ENVIRONMENTAL ANTIGENS
Association between hypersensitivity to environmental antigens (e.g., allergies) and uveitis has been documented. Cases of uveitis have been reported in association with allergies to food,23,24 dust,23 ragweed,24 trees and grasses,25 cat, caterpillar, and tarantula hair.26–30
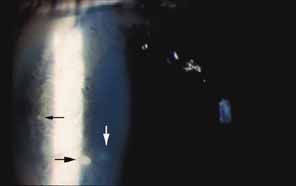
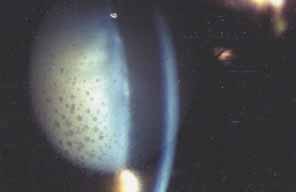
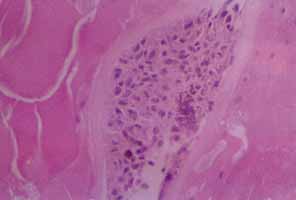
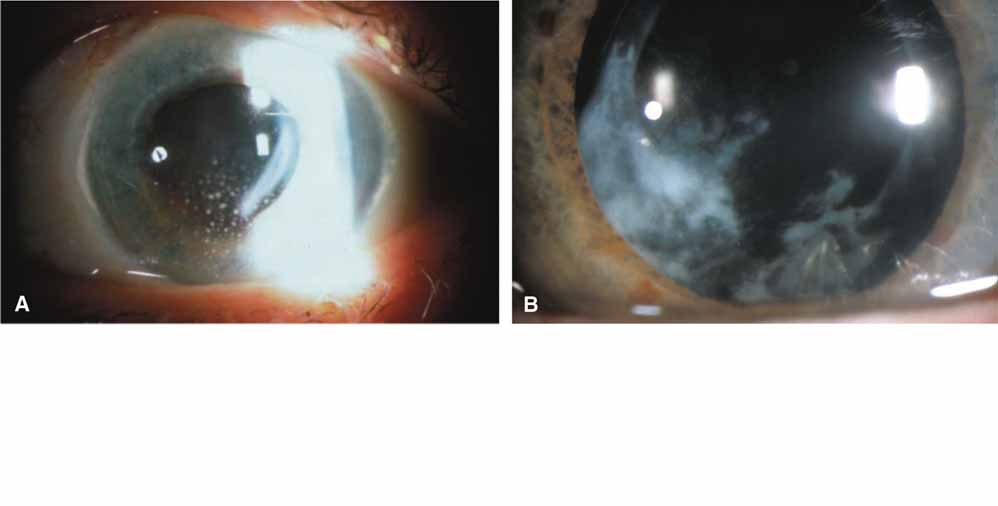
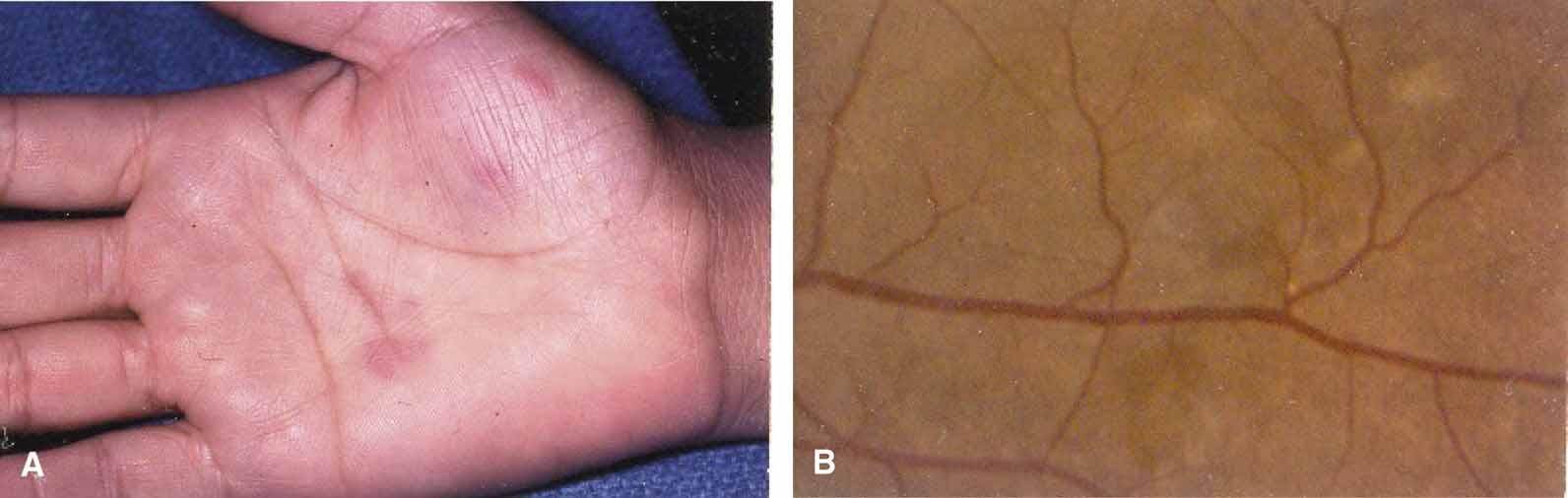
In 1904, Saemisch31 first used the term ophthalmia nodosa to describe the granulomatous nodules formed on the conjunctiva and iris in response to caterpillar hairs or sensory setae. Iritis, occasionally with formation of iris nodules and vitritis, is also described after ocular reaction to caterpillar hairs.32 Ophthalmia nodosa can also be caused by tarantula hairs. Tarantulas are occasionally kept as pets and are usually harmless, but, if threatened, they eject their dorsal barbed hairs with the hair of their hind legs. These hairs are capable of embedding into cornea and skin and inciting an inflammatory reaction. It is usually hard to detect those hairs in the cornea. Even with hairs in the anterior corneal stroma only, anterior uveitis with mutton fat deposits on endothelium may be seen.29 One patient is reported to have developed small peripheral choroidal lesions after 6 months.30 Extensive dissection of the cornea or sclera to remove the hairs is not recommended because successful management is obtainable with topical steroids (Fig. 1).
An asthmatic patient has been described who developed a well-demarcated area of choroiditis after several bouts of urticaria. The authors felt that this was due to IgE-mediated release of vasoactive amines, which have been reported to trigger vasculitic syndromes.32
