UVEAL MELANOMA
Any elderly patient with a unilateral media opacity and inflammation has a uveal melanoma until proven otherwise with appropriate imaging studies and, when necessary, invasive diagnostic techniques.4 Although unapparent intraocular foreign bodies are a much more common cause of unilateral cataract, in as many as 10% of blind eyes submitted to eye pathology laboratories, an undiagnosed uveal melanoma is found. All adults with unexplained unilateral media opacity require a thorough evaluation, including a diagnostic ultrasound examination.5,6 We have managed many very large uveal melanomas that were discovered only after cataract extraction and were not detected at the time of ultrasonic biometry to measure axial length.
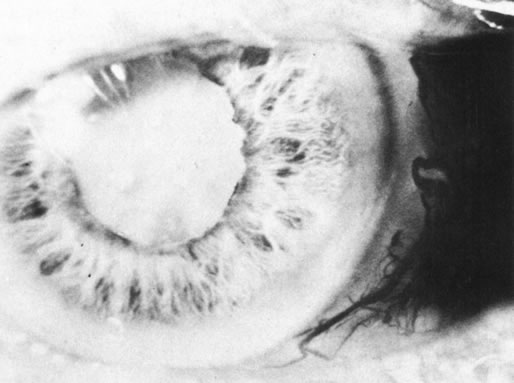
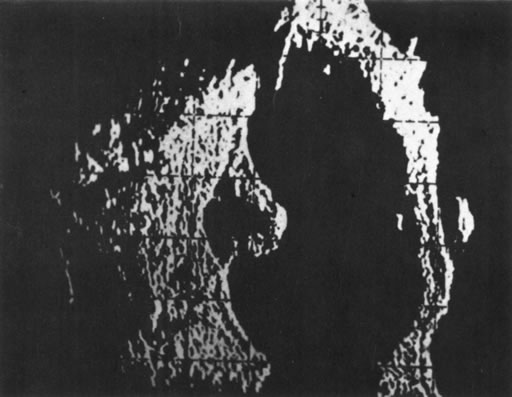
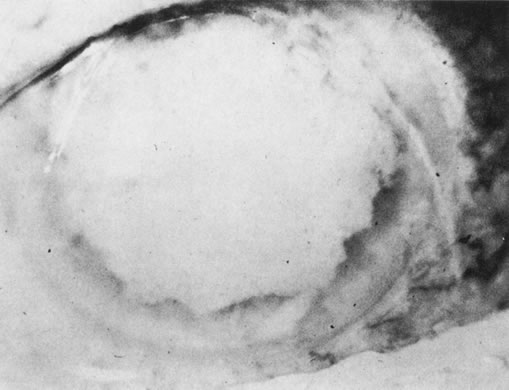
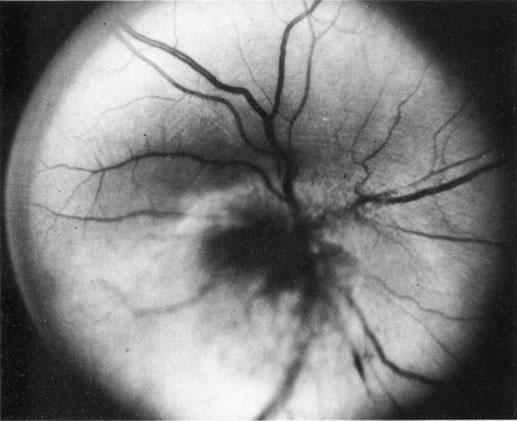
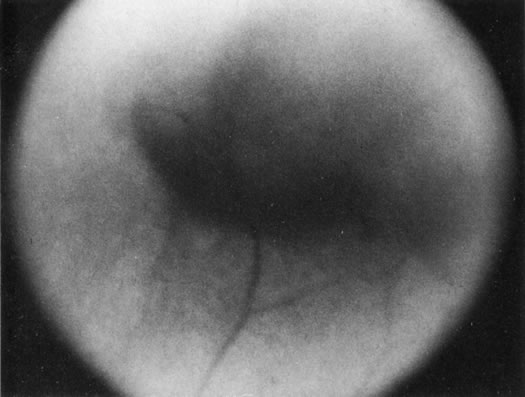
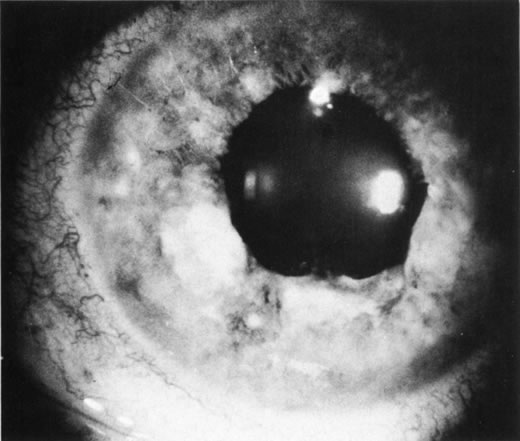
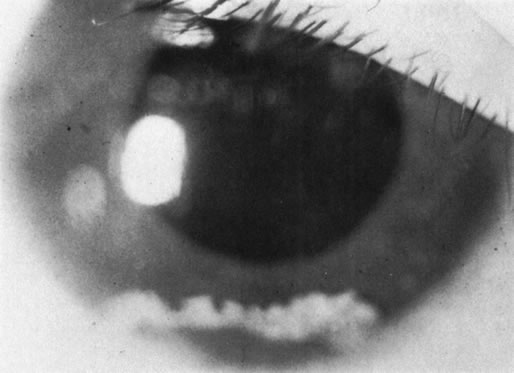
Necrotic melanomas account for approximately 5% of uveal melanomas; in the majority of cases, there is intraocular inflammation accompanying the cataract.7–9Figures 1 and 2 demonstrate a typical case. The patient had a long history of unilateral decreased vision. The eye had become painful 1 month before admission, and he was referred for evaluation of uveitis. Clinically, there was a dense, unilateral cataract with significant intraocular inflammation, which was manifested as a ciliary flush with 2+ cells and flare. Media opacity obscured all fundus detail. An immersion B-scan demonstrated a large intraocular tumor that was most consistent with a uveal melanoma. The eye was removed, and the diagnosis was confirmed histologically.10
