| The evolution of phaco from the initial procedure as described by Kelman9 in the late 1960s to the techniques that we currently practice is nothing
less than remarkable. The contributions of talented ophthalmic surgeons
who persevered throughout these years should be commended, because
they laid the groundwork for our present methods. The major distinction between the phaco techniques practiced today and
the earlier techniques is that modern methods have facilitated phaco of
dense cataracts within the capsular bag, allowing the central endonucleus
to be removed before the epinucleus is encountered. With previous
techniques, we worked from the peripheral portion of the epinucleus/nucleus
complex toward the center. This change was influenced by the recognition
that the nuclear mass of firm and hard lenses could be divided
into smaller pieces for controlled removal within the protective layer
of the epinucleus and that a capsular opening produced by a CCC would
withstand the forces involved in nuclear cracking. Retention of an
intact CCC opening also required sequential microsurgical removal of
the contents of the capsular bag, which is best achieved by performing
phaco in the central and deepest portion of the anterior chamber. DIVIDE AND CONQUER TECHNIQUE Divide and conquer nucleofractis phaco, described by Gimbel,42 was the first nucleofractis (two-instrument) cracking
technique developed. After adequate hydrodissection, a deep crater is
sculpted into the center of the nucleus, leaving a dense peripheral rim
that can later be fractured into multiple sections. It is important
that the crater include the posterior plate of the nucleus; otherwise, fracturing
of the rim is much more difficult. A shaving action is used
to sculpt away the central nuclear material. When the central material
is no longer accessible to the phaco probe, the lens should be rotated
and additional central phaco performed to enlarge and deepen the
crater. The size of the central crater should be expanded for progressively
denser nuclei. Enough of the dense material must be left in place, however, to
allow the phaco probe and second instrument to engage the
rim and fracture the lens into sections. The surgeon uses his experience as a guide to determine how deeply the
central crater should be sculpted. The peripheral nuclear rim stretches
the entire capsular bag and acts as a safety mechanism to prevent the
posterior capsule from suddenly moving anteriorly and being cut by the
phaco probe. For harder nuclei, small sections should be fractured
from the rim. Rather than emulsify the sections as they are broken away, the
sections should be left in place within the rim to maintain the
circular rim and the tension on the capsule. Leaving the sections in
place also facilitates rotation and the progressive fracturing of the
remaining rim. It is sometimes advisable to initially remove one small
section to allow space for fracturing of the other segments of the remaining
rim. If only a small fragment is removed, the remaining segments
can maintain capsular stretch and help to avoid rupture of the capsule. After
the rim is fractured around the entirety of its circumference, each
segment can then be brought to the center of the capsule for
safe emulsification. One must be more cautious at this point because as
more segments are removed, less lens material is available to expand
the capsule and the capsule will have a greater tendency to be aspirated
into the phaco tip, especially if high aspiration flow rates are used. PHACO FRACTURE TECHNIQUE In phaco fracture, a widely used nucleofractis technique described by Shepherd,43 the surgeon sculpts a groove from the 12- to 6-o'clock
position after performing hydrodissection and hydrodelineation. The
width of the groove should be one and a half to two times the diameter
of the phaco tip. Using the phaco handpiece and a second instrument, the
surgeon rotates the nucleus 90 degrees. A second groove is sculpted
perpendicular to the first, in the form of a cross. Sculpting continues
until the red reflex is seen at the bottom of the grooves. Additional
rotations and removal of nuclear material is often necessary to
accomplish adequate grooving. Care should be taken to avoid sculpting
completely through to the cortex peripherally, because this procedure
puts the equatorial and posterior capsule at increased risk of damage. A
