NATURAL HISTORY STUDIES
The natural history study is similar in design to the case series approach. In the ideal natural history study, the subjects are identified as having the disease in question and followed over time for the development of preselected “events,” such as presumed complications of the disease. Although this is ideally done in a prospective fashion, in rare situations it is possible to obtain the necessary information from existing records. In addition to calculating the proportion of the study population that has these “events” in a specified period of time, risk factors can also be identified that make certain persons more likely than others to have an “event.”
A case series of untreated patients that has defined eligibility and exclusion criteria and complete follow-up, either prospective or retrospective, is a natural history study. Unfortunately in many published case series, eligibility and exclusion criteria are not carefully defined and the follow-up is incomplete on a sizable proportion of the patients. An example of a successful prospective natural history study is the natural history portion of the Diabetic Retinopathy Vitrectomy Study. 13,14 The hypothesis was that persons with a specified level of severe diabetic retinopathy have a high risk of losing useful vision. It was necessary to confirm this prior to considering vitrectomy for these patients. Patients with this carefully defined degree of diabetic retinopathy were entered into the study and examined at regularly scheduled visits. Rates of severe visual loss, at specific time intervals, could then be calculated. In addition, certain ocular factors were studied to learn whether they would be predictive of severe visual loss. This study demonstrated that eyes with specific characteristics of diabetic retinopathy are at high risk of developing severe visual loss. These characteristics were then used as the major eligibility criteria for entry into one part of the randomized clinical trial that was evaluating vitrectomy in such eyes.
SURVEYS
Surveys measure the prevalence of a disease (the number of cases of a disease present in a defined population at a specified time). In addition, information on the characteristics of the disease as well as on characteristics of the population may be used to study possible associations between the disease and various risk factors. However, although this type of study can demonstrate associations, one cannot impute cause and effect because the risk factor found may be the cause of the disease, the result of the disease, or may simply coexist with the disease. Associations observed may be used to formulate hypotheses to be tested by an epidemiologic approach.
Since surveys are population-based, they require either the examination of each person in the population or everyone in a specifically defined subgroup. To the extent that some persons may not be available for examination, the accuracy of the prevalence estimate is diminished.
An example of a successful cross-sectional survey is the Wisconsin Epidemiologic Study of Diabetic Retinopathy.15 In this study, attempts were made to identify all known diabetics in several counties in southern Wisconsin. A random sample of this group was then selected and efforts were made to examine each of these identified persons. The main goal of this study was to determine the prevalence of various types of retinopathy in this population of diabetics and to identify risk factors such as the degree of hyperglycemia or hypertension that might be associated with more severe retinopathy.
If there is interest in disease prevalence for a general population rather than a segment of the population such as diabetics, then the sample that must be examined must be drawn from the overall population. The Health and Nutrition Examination Survey16 and the Visual Acuity Impairment Study, 17 both done in cooperation with the Census Bureau, are examples of this type of study. The factors limiting the success of a survey are the techniques used to identify the population to be studied and the methods used to motivate those selected to participate in the examination process and/or interview.
Another example of a successful population-based study is the Baltimore Eye Survey.18 In this study, the prevalence of primary open-angle glaucoma was compared between black and white residents of east Baltimore. Based on previous clinical studies, there was a growing acceptance that blacks were at a higher risk of glaucoma than whites. This clinical impression was confirmed by the Baltimore Eye Survey. Using comprehensive examination techniques in population-based samples of blacks and whites, the rate of primary open-angle glaucoma in black Americans was found to be four to five times higher than whites. The comprehensive examination techniques maximized the sensitivity and specificity of the diagnosis of primary open-angle glaucoma.
COHORT STUDIES
In cohort or follow-up studies (also known as prospective studies), persons who are initially free of the disease under study are followed over a period of time during which some develop the disease. Groups are defined at study entry on the basis of the presence or absence of exposure to a possible risk factor, for example, those who are hypertensive versus those who are not, those with elevated hemoglobin A1C versus those with less elevated hemoglobin A1C.