Bee and wasp stings of the cornea have been associated with significant ocular pathology. Uveitis has been observed after corneal bee sting33 and wasp bite.34 Bee venom is a complex toxin composed of several compounds with different actions. Toxicity is related to nonenzymatic polypeptide toxins (mellitin, apamin, iminimine) and enzymes (phospholipase A and B, hyaluronidase). Mellitin can cause depigmentation of the iris (heterochromia iridis), noted in cases of bee sting.35 Mellitin also causes the release of serotonin, histamine, and other chemical mediators of inflammation. Apamin is a neurotoxin that blocks neurotransmission, and internal ophthalmoplegia and sector iridoplegia have been reported as neurotoxic effects of apamin after corneal bee sting. Optic neuritis, papilledema, and optic atrophy have occurred following bee stings to the other parts of the body.36 The mechanism is thought to be focal demyelination of the optic nerve caused by an acute allergic reaction to the bee venom. Enzymes in the venom have high molecular weight and are highly antigenic, accounting for the immunologic injury to the eye following stings. Type I hypersensitive reaction takes place, with release of chemical mediators of inflammation and manifested by such findings as conjunctival injection, chemosis, and corneal edema.33 Corticosteroids alone, or in conjunction with cycloplegics and antibiotics, are used in corneal bee stings; antihistamines can be included in case of chemosis and conjunctival injection.
The cases above are unusual. In general, the association between allergies and uveitis is rare. Van Metre37 reported no cases due to allergy among 556 cases of uveitis studied. In a series of 1500 cases, Kimura26 did not find any statistically significant association between contact allergy and uveitis. These results also reflect our clinical experience.
INFECTIOUS AGENTS
Infectious causes (e.g., tuberculosis, syphilis, and toxoplasmosis) of uveitis are well known; however, uveitis associated with hypersensitivity to infectious agents is seldom recognized clinically.
Although the specific cause of uveitis remains unknown in many patients, the initiating stimuli for intraocular inflammation can be divided into two major pathways: an antigen specific (infectious agent) immune-mediated inflammatory response and a nonspecific inflammatory response (which is nonantigen specific).