|
|
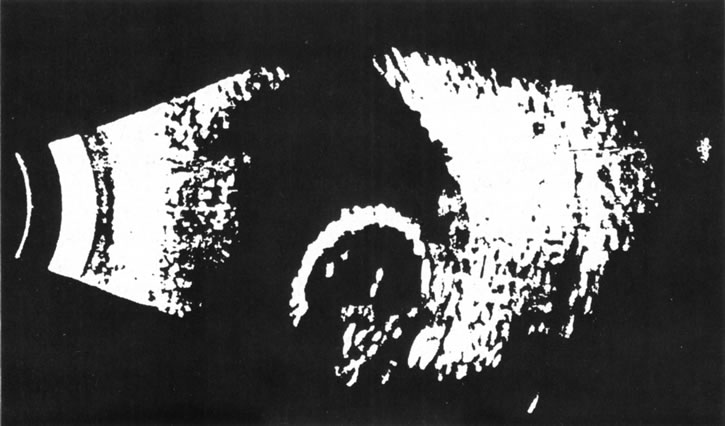
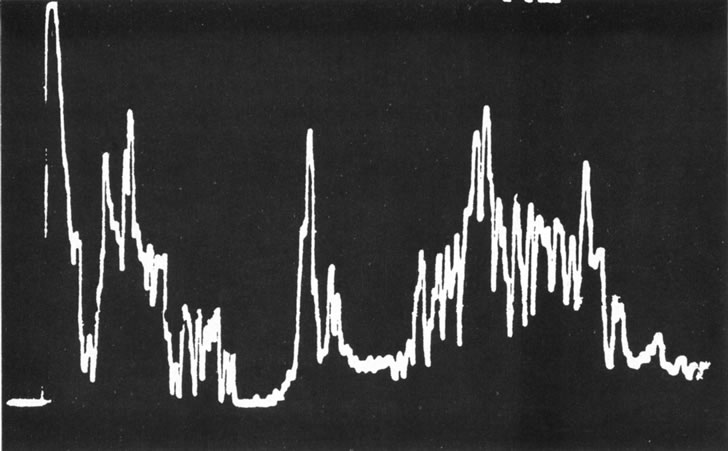
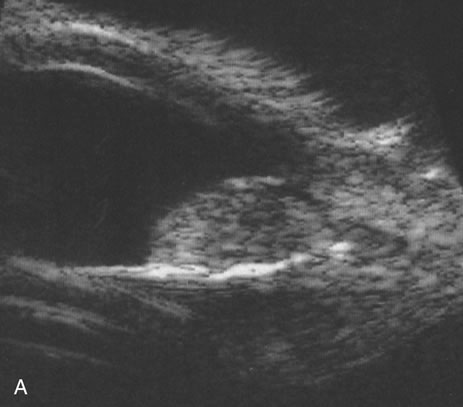
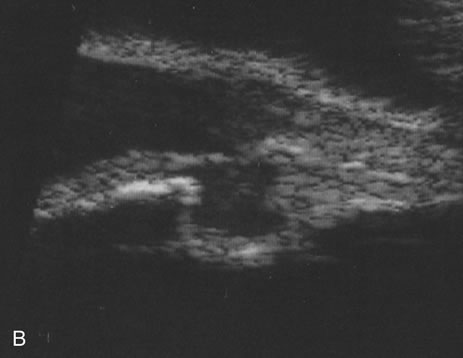
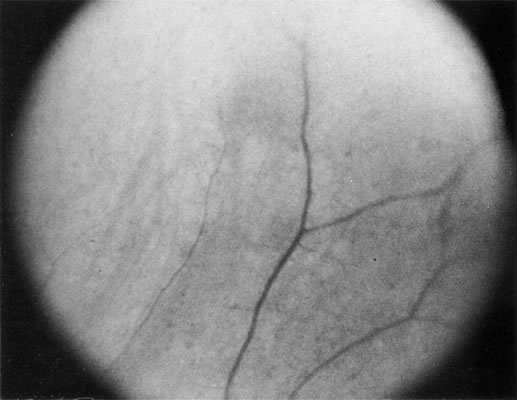
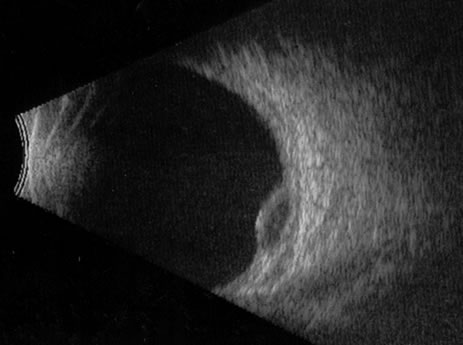
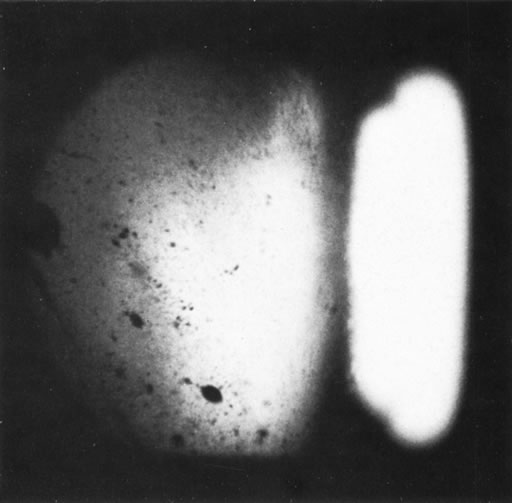
Unfortunately, imaging studies are not always diagnostic. The A- and B-scans shown in Figures 3 and 4 were from a patient with unilateral media opacity referred for evaluation and treatment of a presumed uveal melanoma. Occasionally, if the cataractous lens is scanned tangentially, artifact can occur. A repeat ultrasound at our institution was negative for tumor, and the referral ophthalmologist who performed a cataract extraction noted that no tumor was present and that the patient has an excellent visual outcome. In contrast, the patient shown in Figure 5 was referred with a presumed uveal melanoma with secondary inflammation and a history similar to that described for the patient shown in Figure 111 Ultrasound and CT examinations were not diagnostic. Because the eye was blind and painful, it was enucleated, and an extremely necrotic uveal melanoma was noted histologically. In some necrotic melanomas, the fundus cannot be visualized and the diagnosis cannot be established with imaging studies. Very rarely, a necrotic uveal melanoma can produce sufficient pigment dispersion into the vitreous to obscure the correct diagnosis.12