The analysis of data from cohort studies results in a calculation of the incidence of the disease in the cohort. This is determined by dividing the number of new cases observed during the specified period of follow-up by the number of individuals at risk of developing the disease. The incidence in the group with a positive exposure or with a specific risk factor divided by the incidence in the group without the risk factor is called the relative risk, which is a primary statistic used to assess whether the risk factor is associated with the disease. When the relative risk is significantly greater than 1, the factor is thought to be associated with the development of the disease; when it is significantly less than 1, the factor is associated with not developing the disease.
Since all individuals in a cohort study are disease free at the onset of the study, the researcher can document that exposure to a risk factor predated development of the disease. However, cohort studies require following large numbers of individuals over long periods of time to observe an adequate number of cases developing the disease. This cost, in terms of money and time, is a major drawback of a cohort study. In addition, because individuals must be followed for long periods of time, even as long as decades, there is a great potential for introducing bias due to losses in follow-up. This study design is best suited for relatively common disease; that is, a disease that can be expected to develop in a large proportion of the study group during the follow-up period.
A good example of a cohort study is the Framingham Heart Study.19 In this study, 5209 Framingham residents were identified who were between the ages of 30 and 62, free of cardiovascular disease, and were agreeable to follow-up examinations every 2 years. At the initial examination, study participants were examined for a large number of possible cardiovascular risk factors such as hypertension, history of cigarette smoking, and blood cholesterol levels. Over the next 35 years, all cardiovascular events were ascertained and incidence rates and relative risks were calculated. For example, the incidence rate of myocardial infarction, adjusted for age and sex, among persons with diastolic blood pressure equal to or greater than 90 mm Hg at the first examination was several times the rate among persons with diastolic blood pressure less than 90 mm Hg at the first examination. Some of the earliest data demonstrating the association between the development of cardiovascular disease and hypertension came from this study.
An example of a cohort study in eye diseases is the Wisconsin Epidemiologic Study of Diabetic Retinopathy.20,21 Persons who were diagnosed with diabetes mellitus and examined in the original survey were re-examined four years later. The objectives of this cohort study were to examine the incidence and progression of diabetic retinopathy and to determine the relationships between incidence and progression of diabetic retinopathy and risk factors. In patients who were free of retinopathy at the baseline examination and who were diagnosed with diabetes mellitus at the age of 30 years or more, 47% of insulin users and 34% of nonusers developed retinopathy by the four-year visit. The most potent risk variable for prevalence and incidence of diabetic retinopathy in other studies was the duration of diabetes. This characteristic was found to be an important predictor for this cohort study during the four years of observation but did not have a consistent linear effect on the rate of development or on the rate of progression. A likely explanation for this effect may be the increasing rates of mortality in the older patients. In this cohort study of subjects whose diagnosis of diabetes mellitus was made at 30 years or more of age, 25% had died during the four-year interval; thus only 72% of this group of patients participated in the follow-up visit. In the patients with onset at a younger age, 82% participated in the follow-up examination. Death accounted for the majority of the nonparticipation rate in this cohort study. Nevertheless, the incidence and progression rates for diabetic retinopathy provided important information for future studies, that is, clinical trials, and for planners of health care.
STUDIES OF CASES AND CONTROLS
Cohort studies can be particularly long, expensive, and difficult if the incidence of the disease under study is low. An alternative approach to investigate possible associations between risk factors and disease is the case-control study (also known as a retrospective study). In contrast to a cohort study, which starts with persons who do not have the disease, the case-control study first identifies persons who already have the disease (cases) and a comparable group of persons who do not have the disease (controls). Both groups are then asked questions about past exposure to suspected risk factors, documenting when possible that the exposure predated disease in the group of cases. The groups are then compared with respect to the proportion having a particular characteristic or exposure factor. For example, if cigarette smoking were a possible risk factor for the development of a disease under study, one would try to ascertain the smoking histories in both the cases and controls. Both the case group and the control group are divided into smokers and nonsmokers using this historical information. A statistic called the odds ratio can be calculated from these data. The odds ratio is similar to the relative risk calculated in a cohort study. If it is statistically significantly greater or less than 1.0, the factor is said to be either positively or negatively associated with the disease. If it is not statistically significantly different from 1.0, there is no evidence from this study that the factor and the disease are associated.