Endotoxin-induced uveitis (EIU) is an animal model for nonantigen-specific stimulus induced ocular inflammation. In Lewis rats, intravenous, intraperitoneal, and intrafootpad endotoxin injection38 or intraocular endotoxin injection39 are demonstrated to induce EIU. According to kinetic studies, inflammatory cells migrate first into the eye about 6 hours after endotoxin injection, and ocular inflammation peaks approximately 18 hours later. Inflammation is related to the release of cytokines from activated cells. Tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), IL-6, and IL-8 are inflammatory mediators that appear to be stimulated by endotoxin. Those cytokines and other inflammatory molecules can start the inflammatory cascade with breakdown of the blood-aqueous and blood-retinal barriers, leading to additional cellular infiltration of the eye.40
Antigen-specific ocular immune responses may also take place and are divided into cell-mediated and humoral responses. Both of these require processing of the antigen by specialized antigen-presenting cells (APC). It has been postulated that this results from molecular mimicry between part of the DNA of the various organisms and a portion of HLA-B27, although this is still controversial.
Immune responses against infectious agents may cross-react against ocular antigens and induce uveitis. For example, researchers noted homology between yeast histone and S-Ag.41 A number of forms of uveitis follow an infectious disease but do not seem to be caused by direct infection. Reiter's syndrome is associated with HLA-B27 haplotype. Uveitis in these patients may occur after gram-negative dysentery or after nongonococcal urethritis, as a result of chlamydia trachomatis and ureaplasma urealyticum.40
Post-streptococcal syndrome is an autoimmune disorder precipitated by infection with group A streptococci. The pathologic process is thought to stem from a cross-reaction between antibodies, sensitized lymphocytes, or both, generated against streptococcal antigens with the tissues of the host.42 Manifestations include acute rheumatic fever (ARF), post streptococcal reactive arthritis (PSRA), and acute glomerulonephritis. Recurrent anterior uveitis can occur in patients with a history of post-streptococcal syndrome who have repeated group A streptococcal infection. The intraocular inflammation follows a time course similar to that of the other manifestations of the syndrome, such as recurrent rheumatic fever.43,44 PSRA differs from ARF, in which evidence of carditis is not usually seen and the response of arthritis to aspirin is poor. Two adult patients with PSRA, both of whom developed uveitis, were recently described.45
There are probably multiple initiating infectious stimuli for inflammation in the uvea. Uveitis may result from the replication of the microbes, the host's hypersensitivity to the components of the microbe, or both. Unfortunately, the exact mechanism of uveitis in humans is still unknown.40
DRUG-INDUCED UVEITIS
Drug-induced uveitis is a relatively rare occurrence (reported to be less than 0.5 % in a tertiary referral uveitis clinic).46 Drug-induced uveitis is almost always reversible within weeks of discontinuation of the causative agent and treatment of the inflammation.
Naranjo et al.47 proposed the following seven criteria to establish causality of adverse events by drugs:
- The reaction is a frequently described event that is well documented.
- Recovery occurs upon withdrawal of the drug.
- Other possible causes for the event have been excluded.
- The reaction becomes more severe when the dose of drug is increased.
- The adverse event is documented by objective evidence.
- Similar effects can occur in a given patient with similar drugs.
- The event recurs on rechallenge with the suspected drug.
Several drugs have anecdotally been noted to cause uveitis in single case reports. Very few drugs that have been reported to cause uveitis have had causality confirmed by elimination of confounding variables, double-blind challenge, or rechallenge testing.