bimanual cracking technique is used to create a fracture through the
nuclear rim in the plane of one of the grooves. The nucleus is then
rotated 90 degrees, and additional fractures are made until four separate
quadrants are isolated. The segments are then tumbled toward the center
of the capsule for safe emulsification. A short burst of phaco power
is used to embed the phaco tip into the bulk of the isolated quadrant, and
then with the use of aspiration, the quadrant is gently pulled
into the center for emulsification. Alternatively, the second instrument
can be used to elevate the apex of the wedge to facilitate mobilization
of the nuclear quadrant to the capsule's center. CHIP AND FLIP TECHNIQUE Introduced by Fine,44 and useful for softer grades of nuclei, this procedure relies on a nucleus
that rotates freely within the capsular bag. Initially, a central
bowl is sculpted in the nucleus until a thin central plate remains. The
second instrument introduced through the side port incision engages
the subincisional nuclear rim to move the inferior nuclear rim to toward
the center of the capsule bag. Then clock-hour pieces of the
rim are carefully emulsified as the nucleus is rotated. Once the entire
rim is removed, the second instrument is used to elevate the remaining
central thinned nuclear plate (the chip), which is then
emulsified. The epinucleus is engaged at the 6-o'clock position
with aspiration alone. As the phaco tip is moved superiorly, the
second instrument pushes the epinucleus toward the 6-o'clock
position, thereby tumbling the epinuclear bowl and permitting it
to be aspirated (the flip). CRACK AND FLIP TECHNIQUE Fine and colleagues modified Shepherd's phaco fracture technique by
adding hydrodelineation, resulting in the crack and flip technique.45 Sculpting two deep grooves at right angles to each other that extend to
the golden ring permits bimanual nucleus cracking. Only the endonucleus
cracks, because the epinucleus is separated from it by hydrodelineation. Each
quadrant is then sequentially removed with the use of pulsed
phaco and moderate aspiration. The second instrument elevates the apices
of each quadrant so that the tip of the phaco needle can be totally
occluded to aid in aspiration. Once the nucleus is removed, the epinucleus
is aspirated as with the chip and flip technique. There are several helpful tips in using the crack and flip technique or
a modification of this method. The sculpting portion of the procedure
is performed with minimal vacuum, relatively low aspiration flow, and
low phaco power. The forward passes of the phaco needle only shave the
nuclear material to ultimately sculpt a groove. The phaco tip is never
totally occluded during this phase of the operation. Rather, only a
portion of the phaco needle contacts the nucleus to remove controlled
amounts of lens material. This process is continued until the grooves
are deep. The appropriate depth can be assessed by a brightening of the
red reflex, which suggests that the denser portion of the nucleus has
been emulsified on the region of the grooves. To achieve nuclear cracking, two instruments are placed deeply in the grooves
and moved down and outward. The phaco needle and a second instrument
introduced through the side port, paracentesis, or incision will
crack the nucleus if the grooves are sufficiently deep and the instruments
placed in the depth of the grooves. If cracking does not readily
occur, additional deepening of the grooves is warranted. Phaco energy
is not required during this step. The limit of the grooves is the golden
ring, which represents the perimeter of the endonucleus. The loosened
quadrants of the endonucleus remain within the cushion of the surrounding
epinucleus. Removing the nuclear fragments requires a change in the parameters for
phaco. For this step, it is desirable to have lens material in contact
with the phaco needle. Increasing the aspiration flow slightly directs
lens material to the phaco tip, whereas increasing the vacuum encourages
the nuclear fragments to be aspirated with application of only a
minimum of phaco power. These parameters are influenced by the density
of the nucleus, but in principle, these settings result in successful
nucleus removal. A second instrument guides the control of nuclear fragments. Removing the epinucleus is accomplished as described in the chip and flip
