allows the surgeon to control eye movements. The gonioscopic solution reduces heat buildup and decreases the incidence of corneal burns.
|
|
The excellent optics of these lenses improves the visibility and magnifies the treatment site, thus reducing the depth of field and improving the precision of the laser focusing. All of these factors make these lenses an important adjunct to the successful production of iridectomies.
SURGICAL TECHNIQUE
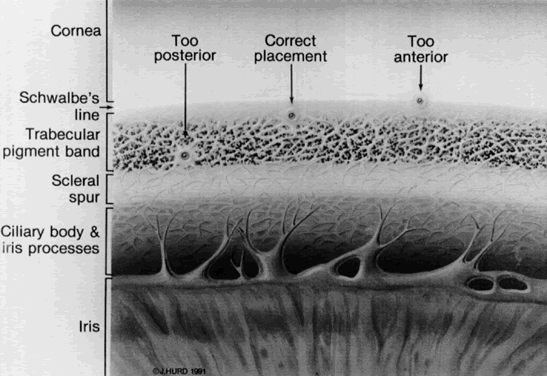
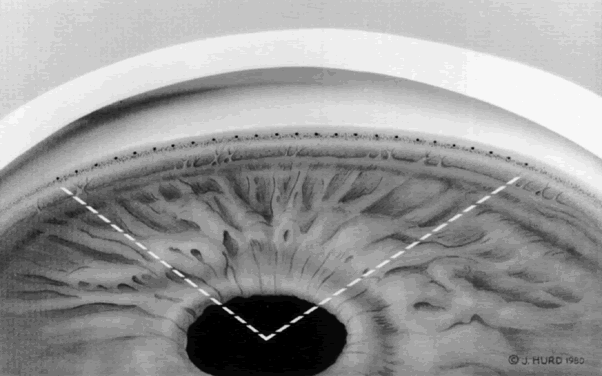
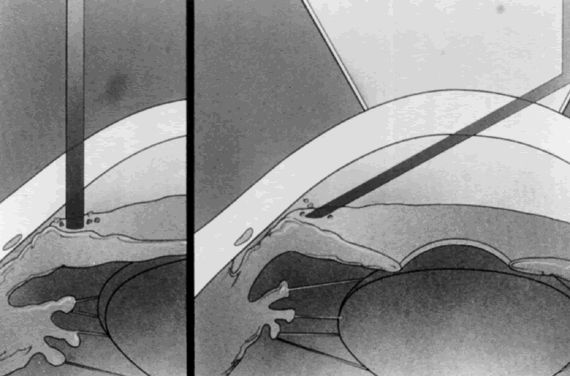
Both the surgeon and patient should be as comfortable as possible. A Velcro head strap helps to keep the patient well positioned with the forehead against the head bar. Patients tend to move backward, away from the slit lamp when the contact lens is placed on their cornea, and this makes precise focusing more difficult. An elbow rest is helpful to steady the surgeon's arm and reduce fatigue. A patient's shirt collar button may be released and tie loosened, and patients should be cautioned not to hold their breath. The laser room should be well ventilated to ensure patient comfort. A drop of topical anesthesia is used in all patients. Retrobulbar anesthesia is not necessary. The contact lens is placed on the eye with the trephined hole superiorly. The location of the iridectomy should be in the midperiphery of a constricted iris, about one third the distance from the limbus to the pupil, usually between the half past 10 and half past 1 o'clock positions (Fig. 3). By placing the iridectomy under the upper lid, the possibility of a postoperative problem with glare or diplopia is reduced. If temporal treatment is being performed, it is important to aim the argon laser beam away from the macula. The aiming beam should be perpendicular to the contact lens surface and through the center of the lens button.
|
Argon Iridectomy
Many authors recommend a variety of techniques to facilitate the performance of an argon iridectomy. Some of these include placing stretch burns in a circle (drumhead technique) and then placing penetrating burns in the center of the circle. Abraham and Miller recommend using stretch burns to cause a hump in the iris and then using penetrating burns to punch through the hump.9 The stretch burns contract the iris, but they are not used for penetration. Stretch burns usually are placed with a 200-μm spot size and a 0.2-second duration, using 200 to 400 mW of power.