A type of case-control study that determines the presence or absence of a risk factor at the time of the examination is called a cross-sectional study. Both case-control and cross-sectional studies are studies of cases and controls. They differ in that within the case-control study format an effort is made to ascertain that the exposure to the suspected risk factor (e.g., history of cigarette smoking) predated the onset of the disease, while in the cross-sectional format the factor and the disease coexist (e.g., current cigarette smoking regardless of past history, or the measurement of current blood pressure or serum cholesterol). Often, retrospective and cross-sectional elements are combined in a single study of cases and controls.
A study of senile macular degeneration by Hyman and associates22 is an example of a study of cases and controls. Patients with senile macular degeneration were identified at 34 ophthalmologists' offices. Presence of the disease was documented photographically. An equal number of age and sex-matched controls were also selected from the same ophthalmologists' offices; absence of disease was photographically documented. Historical information such as family history of senile macular degeneration, history of various medical problems, and exposure to a number of environmental risk factors was ascertained. In addition, data on present conditions such as refractive error, current blood pressure, hand grip strength, and iris color were collected. This study found an association between several of these factors and the presence of senile macular degeneration (e.g., positive family history, light iris color, hyperopia, and history of cardiovascular disease).
A disadvantage of the case-control study is the difficulty of establishing the temporal relationship between the exposure factor and the disease, despite efforts to ascertain risk factors that may have predated the disease. Iris color, for example, is a risk factor that can be assessed after disease diagnosis but that almost certainly predated the development of the disease. Alternatively, intraocular pressure might well be modified by the presence of a disease and cannot be assumed to have been the same prior to the development of the disease. An effective method used in minimizing such problems is the selection of cases prospectively as they occur (incident cases). An example of such a technique is seen in a case-control study of the relative risk of ulcerative keratitis among users of soft contact lenses.23 Cases were defined to be soft contact lenses users with newly diagnosed ulcerative keratitis who were evaluated at six university ophthalmologic centers between November 1986 and November 1987. The use of incident cases provided advantages in that the risk factors examined in the study were more likely to be related to the development of the disease and not to the duration of the disease once it had developed (i.e., prognosis). In addition, the use of incident cases minimized the time between the development of the condition and the interview, which provided more accurate reporting of the information on prior exposures.
Of all the epidemiologic studies, the case-control study has the greatest potential for bias, especially in the selection of cases and controls. It is important to consider possible sources of bias prior to starting a case-control study. In the study of risk factors for cataract (The Lens Opacities Case-Control Study [LOCS]),24 cases were not selected from patients undergoing cataract surgery because the reasons that lead to patient selection and referral for surgery may cause biases that are difficult to assess. In addition, the selection of controls for such cases may be difficult. The cases and controls for this study were selected from the same general eye services of outpatient clinics in order to increase the comparability on socioeconomic, demographic, health utilization, and other factors. Misclassification of these cases and controls were minimized by using a system of lens classification based on clinical examination and centralized grading of lens photographs. Among the factors found to be associated with less extensive lens opacities were use of multivitamins and dietary intake of antioxidant vitamins. This association was further tested and confirmed by adjusting for age, sex, race, and education. This finding demonstrates an association between use of vitamins and lens opacities, but because of possible biases or confounding, does not necessarily imply that decreased intake of vitamins with antioxidant potential causes cataracts, nor does it follow that the disease process will be prevented or retarded by the use of such vitamins. Indeed, such proof is not possible from this type of study. A randomized clinical trial should be designed to address such questions. Furthermore, it is the accumulated weight of evidence, often from markedly different studies (including laboratory and pathologic evidence), that makes investigators willing to ascribe a cause and effect relationship to the association between the suspect risk factor and the disease. This is what has happened in the case of cigarette smoking and lung cancer. A positive association between the risk factor (cigarette smoking) and the disease (lung cancer) has been repeatedly shown in studies of cases and controls; there is laboratory evidence suggesting there are carcinogens in cigarette smoke; and “intervention” studies suggest that discontinuation of smoking helps to mitigate risk of earlier smoking. It is the accumulated evidence that leads to the belief that cigarette smoking causes lung cancer in some persons.
When potential sources of bias in the selection of cases and controls and in the ascertainment of historical information are recognized and minimized both in the design and analysis of the study, case-control studies are a very valuable tool in clinical research. Unlike cohort studies, they are efficient in time and expense. This design is very suitable for studying rare diseases. It is also useful in testing a variety of exposure factors among the diseased and controls, making it particularly suitable for early investigations of the risk factors of a disease.