|
|
The role of MRI in the evaluation of intraocular masquerade syndromes is unclear. There was initial enthusiasm about the possibility of differentiating atypical uveal melanomas from hemorrhagic-simulating lesions based on the unique paramagnetic properties of melanin.13 In relation to vitreous, a uveal melanoma typically has a bright signal on a T1 image and a dark signal on a T2 image. In contrast, hemorrhagic processes tend to be either bright or dark on a T1 image and bright on a T2 image.14
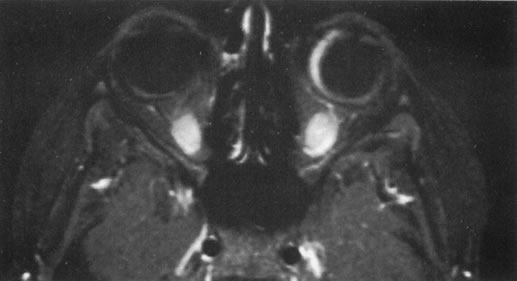
Unfortunately, there are several problems with MRI for uveal melanoma diagnosis. First, several investigators have shown that unless high field-strength, thin-section scans with surface coils are used, the quality of data obtained is suboptimal.15 Second, even in a state-of-the-art center, we have had a number of cases in which we were unable to differentiate between a uveal melanoma and a simulating lesion on the basis of MRI data. We obtain MRI studies in older patients with opaque media, equivocal ultrasonographic data, and large masses. In some of these cases, the MRI is diagnostic of either a melanoma or an extramacular disciform (hemorrhagic) process. It is also a useful adjunctive study if localized extraocular extension is suspected. In equivocal cases, however, we proceed to using invasive diagnostic techniques, such as fine-needle aspiration biopsy (FNAB) (see Metastatic Tumors).