A well-accepted standard technique using the argon laser is to begin initial treatment with a 50-μm spot size and a 0.2-second duration, with about 850 mW of power. The aiming beam must be precisely focused on the surface of the iris to superimpose the burns. These two factors—precise focusing and superimposition—are the keys to success. Often, initial penetration can be achieved within 10 applications, and for any iridectomy, the tissue reaction to the initial burns often is an indicator as to the ease of the procedure. If bubble formation is seen at the site of the burn, this indicates stromal vaporization. The clinician then should focus at the base of the bubble, continuing to deliver energy until the stroma has been penetrated. This is helpful in the light blue iris.
The easiest irides to penetrate with the argon laser are hazel and light brown. The most difficult are light blue irides with minimal pigment and thick, dark brown irides. If good progress is not made at the initial treatment site, the clinician can move to another site. However, if charring is seen, which has the appearance of a black piece of coal, then switching to a chipping technique has been found to be helpful in dark brown irides. The duration is reduced to 0.02 seconds and the power increased to 1 to 1.5 W. This short-duration burn avoids charring at the base and reduces the chance of endothelial burns. However, it may take 100 to 300 applications to penetrate and complete the iridectomy using these short-duration burns. Since the development of the Nd:YAG laser, this has become less of a problem, and if good progress is not being made with the argon laser, it is prudent to switch to the Nd:YAG laser and punch through. In most cases, the Nd:YAG laser is the laser of initial choice.
Light blue irides require a different technique. Blue eyes have little melanin pigment in the stroma, and a longer duration burn provides an increased thermal effect, which is necessary to get through both stroma and pigment epithelium in these lightly pigmented irides. A power setting of 800 to 1000 mW and duration of 0.2 to 0.5 seconds is used with a 50-μm spot size. Table 2 outlines the recommended treatment parameters for argon laser iridectomy. The clinician looks for an iris freckle or an iris crypt that would make penetration easier. The patient's eye usually will not stay still for the full 0.5 seconds, so it is necessary to be able to quickly cut off the laser energy before 0.5 seconds if eye movement is seen. A vaporization bubble sometimes is seen at these settings, and by aiming at the base of the bubble, it is often feasible to achieve penetration in two or three shots of 0.5 seconds' duration each. The signal that the laser beam has penetrated the pigment epithelium is a stream of pigment clumps carried by the posterior chamber aqueous humor into the anterior chamber in a mushroom-cloud configuration. Most of the argon laser treatment is directed toward enlarging the iridectomy and cleaning the pigment out from its edges. The edges of the initial penetration site are treated with laser energy to enlarge the actual opening and reduce the chance of subsequent closure from pigment proliferation. Care must be used because laser energy will be going through the iridectomy, possibly hitting the lens and retina.
TABLE 2. Treatment Parameters for Argon Laser Iridectomy
| Spot Size | Power | Duration | |
| Iris Color | (μm) | (mW) | (s) |
| Hazel | 50 | 850 | 0.2 |
| Brown | 50 | 850 | 0.2 |
| Thick brown | 50 | 1000–1500 | 0.02–0.05 |
| Blue | 50 | 1000 | 0.2–0.5 |
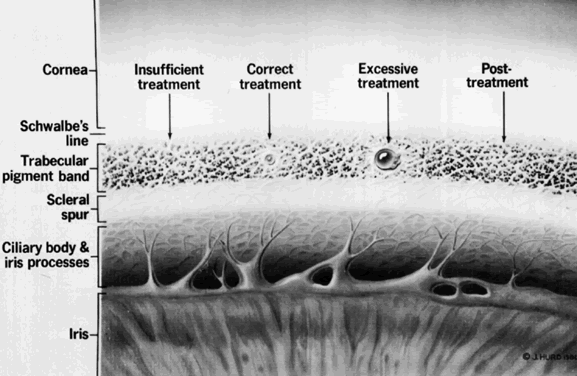
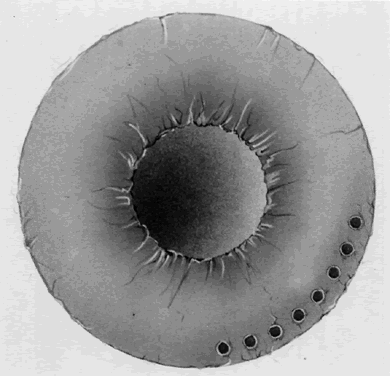
The endpoint of treatment is the direct visualization of the anterior lens capsule with the slit lamp. Transillumination is not a good technique to determine the patency of an iridectomy. There can be extensive transillumination, especially in blue eyes, whereas the iris stroma still may be intact with no through-and-through iridectomy (Fig. 4). After the iridectomy, the central anterior chamber depth usually is not affected; however, deepening of the peripheral anterior chamber is a reliable sign that the iridectomy has achieved its functional purpose.