Systemic Drug-Induced Uveitis
Rifabutin, a derivate of rifampin, is used to treat or to prevent atypical mycobacterial infections in the immunocompromised host. It has been associated with anterior and posterior nongranulomatous uveitis, with or without hypopyon, which may be extremely severe.48,49,50 Rifabutin-associated uveitis, characterized by white-yellow inflammatory opacities in the inferior and posterior vitreous, has also been described by Chaknis et al.51 They described those lesions in three acquired immunodeficiency syndrome (AIDS) patients who were receiving 300 mg of rifabutin daily for 6 or more months for mycobacterium avium complex (MAC) prophylaxis. Three cases of acute uveitis without hypopyon were reported in patients with AIDS who did not have MAC bacteremia and who were taking prophylactic rifabutin.49 Rifabutin-associated uveitis may be an immune reaction to dead mycobacteria, but MAC associated uveitis (without rifabutin) is very rare, and anterior chamber paracentesis of the hypopyon, in these cases, failed to show any organisms on either aqueous cultures or microscopic examinations.52 Rifabutin reaction is probably not T cell-mediated because a paucity of these lymphocytes is one of the hallmarks of AIDS.48 Rifampin is known to be antigenic itself and after binding with serum and tissue proteins. Antibodies against rifampin can circulate or adhere to cellular surfaces and the antigen-antibody complexes induce an inflammatory reaction.53 The high prevalence, incidence of bilaterality, recurrence of uveitis with rechallenges, increasing severity of inflammation with dose escalation, improvement upon withdrawal, and exclusion of other possible causes of uveitis strongly implicate rifabutin as a cause of uveitis.54
Pamidronate sodium (Aredia), an intravenous bisphosphate, inhibits bone resorption and is used in the management of hypercalcemia associated with malignancy, osteolytic bone metastases, paget disease of the bone, and osteoporosis. Seventeen cases of unilateral scleritis and one case of bilateral scleritis have been reported within 6 hours to 2 days after intravenous pamidronate sodium injection, with positive dechallenge and rechallenge data. In 16 cases this occurred anteriorly and in one case, posteriorly.55 The most frequent ocular side effect of serious clinical importance associated with pamidronate is anterior uveitis; both eyes are affected in most patients and onset is within the first 48 hours of drug exposure. In some patients, the drug had to be discontinued, and the outcome was favorable within a few days after topical corticosteroid therapy.55,56 Pamidronate stimulates the production of a distinct group of T cells, which inhibit bone resorption. The activation of T cells releases cytokines, and this may contribute to an immunologic or toxic reaction in patients who develop uveitis or scleritis.57
A more recently developed oral bisphosphate, alendronate sodium (Fosamax), is 100 to 500 times more potent than amino-bisphosphonates and is being used successfully to prevent and to treat osteoporosis in postmenopausal women. Alendronate has also been associated with bilateral anterior uveitis,58 posterior scleritis, anterior scleritis, and orbital myositis that resolves with anti-inflammatory therapy and discontinuation of alendronate.59 There is no rechallenge data for alendronate. But, because this agent is in the same class as pamidronate, has the same pattern of onset, and requires discontinuation of the drug for the scleritis to resolve, a cause-and-effect relationship seems to be almost certain for alendronate.
A bilateral sudden-onset iritis in association with the use of trimethoprim-sulfamethoxazole has been reported by Tilden et al.60 The bilaterality and the recurrence of inflammation with rechallenge are strong evidence that systemic sulfonamides are a cause, albeit rare, of anterior uveitis. The intraocular inflammation may be the result of direct immunogenicity of sulfonamides or, as in the case of Stevens-Johnson syndrome, the result of a systemic, necrotizing vasculitis.54
Diethylcarbamazine (Ivermectin) is an antifilarial agent effective against Oncocerca volvulus, one of the leading causes of blindness in the world. Diethylcarbamazine rapidly and effectively kills microfilaria. Death of the microfilaria that are present in the cornea and anterior chamber liberates an antigenic load that may result in devastating intraocular inflammation. It is Jarisch-Herxheimer reaction, which is a hypersensitivity response, attributed to liberation of endotoxin-like substances or of flarial antigens from the killed or dying microorganisms. This reaction may exacerbate preexisting ocular inflammation, and prophylaxis with corticosteroids may be helpful.54,61,62
There have been old isolated reports of uveitis associated with oral contraceptives.63 There is one case of bilateral anterior uveitis64 and three cases of bilateral posterior uveitis and vasculitis in patients taking norethynodrel and mestranol. The evidence for causality is extremely weak, and there is no rechallenge data. Given the huge number of women using oral contraceptives, these rare cases may have occurred by chance alone. The pathogenesis of uveitis associated with oral contraceptives, if indeed this is a true entity, is unclear.54 Anterior granulomatous uveitis65 and acute nongranulomatous anterior uveitis66 have been reported in patients with hypersensitivity to quinidine.