section. The parameters can be modified such that (1) the
aspiration flow is slightly reduced from the setting used in nuclear
fragment removal, and (2) pulsed phaco power is used. If cortical
cleaving hydrodissection is successful, the cortex is removed, along
with the epinucleus during this step of the procedure. PHACO CHOP The phaco chop technique was initially introduced by Nagahara, who used
the natural fault lines in the lens nucleus to create cracks without
creating prior grooves (Nagahara K, American Society of Cataract
and Refractive Surgery film festival, 1993). The phaco tip is embedded
in the center of the nucleus after the superficial cortex is aspirated. A
second instrument, the phaco chopper, is then passed to the
equator of the nucleus, beneath the anterior capsule, and drawn to the
phaco tip to fracture the nucleus. The two instruments are separated
to widen the crack. This procedure is repeated until several small fragments
are created, which are then emulsified. Koch and Katzen46 modified this procedure because they encountered difficulty in mobilizing
the nuclear fragments. They created a central groove or central crater, depending
on the density of the nucleus. This modification permits
ease of removing the nuclear fragments liberated by the phaco chop
technique. Advocates of these nucleus-dividing techniques have suggested that
high levels of vacuum help remove the nuclear fragments and minimize
the need for ultrasound energy. With some of the newer phaco instruments, higher
vacuum power can be applied with minimal risk of anterior
chamber collapse. CHOO CHOO CHOP AND FLIP Fine described the “choo-choo chop and flip” technique
in 1998.47 Subsequently, Fine, Packer, and Hoffman correlated the reduction of ultrasound
energy with this technique to improvement in uncorrected post
operative day one visual acuity.48 A 30-degree standard bevel down tip is used throughout endonuclear
removal. After aspirating the epinucleus uncovered by the capsulorhexis, a
Fine/Nagahara chopper (Rhein Medical, Tampa, FL) is
placed in the golden ring by touching the center of the nucleus with
the tip and pushing it peripherally so that it reflects the capsulorhexis. The
chopper is used to stabilize the nucleus by lifting and pulling
toward the incision slightly (Fig. 3), after which the phaco tip lollipops the nucleus in either pulse
mode at 2 pulses/second or 80 millisecond burst mode. 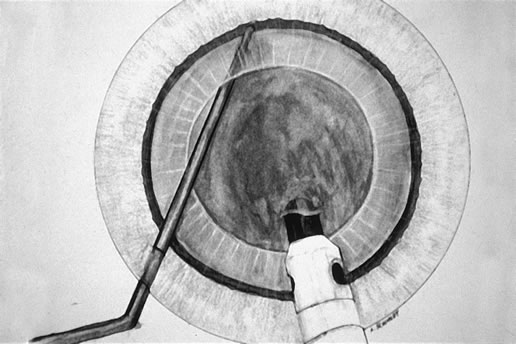 Fig. 3. The Fine-Nagahara chopper supports the endonucleus as the phaco
tip is advanced in foot position three and achieves a firm purchase. Fig. 3. The Fine-Nagahara chopper supports the endonucleus as the phaco
tip is advanced in foot position three and achieves a firm purchase.
|
Burst mode is a power modulation that utilizes a fixed percent power (panel
control), a programmable burst width (duration of
power), and a linear interval between bursts. As one enters foot position 3, the interval between bursts
is 2 seconds; with increasing depressions of the foot pedal in foot
position 3 the interval shortens until at the bottom of foot position 3 there
is continuous phaco. In pulse mode, there is linear power (%) but a fixed interval between pulses, resulting at 2 pulses/sec in a 250-millisecond pulse (linear
power), followed by a 250-millisecond pause in power, followed
by a 250-millisecond pulse, and so on. However, in both
of these modulations with tip occlusion, vacuum is continuous throughout
the pulse and pause intervals. With the energy delivered in this way, ultrasound
energy into the eye is minimized and hold on the nucleus
is maximized as vacuum builds between pulses or bursts. Because of the
decrease in cavitational energy around the tip at this low pulse rate
or in burst mode, the tunnel in the nucleus in which the tip is embedded
fits the needle very tightly and gives us an excellent hold on the
nucleus, thus maximizing control of the nucleus as it is scored and
chopped (Fig. 4) in foot position 2. 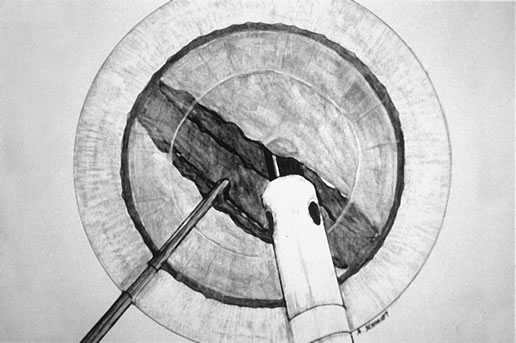 Fig. 4. Vacuum is maintained in foot position 2 as the chopper is brought to the
side of the phaco tip and the two instruments are separated, hemisecting
the endonucleus. Fig. 4. Vacuum is maintained in foot position 2 as the chopper is brought to the
side of the phaco tip and the two instruments are separated, hemisecting
the endonucleus.