|
In eyes with light blue irides, the Nd:YAG laser has distinct advantages and has been a major improvement.
Nd:YAG Iridectomy
Recommended settings for the Nd:YAG laser range from 3 to 7 mJ per burst and from one to three pulses per burst. It is safest to begin with a single pulse of approximately 4 to 6 mJ. As with argon iridectomy, the importance of precise focusing on the anterior stroma cannot be overemphasized. This produces maximum photodisruption and minimizes the possibility of lens injury.
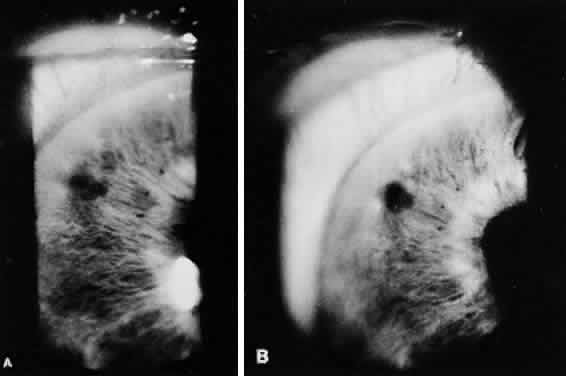
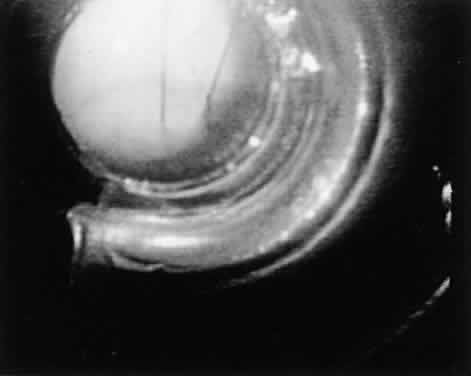
Eyes with thick brown irides are more difficult to penetrate. Sometimes, the iris stroma shreds without a through-and-through opening achieved. It can be helpful to thin out a cryptlike area with the argon laser and then punch through with the Nd:YAG laser energy. In a few cases, the reverse procedure is followed: the Nd:YAG laser is initially used, and the iridectomy opening can be completed with argon laser energy. In thick brown irides, using two to three pulses per burst often is helpful. The iridectomy typically appears slitlike (Fig. 5). In patients who are on anticoagulant therapy or have bleeding disorders, it is advisable to use argon laser energy. Bleeding from the iridectomy site is rare with the argon laser because the tissue destruction involves thermal coagulation. Bleeding at the iridectomy site is common with the use of Nd:YAG laser energy because the photodisruption process (Fig. 6) does not coagulate the iris vessels. Moster and colleagues report bleeding from the iridectomy site in 34% of eyes after Nd:YAG iridectomy but in no eyes after argon laser iridectomy.14
|
POSTOPERATIVE TREATMENT
The major postoperative treatment involves monitoring and controlling IOP and the expected mild postoperative inflammation. Moster and colleagues report that 76% of patients had a maximal elevation of IOP in the first hour, whereas 16% peak at the second hour, and 8% reached maximal elevation during the third hour.14 Thirty-one percent of the Nd:YAG laser-treated eyes and 34% of the argon laser-treated eyes had an IOP rise greater than 8 mmHg above baseline. Therefore, we routinely pretreat all eyes with apraclonidine and monitor IOP after a laser iridectomy procedure. If there has been a pressure spike, the patient is seen the next day; otherwise, the patient is seen between 5 and 7 days later, at which time the second eye can undergo laser treatment if necessary.