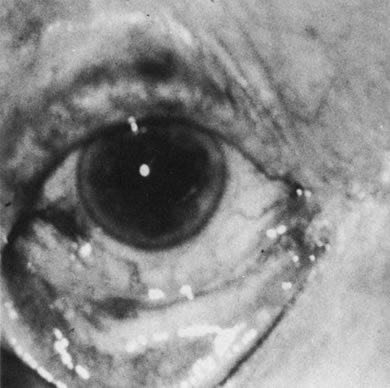
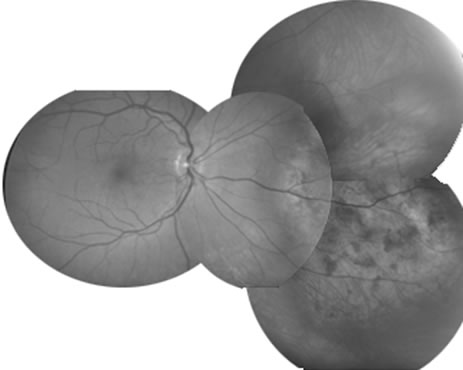
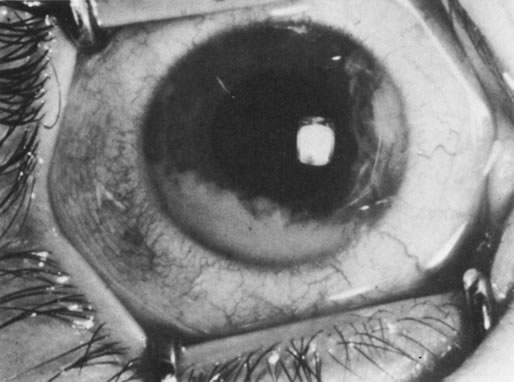
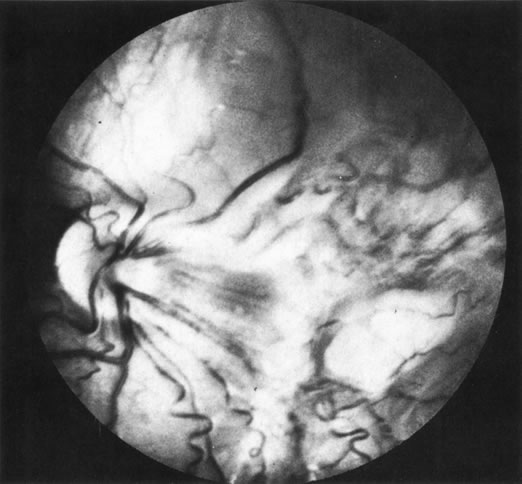
In addition to producing media opacity, inflammation, and cataract, uveal melanomas can also produce other signs that may lead to the incorrect diagnosis of uveitis. Anterior melanomas, especially those that involve the ciliary body, often produce dilated episcleral vessels (sentinel vessels) that occasionally have been misdiagnosed as scleritis (Fig. 6).
|
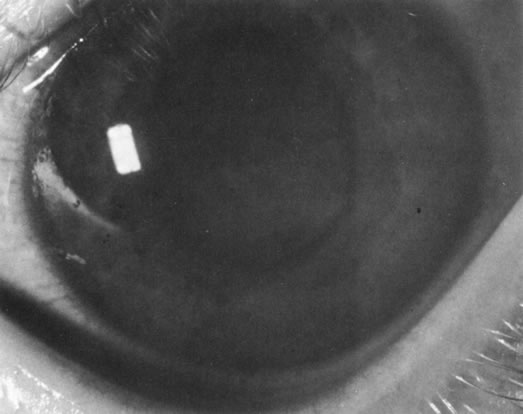
Iris melanomas can produce anterior chamber cell and flare, and if the tumor is located in the extreme iris periphery, it may be missed during a cursory examination. Iris melanomas, especially those in a circumferential or ring configuration, are often difficult to diagnose and may simulate either an anterior uveitis or glaucoma.16 Newer, higher-megahertz (high-frequency) ultrasound equipment is better for imaging anterior uveal tumors (Fig. 7).