|
The Fine/Nagahara chop instrument is grooved on the horizontal arm close
to the vertical “chop” element with the groove parallel
to the direction of the sharp edge of the vertical element. In scoring
the nucleus, the instrument is always moved in the direction the sharp
edge of the wedge-shaped vertical element is facing (as
indicated by the groove on the instrument), thus facilitating scoring. The
nucleus is scored by bringing the chop instrument to the side
of the phaco needle. It is chopped in half by pulling the chopper to
the left and slightly down while moving the phaco needle, still in foot
position 2, to the right and slightly up. Then the nuclear complex
is rotated. The chop instrument is again brought into the golden ring (Fig. 5), the nucleus is again lollipopped, scored, and chopped with the
resulting pie-shaped segment now lollipopped on the phaco tip (Fig. 6). The segment is then evacuated utilizing high vacuum and short bursts
or pulse-mode phaco at 2 pulses/second. The nucleus is continually
rotated so that pie-shaped segments can be scored, chopped, and
removed essentially by the high vacuum assisted by short bursts
or pulses of phaco. The short bursts or pulses of ultrasound energy
continuously reshape the pie-shaped segments that are kept at
the tip, allowing for occlusion and extraction by the vacuum. The size
of the pie-shaped segments is customized to the density of the
nucleus, with smaller segments for denser nuclei. Phaco in burst mode
or at this low pulse rate sounds like “choo-choo-choo-choo”; therefore, the name of this technique. With
burst mode or the low pulse rate, the nuclear material tends to stay
at the tip rather than chatter as vacuum holds between pulses. The chop
instrument is utilized to stuff the segment into the tip or keep it
down in the epinuclear shell. 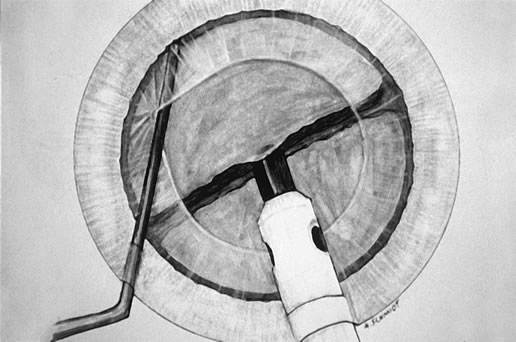 Fig. 5. Following rotation of the endonuclear complex, the chopper is repositioned
in the golden ring and the phaco tip is buried in the distal heminucleus
in preparation for the second chop. Fig. 5. Following rotation of the endonuclear complex, the chopper is repositioned
in the golden ring and the phaco tip is buried in the distal heminucleus
in preparation for the second chop.
|
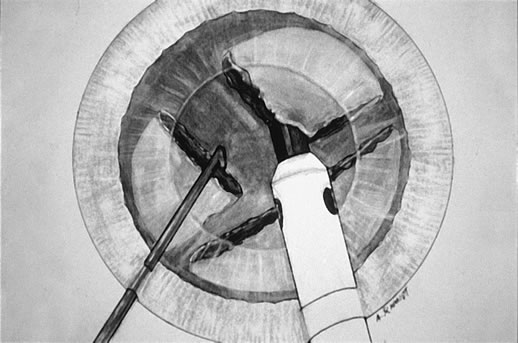 Fig. 6. The second chop is complete. The pie-shaped wedge of nucleus is
positioned on the phaco tip, ready for aspiration by high vacuum and small
pulses of ultrasound as required. The size of this wedge can be adjusted
in inverse proportion to the density of the cataract. Fig. 6. The second chop is complete. The pie-shaped wedge of nucleus is
positioned on the phaco tip, ready for aspiration by high vacuum and small
pulses of ultrasound as required. The size of this wedge can be adjusted
in inverse proportion to the density of the cataract.
|
After evacuation of the first heminucleus, the second heminucleus is rotated
to the distal portion of the bag and the chop instrument stabilizes
it while it is lollipopped. It is then scored (Fig. 7) and chopped. The pie-shaped segments can be chopped a second
time to reduce their size (Fig. 8) if they appear too large to easily evacuate. 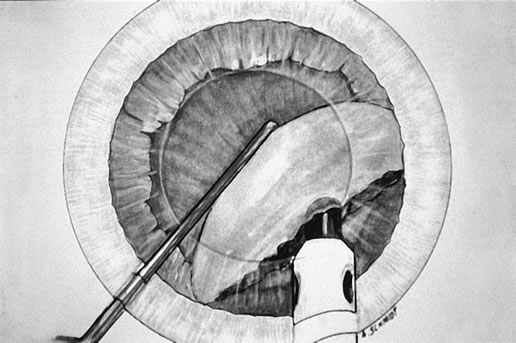 Fig. 7. After extraction of the first heminucleus, the remaining material is rotated
distally and chopped. Fig. 7. After extraction of the first heminucleus, the remaining material is rotated
distally and chopped.