Cidofovir (Vistide), a DNA polymerase inhibitor (HPMPC), has been successfully used for the treatment of cytomegalovirus retinitis and acyclovir-resistant herpes virus infections in patients with AIDS. Cidofovir-associated uveitis (CAU) has been described in 25% to 59% of patients receiving intravenous cidofovir.67,68 The uveitis is usually anterior, associated with posterior synechiae and accompanied by hypotony. It may be unilateral or bilateral, is dose related, and the risk is increased with highly active antiretroviral therapy (HAART) and with rising CD4+ cell counts.69 While CAU occurs mostly in eyes with inactive CMV retinitis, bilateral anterior uveitis was reported in an AIDS patient taking cidofovir because of presumed recurrence of CMV encephalitis. The patient was not on
HAART and had no evidence of CMV retinitis or any other abnormality on fundoscopy.70 Recently, several reports have proposed the use of cidofovir as a treatment for infections with the virus known as JC virus (JC are the initials of the first person diagnosed with this virus) that causes progressive multifocal leukoencephalopathy (PML) in patients with AIDS. Tacconelli et al.,71 in 2003, treated AIDS patients receiving HAART with cidofovir for CMV retinitis or PML. Sixty percent of CMV patients had CAU on the same side as the retinitis, whereas no cases were detected among the patients with PML. It is hypothesized that the retinal action of cidofovir is increased by concomitant retinal alteration caused by retinitis, previous mycobacterial disease, or toxoplasmosis. It is also possible that an increase in a patient's HIV-viremia level (viral load) may result in HIV-associated retinal alteration, which facilitates an increase in the penetration of cidofovir. CAU seems to occur more frequently in patients with retinitis, on HAART, in whom HAART has failed to restore immunity. The concomitant use of probenecid decreases the incidence of uveitis associated with intravitreal and also intravenous cidofovir.72 Probenecid inhibits renal tubular secretion of cidofovir73 and may inhibit secretion from the ciliary body, which shares many of the transport mechanisms in the kidney, resulting in decreased intraocular drug levels.67
Etanercept (Enbrel) inhibits the action of both TNF-α and TNF-γ. It is increasingly being used in the management of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. The development of rheumatoid nodules and leukocytoclastic vasculitis is reported with etanercept.74,75 A case of anterior nongranulomatous uveitis was reported in 2003 following subcutaneous etanercept treatment for ankylosing spondylitis.76 The close temporal association of the start of etanercept and the uveitis and challenge-rechallenge data suggests that etanercept might have provoked the anterior uveitis.
Ibuprofen, a noncorticosteroidal anti-inflammatory drug (NSAID), can cause aseptic meningitis. A patient with aseptic meningitis and bilateral nongranulomatous iridocyclitis related to ibuprofen has been reported. And it has been suggested that a hypersensitivity reaction to NSAID should be considered when a patient has neurologic abnormalities after initiation of NSAID therapy in the presence or absence of iridocyclitis.77
Topiramate (Topamax) is an oral sulfamate medication used primarily as an antiepileptic. It is also used in the treatment of bipolar disorders and pain control of migraine. Cases of acute myopia and bilateral angle-closure glaucoma with ultrasound biomicroscopic signs of ciliochoroidal effusion have been reported associated with topiramate use.78,79 Fluid accumulated in the supraciliary space with ciliary body detachment is the main factor producing anterior rotation of the ciliary body. This rotation pushes the iris anteriorly and closes the angle.80 Acute myopia is explained by the forward displacement of the lens caused by supraciliary effusion, although some authors suggest that ciliary body swelling and lens thickening may also play a role.81
Topical Drug-Induced Uveitis
The nonselective β-adrenergic blocking agent metipranolol (Optipranolol) appears to cause granulomatous anterior uveitis with prominent mutton fat82 or medium-sized keratic precipitates83 and can also cause nongranulomatous anterior uveitis.84 In 1991 Akingbehin et al.85 presented 15 patients who developed granulomatous anterior uveitis (GAU) with characteristic mutton-fat keratic precipitates after using metipranolol. Intraocular pressure elevations more than 5 mm Hg above their baseline levels developed in over half of the eyes with GAU. This rise in intraocular pressure with metipranolol-associated uveitis may be due to inflammatory debris blocking the trabecular meshwork. The authors rechallenged 7 of the original 26 patients using 0.3% metipranolol in one eye and 0.5% timolol in the fellow eye for up to 14 days.86 In all of the eyes that were rechallenged with metipranolol, GAU, marked intraocular pressure increase, blepharoconjunctivitis, or periorbital dermatitis developed within 14 days. Two weeks after discontinuing the medication, all the patients had complete resolution of symptoms. Uveitis did not develop in the eyes treated with timolol. Because of the relatively large number of cases, time of onset, corresponding laterality of occurrence to drug treatment, and recurrence upon rechallenge, this report strongly suggests that metipranolol was the cause of uveitis.54 The etiology of intraocular inflammation from metipranolol remains unclear.