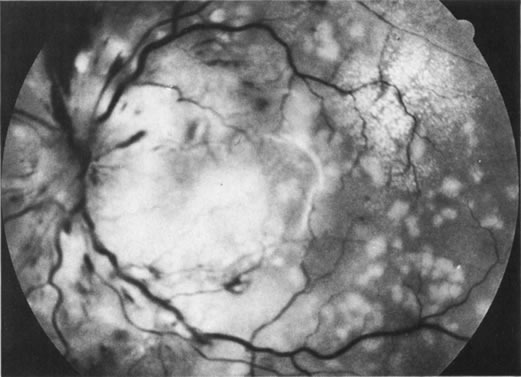
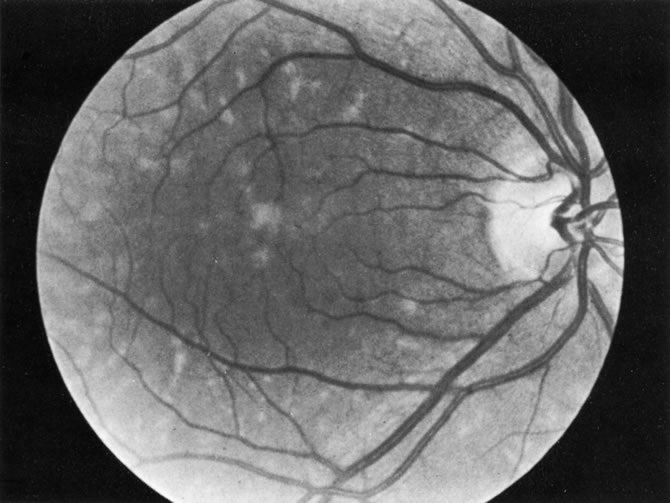
If direct ophthalmoscopy is used, a circumferential peripapillary melanoma can simulate optic neuritis (Fig. 8).
|
Pseudomelanoma
Melanomas can masquerade as uveitis, and some inflammations or hemorrhagic processes can simulate melanoma. These processes include posterior scleritis, focal choroidal hemorrhage or detachments, and focal scleral thinning. Figures 9 and 10 demonstrate focal scleral thinning in the area of the superior rectus insertion in a patient with rheumatoid scleritis that produced a pseudomelanoma. An apparent elevated pigmented mass was noted on upgaze, but no tumor was observed when the eye was in primary position. Posterior scleritis is often not painful, although some of these patients have deep, piercing pain and increased discomfort during eye movement. Ultrasound usually is diagnostic, demonstrating fluid inside of Tenon's capsule (Fig. 11). A focal hemorrhagic choroidal detachment after cataract surgery can simulate a melanoma. These lesions usually have a bright area of hemorrhage at their base, whereas melanomas typically do not have associated hemorrhage until they break through Bruch's membrane (most commonly, such tumors are more than 5 mm thick). The differential diagnosis of uveal melanoma is discussed elsewhere in these volumes.
|
|
|
METASTATIC TUMORS
Choroidal metastases can also produce signs that mimic uveitis. As many as 50% of patients with metastases to the eye may present to the ophthalmologist before the primary malignancy is discovered.17 Patients with renal and lung carcinomas have the highest frequency of presenting with eye symptoms as the initial complaint associated with the neoplasm; in contrast, more than 90% of breast carcinomas have a known primary at the time ocular metastases are noted. Usually, intraocular metastases are solid, posterior, amelanotic, choroidal tumors; however, in less than 10% of cases, they can simulate a benign intraocular inflammation. At least five patterns of inflammatory masquerade syndromes can occur with intraocular metastases.
- Tumors may produce a pseudoscleritis.
- Patients with metastatic carcinoma may present with a diffuse infiltration
of the iris (Fig. 12).
- In rare cases, an optic nerve metastasis can simulate either an optic neuritis
or an acute anterior ischemic neuropathy (Fig. 13).