|
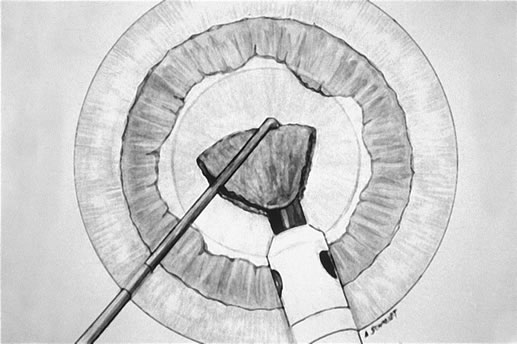 Fig. 8. The final pie-shaped wedge is chopped and extracted. Fig. 8. The final pie-shaped wedge is chopped and extracted.
|
There is little tendency for nuclear material to come up into the anterior
chamber with this technique. Usually, it stays down within the epinuclear
shell, but the chop instrument can control the position of the
endonuclear material. The 30-degree bevel-down tip facilitates
occlusion, as the angle of approach of the phaco tip to the endonucleus
through a clear corneal incision is approximately 30 degrees. This
allows full vacuum to be quickly reached, which facilitates embedding
the tip into the nucleus for chopping and allows mobilization of
pie-shaped segments from above rather than necessitating going
deeper into the endolenticular space as is necessary with a bevel-up
tip. In addition, the cavitational energy is directed downward
toward the nucleus rather than up toward the endothelium. After evacuation of all endonuclear material, the epinuclear rim is trimmed
in each of the three quadrants, mobilizing cortex as well in the
following way. As each quadrant of the epinuclear rim is rotated to the
distal position in the capsule and trimmed, the cortex in the adjacent
capsular fornix flows over the floor of the epinucleus and into the
phaco tip. Then the floor is pushed back to keep the bag on stretch until
three of the four quadrants of the epinuclear rim and forniceal cortex
have been evacuated. It is important not to allow the epinucleus
to flip too early, thus avoiding a large amount of residual cortex remaining
after evacuation of the epinucleus. The epinuclear rim of the fourth quadrant is then used as a handle to flip
the epinucleus. As the remaining portion of the epinuclear floor and
rim is evacuated from the eye, most of the time the entire cortex is
evacuated with it. Downsized phaco tips with their increased resistance
to flow are less capable of mobilizing the cortex because of the decreased
minisurge accompanying the clearance of the tip when going from
foot position 2 to foot position 3 in trimming of the epinucleus. After the IOL is inserted, these strands and any residual viscoelastic
material are removed using the irrigation-aspiration tip, leaving
a clean capsular bag. If there is cortex remaining following removal of all the nucleus and epinucleus, there
are three options. The phacoemulsification handpiece
can be left high in the anterior chamber while the second handpiece strokes
the cortex-filled capsular fornices. Frequently, this results
in floating up of the cortical shell as a single piece and its exit
through the phacoemulsification tip (in foot position two) because
cortical cleaving hydrodissection has cleaved most of the cortical
capsular adhesions. Alternatively, if one wishes to complete cortical cleanup with the irrigation-aspiration
handpiece before lens implantation, the residual
cortex can almost always be mobilized as a separate and discrete shell (reminiscent
of the epinucleus) and removed without ever
turning the aspiration port down to face the posterior capsule. The third option is to viscodissect the residual cortex by injecting the
viscoelastic through the posterior cortex onto the posterior capsule. We
prefer the dispersive viscoelastic device chondroitin sulfate-hyaluronate (Viscoat, Alcon Surgical, Fort Worth, Texas). The
viscoelastic material spreads horizontally, elevating the posterior
cortex and draping it over the anterior capsular flap. At the same
time, the peripheral cortex is forced into the capsular fornix. The posterior
capsule is then deepened with a cohesive viscoelastic device, and
the IOL is implanted through the capsulorhexis, leaving the anterior
extension of the residual cortex anterior to the IOL. Removal of residual
viscoelastic material accompanies mobilization and aspiration
of residual cortex anterior to the IOL, which protects the posterior capsule, leaving
a clean capsular bag. Chop techniques substitute mechanical forces (chopping) for ultrasound
energy (grooving) to disassemble the nucleus. High
vacuum is utilized as an extractive technique to remove nuclear material
rather than utilizing ultrasound energy to convert the nucleus to
an emulsate that is evacuated by aspiration. These techniques maximize
safety, control and efficiency, allowing phaco of harder nuclei, even
in the presence of a compromised endothelium. Chop techniques facilitate
the achievement of two goals: minimally invasive cataract surgery
and maximally rapid visual rehabilitation. LASER PHACOEMULSIFICATION Cataract extraction modalities employing laser energy currently include
the Erbium:YAG Phacolase (Carl Zeiss Meditec, Jena, Germany), the