Methods to reduce the incidence and magnitude of a postlaser iridectomy pressure spike include pretreatment with apraclonidine and miotics if they have not been used in the last 2 hours, instilling a beta blocker if not contraindicated, and considering the use of a topical carbonic anhydrase inhibitor if there is significant cupping and field loss. Robin and associates demonstrated that topical paraaminoclonidine (apraclonidine hydrochloride; Iopidine, Alcon) was effective in reducing significant pressure spikes after argon laser iridectomy.16 Forty-three percent of eyes treated with placebo and no eyes treated with apraclonidine had an IOP rise more than 10 mmHg above baseline. By reducing the incidence and magnitude of the pressure spike, there is less chance for further glaucomatous damage. No significant differences in postoperative IOP spikes have been found between the two types of lasers.
The IOP elevation may be related to the release of pigment and inflammatory debris clogging the trabecular meshwork. In eyes with poorly controlled IOP or eyes that have severely compromised outflow facilities, IOP elevations are more likely to be seen. By monitoring the IOP postoperatively if there is a significant elevation, treatment with pressure-lowering agents can be carried out. Tonographic studies done in monkeys at varying intervals after laser iridectomy have not shown decreases in outflow facility, which indicates the pigment liberated does not permanently clog the trabecular meshwork.17 Topical steroids are used to control iritis, which is seen in all patients. The usual treatment schedule is one drop of prednisolone acetate four times a day, and this usually can be discontinued by 1 week postoperatively. Miotics are continued if the patient has been on them preoperatively. Miotics also are used if there is a question about the patency of the iridectomy.
If there is a patent iridectomy, the pupil is dilated on an early postoperative visit to prevent the formation of posterior synechiae and to visualize the posterior pole and peripheral retina, which might not have been possible before. Also, dilating the pupil postoperatively serves as a mydriatic provocative test to rule out the possibility of a plateau-iris configuration. Gonioscopy always should be done on the first postoperative visit. Gonioscopy is important to assess the effect of the iridectomy on the angle configuration and to determine the extent of permanent synechiae, if present. The patient is observed over the next 6 weeks to watch for pigment proliferation leading to closure of the iridectomy. If closure occurs, the iris is retreated to open the iridectomy and enlarge it.
COMPLICATIONS OF SURGERY
Corneal Changes
Corneal changes include epithelial and endothelial burns. They usually are transient but can impair the ability to complete an iridectomy because the laser energy cannot be delivered accurately and effectively. It helps to position the eye and aim through a noninvolved portion of the cornea. It may be necessary to go to another location if visibility is not adequate. Endothelial burns are caused by the thermal effects of treating the iris, especially in eyes with shallow chambers. They usually resolve within a few weeks but can cause focal endothelial cell loss. Investigators have not been able to demonstrate statistically significant endothelial cell loss during follow-up periods of 1 year.18,19 Nd:YAG iridectomies can cause focal endothelial cell loss if the disruption takes place less than 1 mm from the corneal endothelium.20
Schwartz and coworkers report on five eyes of three patients who developed corneal decompensation after undergoing argon laser iridectomy for angle-closure glaucoma.21 They found that a history of preexisting corneal guttata, episodes of angle-closure glaucoma with elevated IOP and inflammation, and the need for repeat treatments all seemed to predispose to this rare complication.