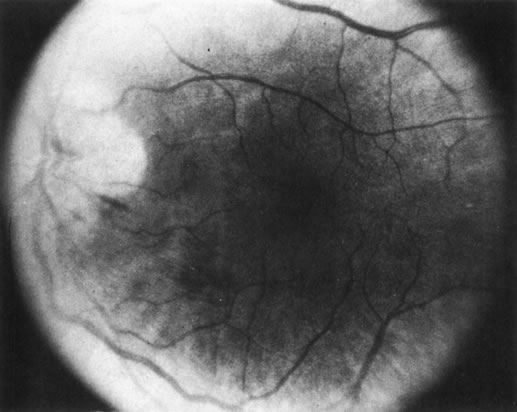 Fig. 13. Bronchiogenic carcinoma metastatic to the optic nerve. (Char DH: Clinical
Ocular Oncology. 2nd ed. Philadelphia, Lippincott-Raven, 1996.)
Fig. 13. Bronchiogenic carcinoma metastatic to the optic nerve. (Char DH: Clinical
Ocular Oncology. 2nd ed. Philadelphia, Lippincott-Raven, 1996.) - Isolated metastatic cells in the vitreous or anterior chamber may occur
as the only manifestation of an ocular metastasis.18
- Figure 14 shows a patient with bilateral pseudohypopyon as the first stigmata of
widespread disease.
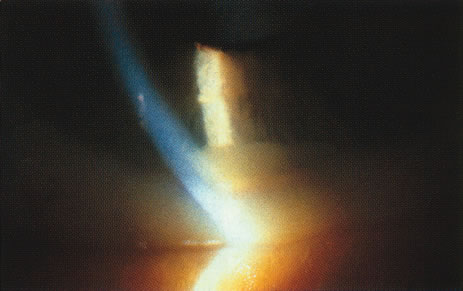 Fig. 14. Bilateral pseudohypopyon as the initial manifestation of widespread metastases.
Fig. 14. Bilateral pseudohypopyon as the initial manifestation of widespread metastases.
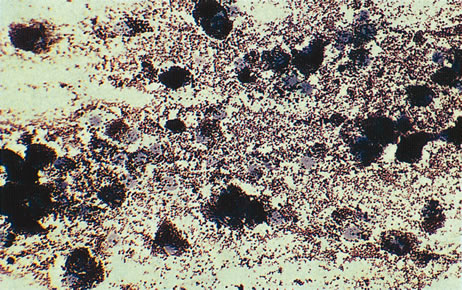
Most commonly, pseudohypopyons in adults develop in association with either a systemic lymphoma or leukemic relapse. Rarely, metastatic cutaneous melanoma can produce this pattern; however, usually these later cells are pigmented (Fig. 15). Much more frequently, vitreous cells in patients with known metastases are benign, originating from either infectious or endogenous uveitis.
A masquerade syndrome secondary to a distant malignancy can take several forms. In some metastatic tumor patients, multiple areas of increased choroidal pigmentation develop. Although these paraneoplastic syndromes are rare, a more common form is autoimmune destruction of retinal photoreceptor or ganglion on cells that more commonly occur either with small cell carcinomas of the lung or metastatic cutaneous melanoma.19–23
The evaluation of patients with metastatic tumors to the eye may be difficult. Approximately 10% of uveal melanoma patients have a history of a systemic malignancy. Approximately 50% of patients with uveal metastases are found to have an elevated plasma carcinoembryonic antigen or cancer antigen 125 level; in contrast, uveal melanoma patients do not have significantly elevated levels.24 Most metastases to the uveal tract can be correctly diagnosed with ultrasonography. In atypical presentations of metastatic tumors in which there is diagnostic uncertainty, we have confirmed the diagnosis with an intraocular FNAB.18
In several ocular oncology units, FNAB has become an important diagnostic adjuvant in difficult cases.25–27 We use FNAB in intraocular oncologic cases that cannot be diagnosed with noninvasive techniques.4 We have observed no false-positives in uveal melanoma diagnosis, and in that malignancy, we can accurately predict prognosis on the basis of the cellular composition (percentage of epithelioid cells) of the aspirate.27 Rarely in a necrotic melanoma, insufficient viable tumor cells are present to establish a diagnosis with this technique. In several lesions that simulate either retinoblastoma or melanoma, cytologic pattern on FNAB is diagnostic. We have found this technique especially useful in atypical metastatic uveal tumors. Often such patients are incorrectly diagnosed as having either a uveal melanoma or a benign lesion. Establishing the correct diagnosis results in optimum treatment. A misdiagnosis of a uveal melanoma would require a subsequently higher radiation dose with a higher likelihood of radiation vasculopathy. Conversely, a misdiagnosis of a benign process would result in ineffective therapy.