Neodymium:YAG Photon Laser PhacoLysis System (Paradigm Medical, Salt
Lake City), and the Dodick Q-switched Neodymium:YAG
laser (ARC GmbH). Several potential advantages over ultrasound
have maintained interest in laser, including relative reduction
in the energy requirement for cataract extraction, the absence of any
potential for thermal injury, and improved protection of corneal endothelial
cells. The erbium:YAG (2940 nm) laser energy is well absorbed by tissues
with high water content and has a penetration depth of less than 1 micron. The
laser energy is delivered though a fiber inside the aspiration
port placed flush with the tip. Hoh and Fischer demonstrated that
erbium laser is safe and effective for mild to moderate nuclear sclerosis.49 Surgeons may employ either a bimanual technique, separating irrigation
from aspiration, or the more familiar coaxial set up. With the latter, Takayuki
Akahoshi's counter prechop technique is used to effectively
disassemble the lens nucleus into multiple wedge-shaped segments.50 A horizontal chopper such as the Fine-Nagahara chopper (Rhein
Medical, Tampa, Florida) is inserted through the side port, touched
against the anterior lens surface, and gently pushed under the
distal anterior capsular flap, where it falls into the golden ring. The
chopper supports the nucleus, while the Akahoshi Prechopper (ASICO, Westmont, Illinois) is passed through the 2.5 mm corneal incision
directly into the core of the nucleus. The chopper in the golden
ring is held in front of the prechopper to preclude rotational movement
of the nucleus. Opening the prechopper then bisects the nucleus. The nucleus is then rotated 90 degrees, and the first heminucleus is bisected
in a similar fashion. The chopper supports the heminucleus from
the golden ring, whereas the prechopper is inserted directly into the
center of the heminucleus and opened. In this manner, the nucleus may
be divided into four or more segments, each of which is a suitable size
for laser phacoemulsification. Nd: YAG photolysis represents a low energy modality for cataract extraction
developed by Dodick.51 Kanellopoulos reported a mean intraocular energy use of 5.65 Joules per
case.52 This level of energy compares favorably with values previously reported
for ultrasound phacoemulsification, and approximates the level of energy
reported for the chop and flip phacoemulsification technique using
power modulations.48 Huetz and Eckhardt found mean total energy of 1.97 Joules for nuclear
sclerosis up to grade 3, 3.37 Joules for Grade 3 and 7.7 Joules for Grade 4.53 Surgeons generally employ a groove and crack technique with the laser, sculpting
in a bimanual fashion and cracking as soon as possible. Once
superficial cortical material is aspirated, the laser tip is used to
ablate and fragment the nucleus. The laser tip should only just touch
the surface of the nucleus, and not be used to impale the cataract. Following
central photofragmentation, the nucleus is handled much as it
is with the classic divide and conquer technique. The total time that
the tip is in the eye varies with the grade of nucleus, from 2.15 minutes
for 1+ nuclear sclerosis to 9.8 minutes for 3+ nuclear sclerosis.52 Using the bimanual Dodick system, a cataract may be completely extracted
through two 1.5-mm incisions. Now, IOL technology is becoming
available to take advantage of this ultrasmall incision. Wehner and Ali
have reported a series of cases implanted with a dehydrated acrylic
IOL through a 1.5-mm incision.54 BIMANUAL ULTRASOUND PHACOEMULSIFICATION The promise of bimanual, ultrasmall incision cataract surgery and companion
IOL technology is becoming a reality through both laser and new ultrasound
power modulations. New instrumentation is available for bimanual
surgery, including forceps for construction of the capsulorhexis, irrigating
choppers, and bimanual irrigation and aspiration sets (Fig. 9). Proponents of performing phaco through two paracentesis-type
incisions claim reduction of surgically induced astigmatism, improved
chamber stability in every step of the procedure, better flow characteristics
due to the physical separation of infusion from ultrasound
and vacuum, and greater ease of irrigation and aspiration with the elimination
of one, hard-to-reach subincisional region. However, the
risk of thermal injury to the cornea from a vibrating bare
phaco needle has posed a challenge to the development of this technique.  Fig. 9. Capsulorhexis forceps designed for bimanual phaco through two paracentesis
incisions (ASICO, Westmont, Illinois). Fig. 9. Capsulorhexis forceps designed for bimanual phaco through two paracentesis
incisions (ASICO, Westmont, Illinois).