RANDOMIZED CLINICAL TRIALS
Randomized clinical trials have been called the “gold standard” of epidemiologic research. They differ from the previously described studies in that they are experimental rather than observational. In a randomized clinical trial, the investigator can allocate one of the two or more exposures (typically treatments) at random to a group of subjects who will be followed prospectively. Randomization helps ensure that the groups of persons studied are similar in all respects other than the assigned treatment. Differences in outcome in the groups are therefore likely to be due to the treatment. Rarely does the introduction of a new treatment or procedure produce such unequivocal results as that of penicillin in the treatment of pneumococcal pneumonia. More often, the effects of therapy are much smaller but are still clinically important and significant. The use of observational studies may fail to demonstrate this beneficial effect, while a randomized clinical trial will reveal the strongest epidemiological evidence of the beneficial effect.
Generally, in a clinical trial the researcher selects a study population that has a high risk of developing the outcome or response variable. Eligibility criteria for inclusion into the study must be clearly defined at the beginning of the study. Treatment, which should be administered in a standard fashion, is ideally allocated by randomization. This helps ensure that the treatment group(s) are similar to the control group with respect to all characteristics except for treatment. Data from baseline and subsequent visits must be collected in a systematic fashion, preferably by trained personnel. The response or outcome variable that is to be measured must also be clearly defined. To prevent bias, the outcome variable should be objectively assessed by someone who is unaware of the treatment assignment.
Sample size calculations must be performed in the initial development phase of all analytic studies but are particularly important in a clinical trial. A trial must have a sufficient sample size to have adequate statistical power to detect differences between groups considered to be of clinical interest. This is an essential part in the planning of a clinical trial.
During follow-up, the proportion of individuals in each study group(s) that develop the predetermined outcome is calculated and the effects of the intervention are compared. Monitoring of noncompliance and adverse side effects is an important aspect of the study. Prevention of loss to follow-up must be addressed when considering the selection of the study population and the nature of the intervention because a high follow-up rate is essential to the success of the study.
The analysis of clinical trials is similar to that for cohort studies, where the comparison is between the rates of the outcome of interest in the treated (exposed) group(s) and the corresponding rates in the control (unexposed) group. As a result of randomization, the comparison groups are more likely to be quite similar in all aspects other than the treatment. An important initial step in analysis involves comparing the groups to ensure that important baseline characteristics are indeed balanced. Once subjects are randomized to a particular group, they will remain in that group for analysis, regardless of whether they may drop out or become noncompliant (“once randomized, always analyzed”). It is important to minimize noncompliance and drop-out rates and to attempt to ascertain complete information.
The disadvantage of intervention studies is the enormous cost in expense and time. However, carefully designed clinical trials, involving adequate numbers of subjects randomized, will provide the most powerful epidemiological evidence of the effects of an intervention.
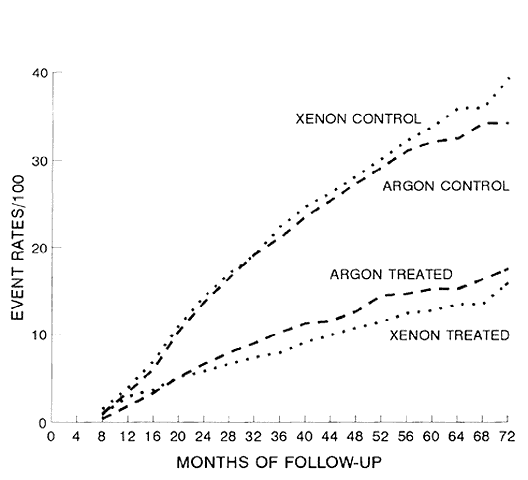
An example of an intervention study in ophthalmology is the Diabetic Retinopathy Study (DRS),25 a randomized, controlled clinical trial to evaluate photocoagulation treatment for proliferative diabetic retinopathy. Between 1972 and 1975, 1758 patients were enrolled, and follow-up was continued until 1979. Eligible patients had proliferative diabetic retinopathy in at least one eye or severe nonproliferative retinopathy in both eyes, and visual acuity of 20/100 or better in each eye. One eye was randomized to immediate photocoagulation with either xenon arc or argon laser photocoagulation, while the fellow untreated eye acted as the control. At four-month intervals, an examiner, who was not aware of the treatment status of the eyes, obtained best-corrected visual acuity (Fig. 1). The primary outcome variable measured was “severe visual loss” defined as visual acuity <5/200 at two or more consecutively completed follow-up visits. In 1976, a change of protocol was