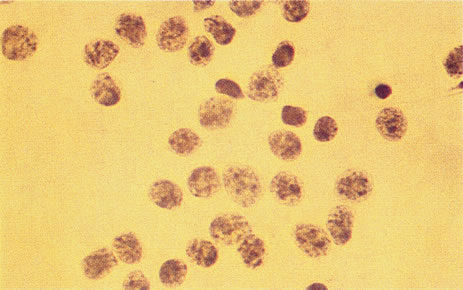
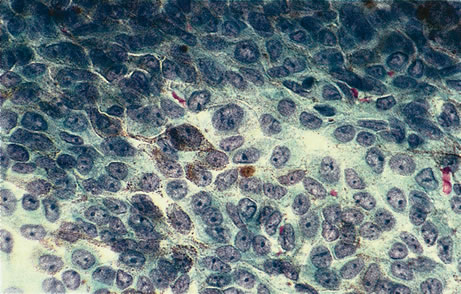
Figure 16 shows a case of what clinically and ultrasonographically appeared to be a uveal melanoma. The FNAB specimen revealed a cellular pattern that was diagnostic of a primary carcinoid (Fig. 17), and the patient responded dramatically with 40 gray of photon radiation. Figure 18 shows the FNAB cytopathology of a typical epithelioid choroidal melanoma; in contrast, a smear of a spindle cell tumor has spindle-shaped cytoplasm and smaller nuclei. Figure 19 shows a benign pigmented mass, such as a retinal pigment epithelial proliferation or a melanocytoma. In contrast to a melanoma, this type of mass has much larger pigment granules and benign cytomorphologic detail. In addition to standard cytopathologic FNAB evaluation, aspirated material may be used for several molecular biology studies (e.g., fluorescence in situ hybridization, comparative genomic hybridization), flow cytometry, special stains, tissue culture, and ultrastructural analyses.
|
|
|
A number of nonuveitis entities can produce vitreous cells, and some of these conditions are listed in Table 1.
Table 1. Vitreous Cells Not Associated With Uveitis
Primary and secondary intraocular lymphomas
Metastatic tumors
Retinoblastoma
Old vitreous hemorrhage (diabetes, retinal detachment)
“Tobacco dust” from retinal detachment
Battered baby syndrome
Retinitis pigmentosa
Asteroid hyalosis
Synchysis scintillans
Familial amyloidosis
INTRAOCULAR LYMPHOMA
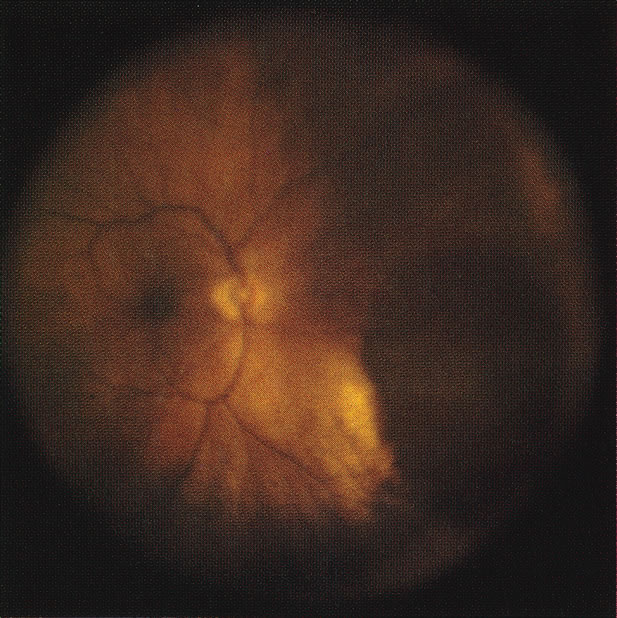
Intraocular lymphoma is usually initially misdiagnosed in older patients as a chronic diffuse uveitis.28 In a series of 828 consecutive patients at the uveitis center, approximately 1.5% of cases (13), had intraocular lymphomas.29 In any patient more than 50 years of age with an onset of a diffuse uveitis or vitritis, the diagnosis of intraocular lymphoma must be excluded. The classification and nomenclature of intraocular lymphomas is in flux.28,30,31 Erroneously termed ocular reticulum cell sarcoma, this neoplastic disease is usually limited to the eye and central nervous system (CNS). This process was initially thought to be due to a proliferation of malignant reticulum cells, but more recent studies have shown that this is usually a malignant B-cell lymphoma.30 Most investigators have adopted the term primary central nervous system lymphoma (PCNSL). The incidence of this disease has increased, although reasons for the rise are unclear.32 Ocular involvement occurs between 12% and 25% of patients. There are three types of intraocular lymphomas.