In 2000, Byles et al.87 suggested that highly selective a-adrenoreceptor agonist brimonidine tartrate (Alphagan) caused granulomatous anterior uveitis in four patients, after 12 months of brimonidine use. In all cases, uveitis settled rapidly after cessation of the drug and recurred on rechallenge testing. Two more cases of granulomatous anterior uveitis as a suspected adverse reaction to topical brimonidine are also reported.88,89
Latanoprost (Xalatan), a prostaglandin analog, has also been reported to cause anterior uveitis. Uveitis improves after cessation of latanoprost with or without corticosteroids,90 and it recurs after rechallenge.91
Anticholinesterase drugs have been used to reduce elevated intraocular pressures and to control accommodative esotropia. Diisopropyl fluorophosphate and phospholine iodide are the most commonly used agents. Vascular congestion secondary to these agents is believed to result in the breakdown of the blood-aqueous barrier, causing mild iritis.54 These drugs may also enhance existing iritis and cause formation of anterior and posterior synechiae and fine keratic precipitates.92
Betaxolol is a cardio-selective β-1 blocker. A 78-year-old female patient with rheumatoid arthritis developed bilateral nongranulomatous anterior uveitis 3 weeks after starting betaxolol. The anterior uveitis in both eyes resolved promptly on stopping betaxolol in conjunction with weak steroid drops and did not reappear on stopping steroids in the follow-up period of 6 months. Rechallenge was not performed, but it was suggested that betaxolol might be responsible for the anterior uveitis in this case.93
Iritis can occur with the use of topical epinephrine, and the presence of antiepinephrine antibody has been documented in patients treated with topical epinephrine.94 Experimentally induced uveitis with the use of topical epinephrine has also been reported.95 Furthermore, topical epinephrine has been known to induce and exacerbate postoperative cystoid macular edema, supporting the possibility that topical epinephrine may induce inflammation in the eye.
Corticosteroid withdrawal may be associated with the development of ocular inflammation.Two patients with ocular hypertension developed unilateral ocular hypotony during a period of provocative testing with topical dexamethasone and a unilateral anterior uveitis 3 to 5 days after discontinuation of dexamethasone.96 In another series involving topical dexamethasone testing, 7 of 401 glaucoma patients and 9 of the 220 apparently healthy individuals developed acute anterior uveitis within a few days of discontinuation of corticosteroid drops. The incidence of uveitis was significantly higher in blacks than in whites; 5.4% compared to 0.5%. In two cases symptoms began while the patients were still using the dexamethasone drops. In the majority of cases, a sharp drop in intraocular pressure to a level lower than the baseline pressure was also seen. Treatment, when necessary, consisted of mild cycloplegia. Almost all patients recovered completely within a period of 3 to 10 days. Since a decrease in intraocular pressure occurs simultaneously with the development of uveitis, and the uveitis occurs after termination of the corticosteroid treatment, the drop in the intraocular pressure may be attributed to the decrease in aqueous humor production caused by the uveitis.97 In another prospective study, Mindel et al.98 compared the intraocular pressure response to four different topically applied corticosteroid esters. They found that acute iritis developed within 72 hours of discontinuation of topical corticosteroids in three eyes of 2 volunteers among 54 treated for 6 weeks. Both patients were black. Each eye had received a different corticosteroid ester. This report confirmed that withdrawal of different topical corticosteroids may be associated with intraocular inflammation. The explanation for this corticosteroid-induced uveitis is not clear; a toxic effect of corticosteroids may weakly explain uveitis.97 Because the incidence of corticosteroid-induced uveitis appears to be much higher in blacks than in whites, melanin may play a role.54 Reactivation of a latent ocular infection is one other possible explanation; that is, corticosteroids might have increased ocular susceptibility to an already existing subclinical intraocular infection.96,97
Intraocular Drug-Induced Uveitis