implemented, because the incidence of severe visual loss in the treated eyes was reduced by 50% or more in comparison to the control eyes. At this change of protocol, eyes with certain “high-risk characteristics” were observed to be at a particularly high risk of severe visual loss. The data and safety monitoring committee recommended that treatment no longer be withheld from these eyes with high-risk characteristics that had initially been randomized to no treatment. Adverse side effects, such as visual field loss and persistent decrease of visual acuity associated with argon and xenon photocoagulation, were also carefully documented in the DRS. The rates of adverse side effects for these two different types of treatments were also compared; the xenon group was found to have greater rates of harmful effects. Thus the DRS, a randomized, controlled clinical trial, successfully demonstrated the efficacy of photocoagulation in the treatment of proliferative diabetic retinopathy and identified a “high risk” group of persons with diabetes for whom photocoagulation was indicated.26
The subsequent clinical trial of treatment of diabetic retinopathy, the Early Treatment Diabetic Retinopathy Study (ETDRS),27 evaluated the timing of photocoagulation during the course of diabetic retinopathy, the efficacy of photocoagulation for diabetic macular edema, and the role of aspirin in altering the course of diabetic retinopathy. The aspirin portion of this study consisted of randomly assigning each patient to 650 mg of aspirin or placebo. Compliance with study medication was assessed with laboratory studies such as serum thromboxane B2 and urine salicylate levels at annual visits and with counts of pills and patient interviews at each clinic visit. The results of these tests showed that 80% of the patients were taking prescribed study medications, a compliance level similar to that reported for other trials of aspirin. The primary endpoint for assessing the effect of aspirin on the course of diabetic retinopathy was the development of high-risk proliferative retinopathy. The relative risk of developing high-risk proliferative retinopathy for patients assigned to aspirin compared with patients assigned to placebo is 0.97 with 99% confidence interval of 0.85 to 1.11. Thus there was no difference between the treated and untreated group as the confidence interval included one. The power of this study to detect a 25% treatment effect as estimated during the design phase of the study was 99%, given the sample size and projected event rates of 40%. After adjusting for noncompliance with study medications and the actual outcome rates, the study had power to detect a 25% treatment effect. When negative results of a clinical trial are presented, it is important to report the study's power to detect the proposed clinical difference.
Another clinical trial in ophthalmology evaluated the common use of corticosteroids to treat optic neuritis.28 The following research questions were posed by the investigators:
Does treatment with either oral prednisone or intravenous methylprednisolone improve visual outcome in acute optic neuritis?
Does either treatment speed the recovery of vision?
What are the complications of treatment in relation to its efficacy?
Visual field, the primary measure of outcome, was graded in a masked fashion by a central reading center. Best-corrected visual acuity was obtained by technicians who were unaware of the treatment assignment. Results of this clinical trial showed that intravenous methylprednisolone followed by oral prednisone accelerated the recovery of visual loss due to optic neuritis but resulted in slightly better vision at six months. Oral prednisone alone actually increased the risk of new episodes of optic neuritis. These findings were somewhat surprising since a mail survey of ophthalmologists and neurologists performed in 1986 in Michigan and Florida indicated that 65% of the ophthalmologists and 90% of the neurologists prescribed corticosteroids for optic neuritis.
Such clinical trials cannot be used to evaluate all risk factors or treatments. A clinical trial of cancer and cigarette smoking, although possible in animals, is not possible in humans. This is because it is not possible to force people at random to smoke or not smoke. The only alternative is to take advantage of the fact that populations of smokers, nonsmokers, and ex-smokers exist. The rates of disease in these different populations or the rates of smoking in people with or without the disease can be observed. The observational approach is often the only method of investigation available in human research. When feasible, however, the randomized clinical trial is likely to provide more convincing evidence than can be obtained from any of the studies employing the observational approach.