- The intraocular findings in primary intraocular–CNS lymphoma may
simulate a diffuse uveitis, usually with a plethora of vitreous cells
and often with virtually pathognomonic yellowish white chorioretinal infiltrates (Fig. 20). Less common clinical presentations can mimic toxoplasmosis, acute
retinal necrosis, a branch vein occlusion, or various forms of retinal
vasculitis.
- A less common form of primary ocular lymphoma consists of lesions in the
choroid with fewer vitreous cells. Systemic, non-CNS lymphoma
usually develops in these patients.33 Rarely, we have observed a variant of this second group, in which a patient
has a solid intraocular lymphoma that involves the choroid as the
first manifestation of widespread disease.34
- Intraocular involvement may occur as a sequela of systemic lymphoma, often
as the first sign of reactivation after therapy. Such patients usually
present with a malignant hypopyon (Fig. 21). In very rare cases, a patient with mycosis fungoides, a cutaneous
T cell lymphoma, is found to have an associated anterior or posterior
uveal involvement.
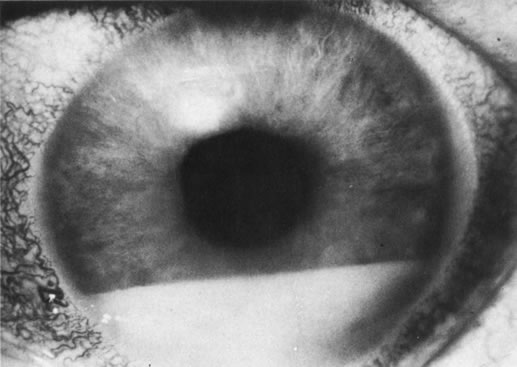 Fig. 21. Malignant hypopyon in a patient with systemic lymphoma.
Fig. 21. Malignant hypopyon in a patient with systemic lymphoma.
In all three forms of intraocular malignant lymphoma, the diagnosis is made with FNAB. In rare instances, a chorioretinal biopsy is necessary.35,36 In 15% of cases, a single vitreous biopsy may not demonstrate malignant cells, and multiple biopsies may be required.28
All patients with intraocular lymphoma require a thorough systemic evaluation, including examination of cerebral spinal fluid cytology, gadolinium-enhanced brain MRI, abdominal-chest CT, routine blood studies, and bone marrow biopsies. Probably, positron emission tomography scans will supplant some of these studies, but at present, data from this entity are insufficient. Historically, primary intraocular–CNS lymphomas were almost uniformly fatal, but newer, aggressive therapies with either intrathecal or systemic chemotherapy plus eye and CNS irradiation have produced long-term survival in some patients. Early diagnosis and treatment are associated with better prognosis, so patients should be evaluated promptly.28
The diagnostic evaluation of these patients continues to evolve. Superb cytologic analysis remains the central diagnostic test. Several approaches can be used to assess monoclonality (usually associated with lymphoma) versus polyclonality (usually associated with benign processes). The major problem with all of these techniques is that the vitreous sample is limited, and all of these ancillary assays may have false-positive and false-negative results. Flow cytometric features of lymphoma include an abnormal kappa-lambda ratio of either greater than 3 or less than 0.33, and monoclonal expansion of either B cells (more common) or T cells.37,38 A more elegant technique is to adapt a variation of polymerase chain reaction assay to identify a clonal B or T cell expansion, gene rearrangements, or oncogenes. However, errors do occur even with these sensitive assays.39,40 A few vitrectomy specimens that were not diagnosed cytologically were correctly diagnosed with these techniques.41,42 In addition, often the vitreous ratio of two cytokines, interleukin (IL)-10 and IL-6, is increased in lymphoma; however, exceptions occasionally have been reported.43,44
Pseudolymphoma
A benign variant of intraocular lymphoid tumors may occur in either an isolated form or, more commonly, a diffuse form involving both the intraocular contents and contiguous sclera and orbit.45 Usually these lesions simulate a solid intraocular neoplasm (e.g., melanoma), and they may occur with or without pain (Fig. 22). Most of these patients have an ultrasound pattern consistent with a melanoma, and the correct diagnosis is established with FNAB. As Cockerham and colleagues46 noted, the episcleral lesion may appear benign, whereas the intraocular component demonstrates lymphoma.
|
Rarely, after liver transplantation, patients develop a spectrum of post-transplantation lymphoid proliferative disorders that may range from benign to malignant lymphomas and have involved the eye.47–49