Drugs administered directly into the anterior chamber or vitreous cavity can cause inflammation, with breakdown of the blood-aqueous or blood-retinal barrier. However, the act of simply placing a sterile needle inside the anterior chamber, that is, without injecting any drug, may also produce iritis. Nearly all antibiotics injected intracamerally have been reported to produce inflammation. This anterior segment inflammation is usually transient and mild.54 Aminoglycosides have a particularly toxic effect on ganglion and other neural cells of the retina.99
Urokinase, a plasminogen activator, is a clot-specific fibrinolytic agent that has been successfully used to treat fibrin membrane formation. Tissue plasminogen activator has been used to treat impending pupillary block glaucoma in patients with acute fibrinous HLA-B27 positive iridocyclitis.100 Intravitreal injection of urokinase has been reported to produce a sterile hypopyon-like reaction in up to 50% of patients with vitreous hemorrhages. The hypopyon was probably not due to inflammation because it was not associated with conjunctival injection, chemosis, or ocular pain. Resolution occurred within 6 days, regardless of whether or not topical corticosteroid therapy was administered.101
Cidofovir, previously described in the section on systemic agents, may also cause uveitis after intravitreal injection. Chavez de la Paz et al.,72 in 1997, reported the incidence and findings of an anterior nongranulomatous uveitis that occurred after intravitreal injection of 20 μg of cidofovir in 46 patients with AIDS and cytomegalovirus retinitis. Of the 130 injections in 69 eyes, 30 cases (23%) developed iritis after a median post injection period of 4 days. Posterior synechiae occurred in 37% of eyes. Oral probenecid treatment before cidofovir injection was associated with a statistically significantly lower incidence of iritis. It is hypothesized that oral probenecid might decrease or suppress the absorption of cidofovir into the ciliary body and reduce the incidence of iritis.73
Sterile endophthalmitis after intravitreal injection of triamcinolone diacetate (Kenolog) has been reported. This may be a toxic reaction to preservatives in the diluents or possibly a poorly understood immune reaction.102
Vaccination-Induced Uveitis
Bacilli Calmette-Guérin (BCG) vaccine is used to immunize against tuberculosis and to treat carcinoma in-situ of the bladder. Donaldson et al.103 reported two cases of bilateral uveitis associated with vitiligo in patients injected subcutaneously with multiple 0.1 mL aliquots of BCG for metastatic malignant melanoma. There was associated severe atrophy of both irides of the two patients. Arthritis and unilateral iritis developed within 4 weeks in three patients who were treated with intravesical BCG therapy for superficial bladder carcinoma.104,105,106
One patient was HLA-B27 positive.105 The BCG vaccine appeared to be an adjuvant for melanin-induced uveitis.105,107 The second possible mechanism is a direct toxic effect of melanin on the uvea. Melanin is abundant in patients with metastatic malignant melanoma. Therapies that destroy melanoma tissue will release this pigment. Melanin toxicity has been implicated in the development of intraocular inflammation.107 Melanin may also act as a depot for antigens and thus hold antigens and promote inflammation in the eye.108
Panuveitis has been reported in patients after tuberculin challenge. Lish and Berman109 reported development of bilateral anterior segment inflammation and vitritis 2 weeks after a tuberculin (purified protein derivative) skin test. The time course suggested a cell-mediated immune response (type IV delayed-type hypersensitivity reaction) to the skin test. The ocular inflammation improved after 4 weeks of treatment with systemic antimycobacterial medications and 8 weeks of treatment with prednisone. Nussenblatt and Palestine110 described a multifocal choroiditis resembling Vogt-Koyanagi-Harada syndrome 5 days after tuberculin challenge in a woman who was known to be tuberculin positive in the past. The uveitis was characterized by serous retinal detachments and choroidal Dalen-Fuchs-like nodules throughout the fundus. This patient also improved with systemic corticosteroids. In these patients the uveal tract may have been sensitized by hematogenous spread of mycobacteria or tuberculous proteins during the initial clinical phase of the pulmonary tuberculosis.109