|
In the 1970s, Girard attempted to separate infusion from ultrasound and
aspiration, but abandoned the procedure because of thermal injury to
the tissue.55, 56 Shearing and colleagues successfully performed ultrasound phaco through
two 1.0-mm incisions using a modified anterior chamber maintainer
and a phaco tip without the irrigation sleeve.57 They reported a series of 53 cases and found that phaco time, overall
surgical time, total fluid use, and endothelial cell loss were comparable
to those measured with their standard phaco techniques. Crozafon described
the use of Teflon-coated phaco tips for bimanual high-frequency
pulsed phaco, and suggested that these tips would reduce
friction and therefore allow surgery with a sleeveless needle . 58 Tsuneoka, Shiba, and Takahashi determined the feasibility of using a 1.4-mm (19-gauge) incision and a 20-gauge
sleeveless ultrasound tip to perform phaco.59 They found that outflow around the tip through the incision provided adequate
cooling, and performed this procedure in 637 cases with no incidence
of wound burn.60 Additionally, less surgically induced astigmatism developed in the eyes
operated with the bimanual technique. Agarwal and colleagues developed
a bimanual technique, “Phakonit,” using an irrigating chopper
and a bare phaco needle passed through a 0.9 clear corneal incision.61 They achieved adequate temperature control through continuous infusion
and use of “cooled balanced salt solution” poured over the
phaco needle. Soscia, Howard, and Olson have shown in cadaver eye studies that phacoemulsification
with the Sovereign WhiteStar system (AMO, Santa Ana, California), using
a bare 19-gauge aspiration needle, does
not produce a wound burn at the highest energy settings unless all
infusion and aspiration are occluded.62, 63 WhiteStar represents a power modulation of ultrasonic phacoemulsification
that reduces the production of thermal energy by limiting the duration
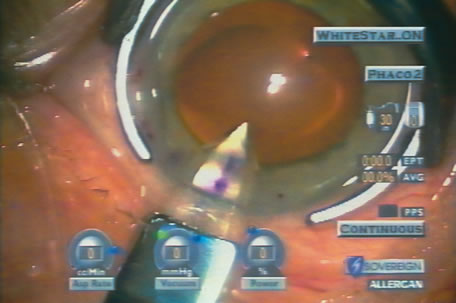
of energy pulses to the millisecond range (Fig. 10). 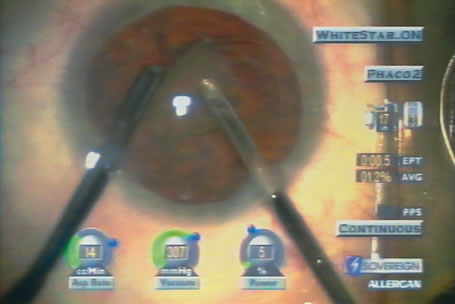 Fig. 10. Bimanual phaco through two paracentesis incisions with WhiteStar (AMO, Santa
Ana, California) and an irrigating chopper (MicroSurgical
Technology, Redmond, Washington). The final pie-shaped
segment is ready for chopping, as shown in figure 8. High vacuum (307 mm
Hg) and low levels of ultrasound (current 5%, total
Effective Phaco Time to this point in the procedure, 0.05 second
at 1.2% Average Power) create efficient nuclear removal. Fig. 10. Bimanual phaco through two paracentesis incisions with WhiteStar (AMO, Santa
Ana, California) and an irrigating chopper (MicroSurgical
Technology, Redmond, Washington). The final pie-shaped
segment is ready for chopping, as shown in figure 8. High vacuum (307 mm
Hg) and low levels of ultrasound (current 5%, total
Effective Phaco Time to this point in the procedure, 0.05 second
at 1.2% Average Power) create efficient nuclear removal.
|
|