Lens Opacities
Focal anterior subcapsular lens opacities at the iridectomy site frequently are seen with argon laser iridectomies and also can be found after Nd:YAG iridectomy (Fig. 7). Long-term follow-up demonstrates that the opacities are nonprogressive and the visual loss from cataracts in patients who have undergone laser iridectomies is similar to an age-matched control group.22 Breaks in the anterior lens capsule in eyes undergoing Nd:YAG iridectomy have been demonstrated histopathologically in patients subsequently undergoing intracapsular cataract extraction.23 However, the widespread concern that the Nd:YAG laser may cause the rapid development of cataracts because of inadvertent rupture of the anterior lens capsule fortunately has not been shown. No differences in cataract formation were demonstrated in rabbit eyes when comparing argon and Nd:YAG laser iridectomies.24
|
Treatment parameters of one or two bursts and less than 6 mJ reduce the incidence of lens opacities in monkeys.25 It also is critical to focus on the anterior iris stroma and to perform the YAG iridectomy peripherally, where the patient's convex lens is further away from the pigmented epithelium of the iris. If a capsule break should occur, animal studies show that fibrous proliferation covers the anterior capsule break.25
Closure of the Iridectomy
Closure of a patent laser iridectomy may be either immediate or delayed. It is important to recheck the iridectomy site at the same time that the patient's IOP is monitored postoperatively because the iridectomy site may be occluded by the landsliding of pigment epithelium into it from its edges. The opening can be enlarged, if necessary, with a few more applications of laser energy. If late closure occurs, it usually occurs by 6 weeks and is a result of pigment proliferation. Performing a large, clean initial iridectomy reduces the likelihood of an iridectomy closing and the need for retreatment. Blue irides treated with the argon laser had a 35% incidence of retreatment in our series of over 200 eyes treated between 1977 and 1982, whereas brown irides had only a 15% retreatment rate. The blue eyes had the greater need for retreatment because of the difficulty of obtaining a large iridectomy and tendency for pigment proliferation to fill in the iridectomy (Fig. 8). One of the major advantages of the Nd:YAG laser is its much reduced incidence of iridectomy closure. With photodisruption, there is not the same tendency for iris pigment proliferation, and this is a distinct advantage.
|
Diplopia and Glare
Occasionally, patients may complain of an extra image or a light reflex from the iridectomy site. By placing the iridectomy under the upper lid, these complications are significantly reduced. However, some iridectomies have been placed inferiorly with no problems. If the iridectomy is located right at the border of the upper lid margin, these symptoms seem more likely to occur.
ADVANTAGES
It is no longer necessary to compare the advantages of laser iridectomy with surgical iridectomy. Laser iridectomy has revolutionized the management of angle-closure glaucoma. It has unburdened both the patient and the ophthalmologist. In the past, one of the hardest tasks was to convince a patient who had just experienced an acute congestive attack of angle-closure glaucoma to undergo an intraocular surgical procedure in a nonsymptomatic “healthy eye” while the acute eye was quieting down.
Another difficult scenario was that of the patient with creeping angle-closure glaucoma who was totally asymptomatic and needed bilateral surgical iridectomies. In patients with combined-mechanism glaucoma, a laser iridectomy can be performed to determine if it alone will control the IOP without having to subject the patient to two intraoperative procedures if filtering surgery was to be eventually necessary. Since laser iridectomies are relatively safe and simple to perform, it is a concern that the indications can be easily abused, and that patients who do not need an iridectomy will be offered it. This may be a result of an inaccurate gonioscopic examination, a subjective examination, or one not uniformly mastered. Laser iridectomy also may be unnecessarily performed if the mechanism of primary angle closure is not truly understood. It then might be performed in eyes with peripheral anterior synechiae that are secondary to uveitis, rubeosis, or iridocorneal endothelial syndrome, disorders caused by a nonpupillary block angle-closure mechanism.
DISADVANTAGES
Compared with surgical iridectomy, there are no disadvantages to laser iridectomy. However, it may not be possible to perform a laser iridectomy if there is significant corneal edema or a flat chamber. If penetration cannot be achieved, a surgical iridectomy then must be performed. With argon laser energy there is a risk of a macular burn, and with both types of laser treatment there is the risk of a postoperative IOP spike and possible visual field loss involving fixation. Laser surgery requires the use of costly equipment that must be well maintained, and this may become more of a problem unless the cost of laser instruments decreases to match changes in reimbursement, an unlikely event.