A 77-year-old woman developed recrudescence of iritis, vitreous haze, and clinical cystoid macular edema within 2 weeks after receiving influenza vaccine.111 Whether the effect was the result of circulating immune complexes, some antigenic reaction with inflamed tissue or other mechanisms is unknown at this time. There have been three reports of uveitis after influenza vaccination involving a total of five patients. Blumberg et al.112 reported development of optic neuritis in one eye and iritis in the fellow eye after vaccination with a split product of A-Victoria influenza. This patient did not have a previous history of uveitis in either eye. No rechallenge was given. The authors suggested that small-vessel vasculitis may have been the mechanism for the iritis and optic neuritis. Knopf111 reported one case of recurrent uveitis after influenza vaccination, and this was followed by a report of three additional cases.113 All four had a history of unilateral recurrent intraocular inflammation prior to vaccination. One patient had had recurrent iritis and scleritis for 25 years prior to vaccination. Another had had a single episode of iritis 5 months earlier. Two other patients had had previous cataract surgery complicated by vitreous loss, recurrent iritis, and vitritis from 7 to 12 months prior to influenza vaccination. Patients ranged in age from 51 to 77 years. Systemic and laboratory evaluations were performed in two patients and were negative. Three patients developed iritis and one developed iritis and vitritis in the previously inflamed eye within 2 weeks of vaccination. In none of the patients did iritis develop in the fellow eye after vaccination. In three patients the iritis responded to topical corticosteroids and nonsteroidal anti-inflammatory agents. One patient required both topical and systemic corticosteroids. No rechallenge was administered to any of the four patients.113 Because four of these five patients had a history of prior uveitis, the vaccination may have been coincidental and unrelated to the uveitis. Alternatively, the vaccination may have been a nonspecific stimulant to a preexisting intraocular inflammation. As the fellow eye was not involved in any of the patients, the authors speculated that a low-grade inflammatory response produced by the vaccine was additive to an already compromised blood-ocular barrier, leading to the reactivation of uveitis.111,113
Uveitis secondary to live attenuated varicella virus vaccine was reported in a 16-year-old girl 7 days after receiving vaccine. The incidence of vaccine-induced chickenpox is estimated at about 3% to 5%. Cutaneous eruptions may occur after varicella vaccine immunization, including vaccine-strain varicella, which occurs within 30 days of vaccination; wild strain varicella, which occurs more than 30 days after vaccination because of incomplete immunity after vaccination; and zoster caused by reactivation of the vaccine or wild-strain varicella.114 In 2003, a 9-year-old boy presented with herpes zoster ophthalmicus 3 years following vaccination with live attenuated varicella vaccine (Oka strain). Amplified DNA from fluid taken from the base of a cutaneous vesicle produced wild-type varicella zoster virus (VZV) DNA not Oka strain. HZV infection therefore needs to be considered in all patients who present with scleritis, keratitis, or anterior uveitis, regardless of their varicella vaccine status.115
Two children developed anterior uveitis 4 to 6 weeks after having received the combined vaccination for measles, mumps, and rubella (MMR). During immunization viral antigen or tissue culture products may have initiated the early events in the immune activation pathway to induce anterior uveitis by antigen mimicry.116
Iridocyclitis, chorioretinitis, and peripheral periphlebitis have occurred after antityphoid vaccination.117,118 Nongranulomatous iritis after administration of diphtheria antitoxin and antitetanus vaccination has also been described.119
An acute bilateral posterior uveitis, which occurred after first and second boost of a hepatitis B vaccination, has been reported. It was suggested that the surface antigen of the vaccine and HBs antibodies of the immune response after vaccination might have formed immune complexes, which initiated bilateral uveitis.120
Although vaccine induced uveitis is rare, parients with inflammatory eye disease should be questioned carefully about recent immunizations.